Abstract
Background: Obstructive sleep apnea (OSA) activates several pathophysiological mechanisms which can lead to the development of vascular diseases. Endothelial dysfunction (ED) is an initial step in the development of atherosclerosis. The association between ED and OSA has been described in several studies, even in previously healthy subjects. High-density lipoproteins (HDL) were generally considered to be atheroprotective, and low-density lipoprotein (LDL) to be an atherogenic component of lipoproteins. However, recent findings suggest a pro-atherogenic role of small HDL subfractions (8–10) and LDL subfractions (3–7). This study aimed to evaluate the relationship between endothelial function and lipid subfractions in previously healthy OSA subjects. Material and Methods: We prospectively enrolled 205 subjects with sleep monitoring. Plasma levels of triacylglycerols, total cholesterol, LDL, HDL, and their subfractions were assessed. Endothelial function was determined using peripheral arterial tonometry, and reperfusion hyperemia index (RHI) was assessed. Results: Plasma levels of small and intermediate HDL subfractions have statistically significant pro-atherogenic correlations with endothelial function (p = 0.015 and p = 0.019). In other lipoprotein levels, no other significant correlation was found with RHI. In stepwise multiple linear regression analysis, small HDL (beta = −0.507, p = 0.032) was the only significant contributor in the model predicting RHI. Conclusions: In our studied sample, a pro-atherogenic role of small HDL subfractions in previously healthy subjects with moderate-to-severe OSA was proven.
1. Introduction
Obstructive sleep apnea (OSA) is a common disorder that occurs in approximately one-quarter of adults. The prevalence of OSA (apnea/hypopnea index (AHI) over 5) is about 24% in men and 9% in women [1]. Unrefreshing sleep with excessive sleepiness is the most common presenting symptom of OSA. Patients experience snoring as well as awakenings accompanied by gasping or choking. Effective therapeutic approaches include weight loss, positive airway pressure, oral appliances, surgical modification of the pharyngeal soft tissues or facial skeleton, and hypoglossal nerve stimulation in selected cases [2]. OSA is characterized by repeated partial or complete obstructions in the upper airways, leading to large changes in intrathoracic pressure, consequent hemodynamic changes, chronic intermittent hypoxia, and sleep fragmentation [3]. Intermittent hypoxemia with concomitant hypercapnia activates the sympathetic nervous system and contributes to elevation of blood pressure. Repetitive respiratory events increase reactive oxygen species, which may also contribute to vascular disease [3]. OSA activates several other pathophysiological mechanisms which can lead to the development of vascular diseases including endothelial dysfunction, systemic inflammatory response, and impaired glucose and lipid metabolism [4,5]. Endothelial dysfunction is an important underlying pathophysiological mechanism of atherogenesis that occurs at the early stages of vascular diseases and is associated with the occurrence of vascular events in the future [6,7]. Multiple mechanisms underlying OSA, including oxidative stress and systemic inflammation, are also important mechanisms in the pathogenesis of decreased nitric oxide bioavailability and subsequent endothelial dysfunction [8]. Endothelial dysfunction can be assessed by various methods, including peripheral arterial tonometry (PAT). The reperfusion hyperemia index (RHI) measured by PAT is a validated marker of endothelial function and a predictor of future vascular events [9,10,11]. Endothelial dysfunction is defined by RHI as lower than 1.67. Endothelial dysfunction is present also in previously healthy subjects with newly diagnosed OSA, and OSA is associated with the severity-dependent impairment of endothelial function assessed by RHI in both adults and children [12,13,14]. Dyslipidemia, defined as an excessive increase in total cholesterol (TC) or triacylglycerols (TAG), with or without a concomitant decrease in high-density lipoproteins (HDL), leads to the acceleration of the atherosclerotic process in predisposed individuals and is also one of the most important risk factors for vascular disease [15]. Along with increased sympathetic nervous system activity, oxidative stress, systemic inflammation, subsequent hypertension and glucose metabolism impairment [16], dyslipidemia is one of the possible mechanisms linking OSA with increased vascular morbidity [17]. The mechanisms of dyslipidemia induced by OSA include the up-regulation of lipoprotein biosynthesis, increased lipolysis, impaired clearance of lipoproteins, lipoprotein peroxidation, and HDL dysfunction [18]; this may represent a pathway by which the increase in cardiovascular risk is mediated [19]. There is also increasing evidence that small dense LDL and HDL subfractions have pro-atherogenic properties [20,21]. This study aimed to evaluate the relationship between endothelial function and lipid subfractions in previously healthy OSA subjects.
2. Materials and Methods
2.1. Study Population
A total of 205 patients were included in this prospective monocentric study. The cohort includes patients who were suspected of suffering from OSA and were hospitalized in the sleep laboratory of the 1st Department of Neurology, Comenius University, and University Hospital Bratislava. The study was approved by the Ethics Committee of the Old Town Hospital, University Hospital Bratislava (with reference number 26/2021). All participants signed informed consent before enrollment.
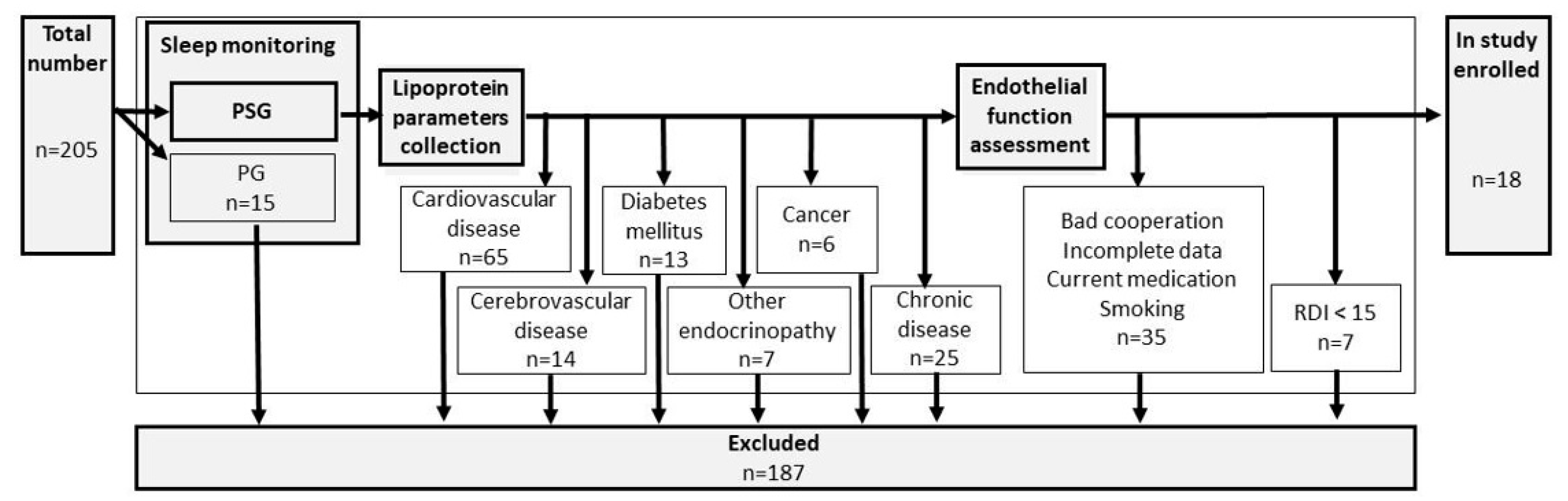
Inclusion criteria were set up as follows: suspicion of OSA, sleep monitoring by polysomnography, and aged over 18. A detailed search for premorbid diseases was performed and exclusion criteria included: cardiovascular disease, cerebrovascular disease, diabetes mellitus, other endocrinopathies, cancer, or any other chronic diseases. Sleep monitoring by limited polygraphy (PG), bad cooperation, incomplete data, use of any current medication, or smoking belonged to the additional exclusion criteria. For details, see flowchart Figure 1.
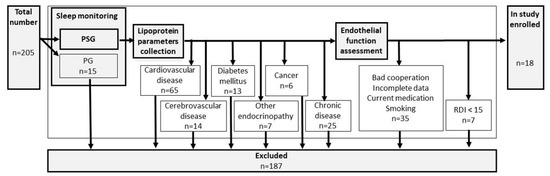
Figure 1.
Flowchart of the study.
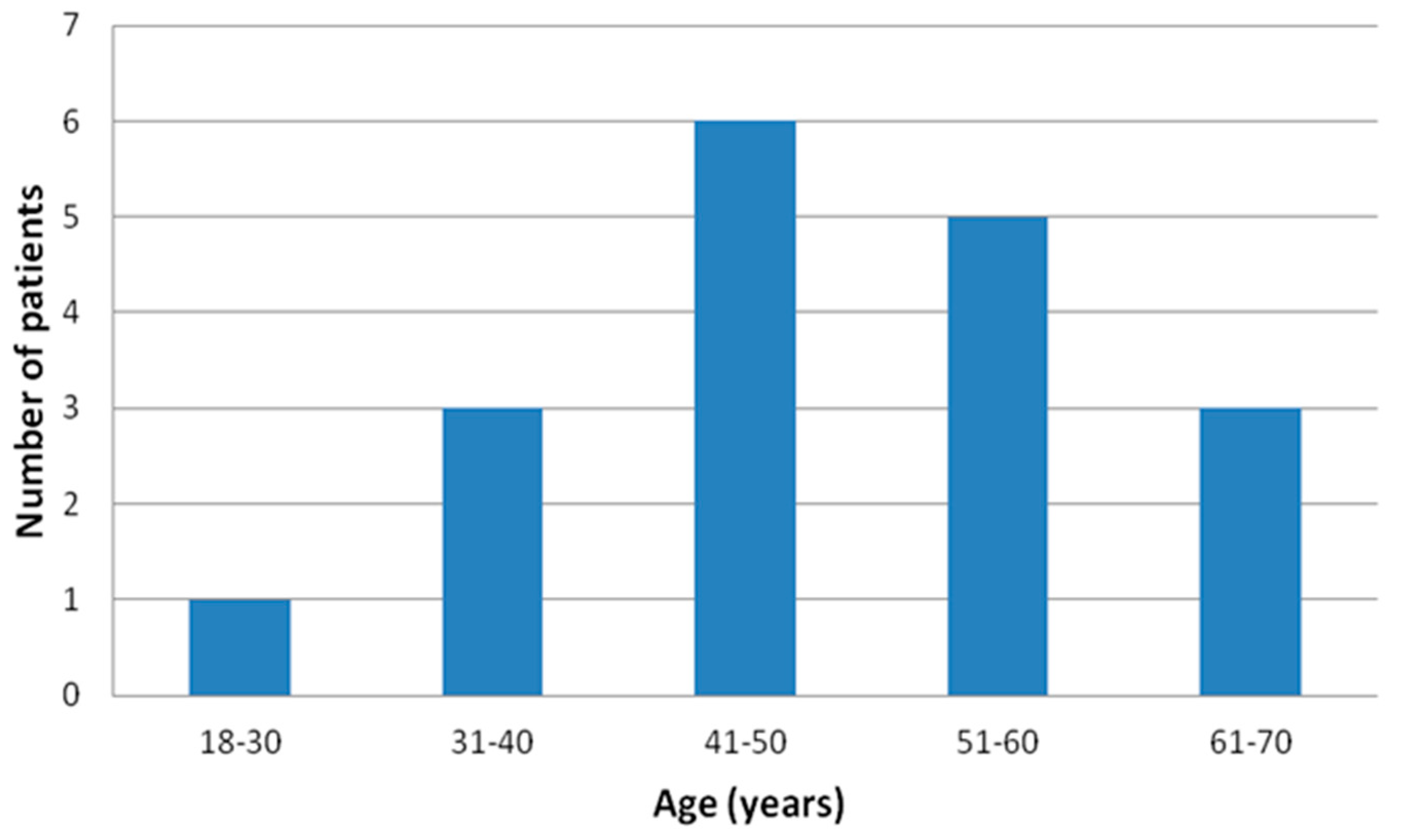
Finally, apparently healthy 18 male subjects, the “real-world” population with no previous history of sleep apnea, age 47.9 ± 11.5 (Figure 2), body mass index (BMI) 32.3 ± 3.8 kg/m2, investigated in this tertiary hospital from March 2021 to March 2022 were prospectively enrolled.
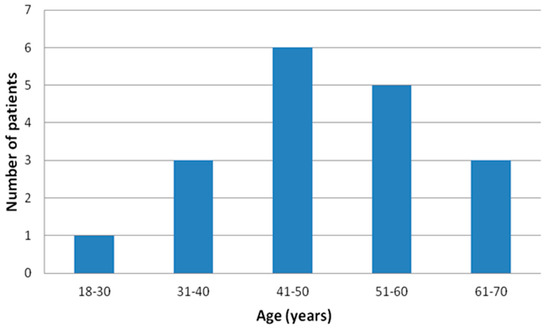
Figure 2.
Age distribution of the final enrolled patients’ sample.
2.2. Methods
- Sleep monitoring
All subjects underwent overnight sleep monitoring. In the study, only patients with polysomnography (Alice 6 device, Philips-Respironics, Murrysville, PA, USA) and recorded respiratory disturbance index (RDI) were enrolled. Only subjects with RDI ≥ 15 were included for further assessment. Other recorded indices included oxygen desaturation index (ODI), arousal index, average nocturnal O2 saturation, and minimal nocturnal O2 saturation. Standardized criteria were used for the scoring of sleep characteristics and respiratory events [22].
Monitored variables:
- BMI (body mass index)—defined as body weight divided by the square of height; a measure of the degree of obesity.
- Apnea—defined as the reduction in airflow ≥90% (or the airflow cessation) lasting >10 s.
- Hypopnea—defined as a reduction in airflow ≥30% lasting >10 s with oxygen desaturation ≥3% or arousal.
- Respiratory disturbance index (RDI)—defined as an average number of apneas, and hypopneas per 1 h of sleep.
- ODI (oxygen desaturation index)—defined as a number of desaturations ≥3% with a duration of >10 s per hour of sleep.
- Arousal index—defined as the total number of arousals per hour of sleep.
- Average nocturnal O2 saturation—defined as the mean O2 saturation during sleep.
- Minimal nocturnal O2 saturation—defined as the lowest single O2 saturation seen in the recording.
- Time with O2 saturation <90% (T90)—defined by the percentage of sleep time below 90% O2 saturation.
- Lipoprotein parameters
Blood plasma samples were obtained in the morning after polysomnography and after overnight fasting. Blood samples with ethylenediaminetetraacetic acid (EDTA) were collected. Immediately after the collection of plasma samples, levels of TAG, TC, LDL, and HDL were determined in a local certified hospital laboratory with an enzymatic method (Roche Diagnostics, Mannheim, Germany). The quantitative analysis of lipoprotein families and lipoprotein subfractions including very low-density lipoprotein (VLDL), intermediate-density lipoprotein (IDL), and plasma lipoprotein subfractions were analyzed by the Lipoprotein system (Quantimetrix Corp., Redondo Beach, CA, USA) using a polyacrylamide gel electrophoresis [23]. The following subfractions were evaluated: large LDL subfractions 1–2 (which are considered atheroprotective), small dense LDL subfractions 3–7 (which are considered atherogenic), large HDL subfractions 1–3 (which are considered atheroprotective), small dense HDL subfractions 8–10 (which are considered atherogenic), and intermediate HDL subfractions 4–7 (their atherogenic/atheroprotective role remains controversial) [20,21].
- Assessment of endothelial function
Endothelial function was assessed by PAT (EndoPAT 2000 device, Itamar Medical Ltd., Caesarea, Israel) as previously described [14]. RHI was calculated as the ratio of the average amplitude of the PAT signal post-to-pre occlusion of the tested arm, normalized to the concurrent signal from the contralateral finger. Calculations were performed using the computer algorithm (software 3.1.2) supplied with the device. RHI value < 1.67 indicated endothelial dysfunction [14,24].
- Statistical analysis
Statistical analyses were performed by SPSS ver. 18 (SPSS Inc., Chicago, IL, USA). The results of normally distributed data are expressed as a mean ± standard deviation, and the results of not normally distributed data are expressed as median, interquartile range, minimal and maximal values. Pearson or Spearman correlation coefficients were used to determine the relationships between RHI and the baseline characteristics of the study population. We used stepwise multiple linear regression to create the prediction model and identify the most important contributors to this model. A model with the highest number of significant predictors was chosen. The dependent variable in the model was RHI, independent variables in the model were anthropometric characteristics (age, gender, BMI), sleep characteristics (T90, RDI, ODI, arousal index, average, and minimal nocturnal O2 saturation), and lipoprotein levels (TAG, TC, LDL, HDL, VLDL, IDL, large LDL, small LDL, large HDL, intermediate HDL, and small HDL). Each model was assessed for the presence of multicollinearity of included variables. The variance inflation factor (VIF) ≥5 was indicative of multicollinearity. The p value < 0.05 was considered statistically significant.
3. Results
The average age of participants was 47.9 years, the average BMI value was 32.31 kg/m2. All participants were in the category of moderate-to-severe sleep apnea with a mean RDI of 45.7. The average nocturnal O2 saturation was 87.8, the average minimal nocturnal O2 saturation was 76.2. None of these monitored variables were statistically significantly correlated with RHI. The results of normally distributed data are expressed as a mean ± standard deviation, and the results of not normally distributed data are expressed as median, interquartile range, minimal and maximal values. For details, see Table 1.

Table 1.
Sleep monitoring indices and their correlations with reperfusion hyperemia index.
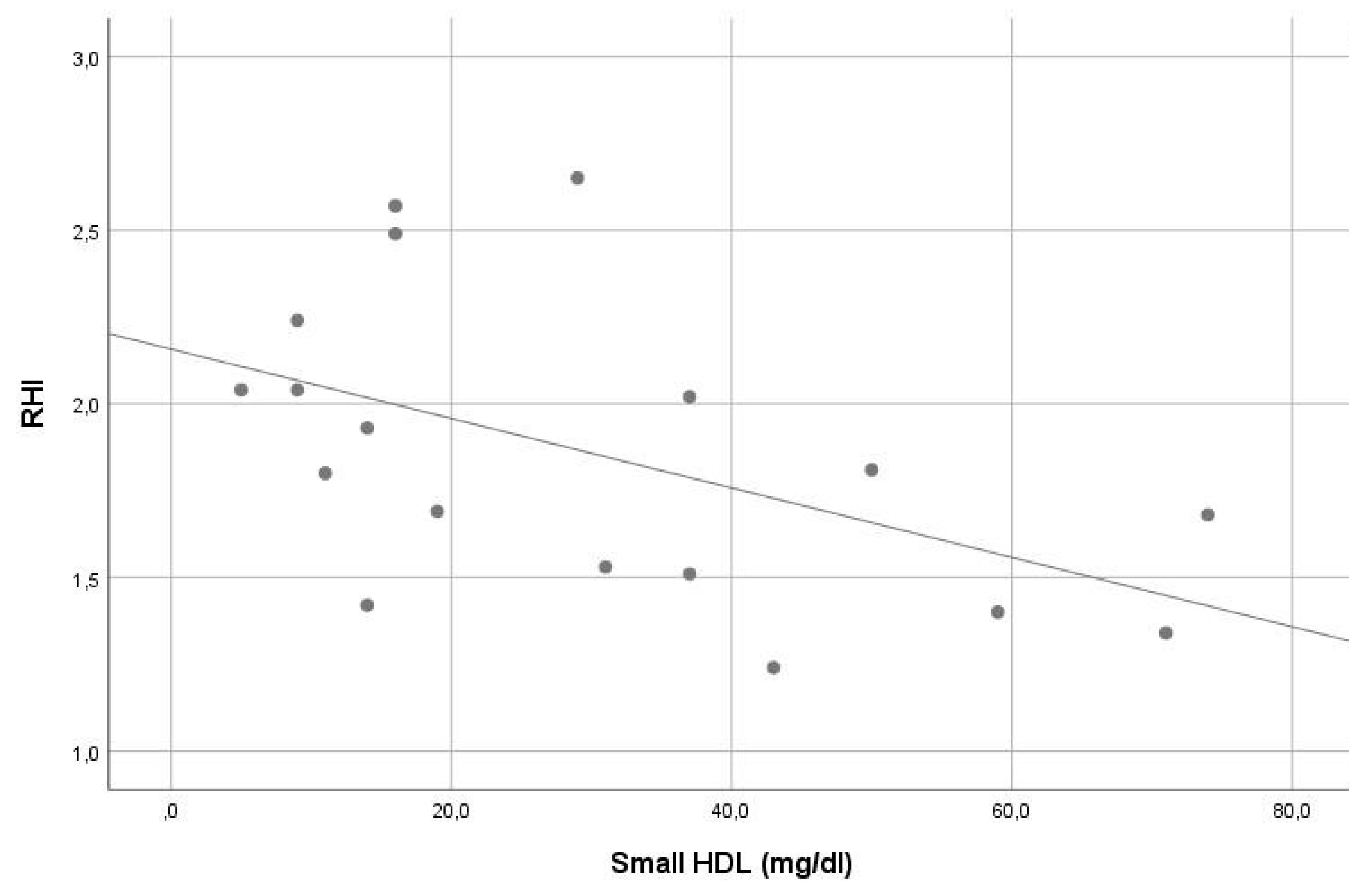
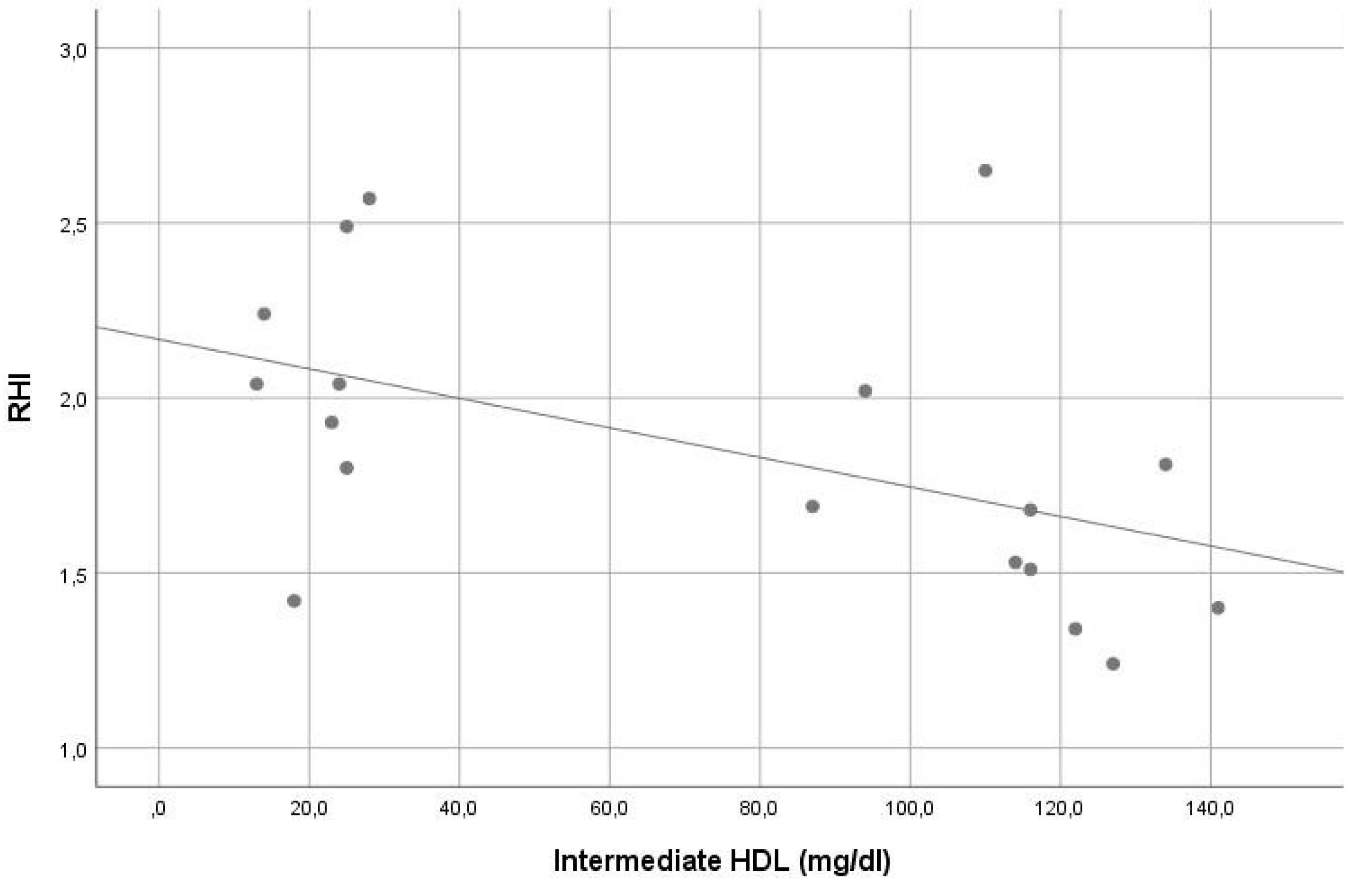
In our sample, the average RHI was 1.9. Except for the significant inverse correlation of small HDL and intermediate HDL with RHI (r = −0.561, p = 0.015 and r = −0.548, p = 0.019, consecutively), no other significant correlation was found between RHI and other lipoprotein levels (see Table 2). In stepwise multiple linear regression analysis, small HDL (beta = −0.507, p = 0.032) was the only significant contributor in the model predicting RHI. The VIF of all variables assessed in this model was <5. For details, see Table 1 and Table 2, Figure 3 and Figure 4.

Table 2.
Baseline laboratory characteristics and correlations of the reperfusion hyperemia index with variables.
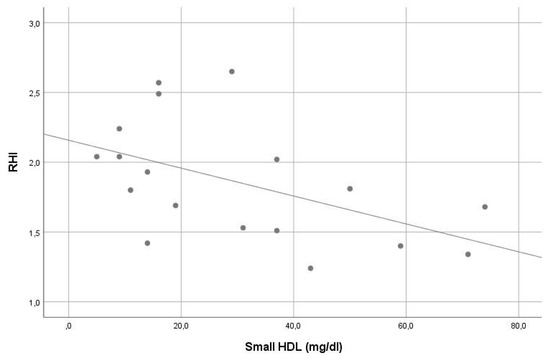
Figure 3.
Correlation of small high-density lipoprotein levels with reperfusion hyperemia index (r = −0.561, p = 0.015).
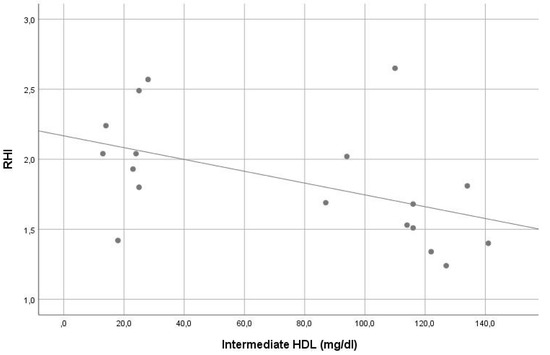
Figure 4.
Correlation of intermediate high-density lipoprotein levels with reperfusion hyperemia index (r = −0.548, p = 0.019).
4. Discussion
OSA activates several pathophysiological mechanisms that lead to the development of vascular diseases, as discussed in recent studies by Ott et al. in 2017 [25], Kollar et al. in 2021 [21], and many other authors [26,27,28]. The potential underlying pathomechanism linking OSA with the development of vascular diseases include endothelial dysfunction [29,30], activation of the sympathetic nervous system [3], oxidative stress [31], metabolic dysregulation [32], activation of inflammatory processes [29,33], and alteration of the coagulation cascade [29]. Levy et al. in 2009 [34] already described, in agreement with Seiler et al. in 2019 [35], that acceleration of atherogenesis could be one of the most important mechanisms involved in the development of vascular diseases in OSA patients. Endothelial dysfunction as the initial step and key process of atherogenesis was proven, for example by Bonetti et al. in 2003 or Gimbrone et al. in 2016 [36,37]. The association between endothelial dysfunction and OSA has been described in several studies [12,13,14] and was found also in patients with OSA who are not treated for any other diseases, like the findings presented in the work of Ip et al. in 2004 or Siarnik et al. in 2014 [14,38].
Our study was based on a strict exclusion process, and the final sample of apparently healthy subjects of our “real-world” population with no previous history of sleep apnea had only 18 responders; at the time, this was a pilot project on the topic of the possible association of lipoprotein subfractions with endothelial function in previously healthy individuals (with newly diagnosed sleep apnea). Juházs in 2014 [17] suggested dyslipidemia as one of the possible mechanisms linking OSA with increased vascular morbidity. The same opinion was presented by Helkin et al. in 2016 [17,39]. LDL is generally considered to be atherogenic and HDL to be atheroprotective. However, there are increasing data that small LDL (3–7) and small HDL (8–10) subfractions have atherogenic properties [20,21,40]. Our results suggest a pro-atherogenic role of small HDL subfractions in previously healthy subjects with moderate-to-severe OSA. Small HDL (beta = −0.507, p = 0.032) was the only significant contributor in the model predicting RHI—a measure of endothelial function, in stepwise multiple linear regression analysis (see Results). The pro-atherogenic lipoprotein phenotype characterized by increased levels of atherogenic lipoprotein subfractions and reduced levels of atheroprotective subfractions was found in individuals with OSA. In this population, significantly lower levels of atheroprotective LDL1 and large HDL subfractions were detected as well as significantly higher levels of atherogenic small dense LDL 3–7 subfractions [21]. Our results are consistent with the findings of previously mentioned studies [17,41,42,43,44,45]. Among our previously healthy patients with newly diagnosed moderate-to-severe OSA, small HDL was the only significant predictor of RHI, suggesting a pro-atherogenic role of small HDL subfractions in this population. We are not aware of any similar study so far.
Although only previously healthy subjects were enrolled and the use of any current medication or smoking belonged to additional exclusion criteria, the co-administration of other supplements as antioxidants and anti-inflammatory substances with beneficial effect on vascular status were not taken in consideration. This fact limits the findings of the current study. For example, the use of micronized purified flavonoid fraction of Rutaceae aurantiae in type 2 diabetic patients proved to reduce the risk of cardiovascular disease [46]. In another study, omega-3 proved its beneficial effect on serum lipid profile and oxidative stress [47]. For endothelial dysfunction, it was found that ramipril [48] as well as febuxostat have a direct ameliorating effect on inflammation and oxidative stress in patients with endothelial dysfunction, which is an important risk factor for cardiovascular diseases [49,50]. Similarly, the oral cholecalciferol effect on vascular calcification and 25(OH)D levels was investigated, which significantly increased serum levels of 25(OH)D and fetuin-A [51].
The enrollment of previously healthy subjects with newly diagnosed sleep apnea is the strength of the current study as it limits the effect of other possible pro-atherogenic confounders. However, a strict exclusion process leads to a small sample of respondents. This pilot study shows that in future large multicenter prospective studies with detailed blood pressure assessment, glycemia testing, a search for anthropometric parameters and physical activity measures, as well as a search for the effect of CPAP on lipoprotein subfractions and endothelial function measures should be beneficial. The effect of lifestyle interventions on LDL and HDL subfractions is known from the results of previous studies [52,53,54,55].
5. Conclusions
In our studied sample of previously healthy subjects with moderate-to-severe OSA, the plasma levels of small and intermediate HDL subfractions have statistically significant pro-atherogenic correlations with endothelial function (p = 0.015 and p = 0.019), but after stepwise multiple linear regression analysis, we conclude that a pro-atherogenic role was proven only for small HDL. Small HDL was the only significant contributor in the model predicting RHI (beta = −0.507, p = 0.032). We are not aware of any similar findings so far. No other significant lipoprotein level correlation was found.
Author Contributions
Conceptualization, P.S. (Pavel Siarnik), B.K., and P.T.; methodology, I.Z., K.K. (Katarina Klobucnikova), A.H.; investigation, A.H., I.Z., M.H., M.D., K.K. (Katarina Konarikova); resources, P.S. (Pavel Siarnik), M.D., M.P.; writing—original draft preparation, A.H., P.K., P.S. (Petr Skopek); writing—review and editing, P.T., M.T., M.U.; supervision, B.K.; project administration, I.Z., P.S. (Pavel Siarnik). All authors have read and agreed to the published version of the manuscript.
Funding
VEGA Grant 1/0022/23 supported this work.
Institutional Review Board Statement
This study was approved by the Ethics Committee of the Old Town Hospital, University Hospital Bratislava on 15 February 2021, with reference number 26/2021. The study was conducted according to the guidelines of the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| BMI | body mass index |
| CPAP | continuous positive airway pressure |
| EDTA | ethylenediaminetetraacetic acid |
| HDL | high-density lipoprotein cholesterol |
| IDL | intermediate-density lipoprotein |
| LDL | low-density lipoprotein cholesterol |
| ODI | oxygen desaturation index |
| OSA | obstructive sleep apnea |
| PAT | peripheral arterial tonometry |
| PG | polygraphy |
| PSG | polysomnography |
| RDI | respiratory disturbance index |
| RERAs | respiratory effort-related arousal |
| RHI | reactive hyperemia index |
| VLDL | very low-density lipoprotein |
References
- Hankey, G.J.; Wardlaw, J.M.; Gorelick, P.B.; Testai, F.D. Hankey’s Clinical Neurology preface. In Hankey’s Clinical Neurology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2014; p. 9. [Google Scholar]
- Gottlieb, D.J.; Punjabi, N.M. Diagnosis and Management of Obstructive Sleep Apnea: A Review. JAMA 2020, 323, 1389–1400. [Google Scholar] [CrossRef]
- Dempsey, J.A.; Veasey, S.C.; Morgan, B.J.; O’Donnell, C.P. Pathophysiology of sleep apnea. Physiol. Rev. 2010, 90, 47–112. [Google Scholar] [CrossRef] [PubMed]
- Drager, L.F.; Polotsky, V.Y.; O’Donnell, C.P.; Cravo, S.L.; Lorenzi-Filho, G.; Machado, B.H. Translational approaches to understanding metabolic dysfunction and cardiovascular consequences of obstructive sleep apnea. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1101–H1111. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Cardell, J.; Ariel, D.; Lamendola, C.; Abbasi, F.; Kim, S.H.; Holmes, T.H.; Tomasso, V.; Mojaddidi, H.; Grove, K.; et al. Abnormalities of lipoprotein concentrations in obstructive sleep apnea are related to insulin resistance. Sleep 2015, 38, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.M.; Zalos, G.; Halcox, J.P.; Schenke, W.H.; Waclawiw, M.A.; Quyyumi, A.A.; Finkel, T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N. Engl. J. Med. 2003, 348, 593–600. [Google Scholar] [CrossRef]
- Perticone, F.; Ceravolo, R.; Pujia, A.; Ventura, G.; Iacopino, S.; Scozzafava, A.; Ferraro, A.; Chello, M.; Mastroroberto, P.; Verdecchia, P.; et al. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation 2001, 104, 191–196. [Google Scholar] [CrossRef]
- Victor, V.M.; Rocha, M.; Sola, E.; Banuls, C.; Garcia-Malpartida, K.; Hernandez-Mijares, A. Oxidative stress, endothelial dysfunction and atherosclerosis. Curr. Pharm. Des. 2009, 15, 2988–3002. [Google Scholar] [CrossRef]
- Flammer, A.J.; Anderson, T.; Celermajer, D.S.; Creager, M.A.; Deanfield, J.; Ganz, P.; Hamburg, N.M.; Lüscher, T.F.; Shechter, M.; Taddei, S.; et al. The assessment of endothelial function: From research into clinical practice. Circulation 2012, 126, 753–767. [Google Scholar] [CrossRef]
- Rubinshtein, R.; Kuvin, J.T.; Soffler, M.; Lennon, R.J.; Lavi, S.; Nelson, R.E.; Pumper, G.M.; Lerman, L.O.; Lerman, A. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur. Heart J. 2010, 31, 1142–1148. [Google Scholar] [CrossRef]
- Matsuzawa, Y.; Sugiyama, S.; Sugamura, K.; Nozaki, T.; Ohba, K.; Konishi, M.; Matsubara, J.; Sumida, H.; Kaikita, K.; Kojima, S.; et al. Digital assessment of endothelial function and ischemic heart disease in women. J. Am. Coll. Cardiol. 2010, 55, 1688–1696. [Google Scholar] [CrossRef]
- Azuma, M.; Chihara, Y.; Yoshimura, C.; Murase, K.; Hamada, S.; Tachikawa, R.; Matsumoto, T.; Inouchi, M.; Tanizawa, K.; Handa, T.; et al. Association between endothelial function (assessed on reactive hyperemia peripheral arterial tonometry) and obstructive sleep apnea, visceral fat accumulation, and serum adiponectin. Circ. J. Off. J. Jpn. Circ. Soc. 2015, 79, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Kheirandish-Gozal, L.; Etzioni, T.; Bhattacharjee, R.; Tan, H.L.; Samiei, A.; Molero Ramirez, H.; Abu Eta, B.; Pillar, G. Obstructive sleep apnea in children is associated with severity-dependent deterioration in overnight endothelial function. Sleep Med. 2013, 14, 526–531. [Google Scholar] [CrossRef]
- Siarnik, P.; Carnicka, Z.; Krizova, L.; Wagnerova, H.; Sutovsky, S.; Klobucnikova, K.; Kollar, B.; Turcani, P.; Sykora, M. Predictors of impaired endothelial function in obstructive sleep apnea syndrome. Neuro Endocrinol. Lett. 2014, 35, 142–148. [Google Scholar] [PubMed]
- Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421. [CrossRef]
- Gottlieb, D.J. Sleep Apnea and Cardiovascular Disease. Curr. Diabetes Rep. 2021, 21, 64. [Google Scholar] [CrossRef]
- Juhász, J. Dyslipidemia: Another brick in the wall. A feasible link in the OSA-cardiovascular disease axis. Sleep Breath. Schlaf. Atm. 2014, 18, 5–6. [Google Scholar] [CrossRef][Green Version]
- Šiarnik, P.; Klobučníková, K.; Mucska, I.; Černá, K.; Kollár, B.; Turčáni, P. Obstructive sleep apnea and dyslipidemia. Vnitr. Lek. 2018, 64, 934–938. [Google Scholar] [CrossRef]
- Drager, L.F.; Jun, J.; Polotsky, V.Y. Obstructive sleep apnea and dyslipidemia: Implications for atherosclerosis. Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Oravec, S.; Dostal, E.; Dukát, A.; Gavorník, P.; Kucera, M.; Gruber, K. HDL subfractions analysis: A new laboratory diagnostic assay for patients with cardiovascular diseases and dyslipoproteinemia. Neuro Endocrinol. Lett. 2011, 32, 502–509. [Google Scholar] [PubMed]
- Kollar, B.; Siarnik, P.; Hluchanova, A.; Klobucnikova, K.; Mucska, I.; Turcani, P.; Paduchova, Z.; Katrencikova, B.; Janubova, M.; Konarikova, K.; et al. The impact of sleep apnea syndrome on the altered lipid metabolism and the redox balance. Lipids Health Dis. 2021, 20, 175. [Google Scholar] [CrossRef]
- Berry, R.B.; Brooks, R.; Gamaldo, C.E.; Harding, S.M.; Marcus, C.; Vaughn, B.V. The AASM manual for the scoring of sleep and associated events. Rules Terminol. Tech. Specif. Darien Ill. Am. Acad. Sleep Med. 2012, 176, 2012. [Google Scholar]
- Hoefner, D.M.; Hodel, S.D.; O’Brien, J.F.; Branum, E.L.; Sun, D.; Meissner, I.; McConnell, J.P. Development of a rapid, quantitative method for LDL subfractionation with use of the Quantimetrix Lipoprint LDL System. Clin. Chem. 2001, 47, 266–274. [Google Scholar] [CrossRef]
- Moerland, M.; Kales, A.J.; Schrier, L.; van Dongen, M.G.; Bradnock, D.; Burggraaf, J. Evaluation of the EndoPAT as a Tool to Assess Endothelial Function. Int. J. Vasc. Med. 2012, 2012, 904141. [Google Scholar] [CrossRef] [PubMed]
- Ott, S.R.; Korostovtseva, L.; Schmidt, M.; Horvath, T.; Brill, A.K.; Bassetti, C.L. Sleep-disordered breathing: Clinical features, pathophysiology and diagnosis. Swiss. Med. Wkly. 2020, 147, w14436 . [Google Scholar] [CrossRef]
- Slouka, D.; Windrichova, J.; Rezackova, H.; Houfkova, K.; Kucera, R.; Cerna, V.; Kostlivy, T.; Topolcan, O.; Pesta, M. The potential of miR-499 plasmatic level as a biomarker of obstructive sleep apnea syndrome. Biomark. Med. 2021, 15, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Javaheri, S.; Barbe, F.; Campos-Rodriguez, F.; Dempsey, J.A.; Khayat, R.; Malhotra, A.; Martinez-Garcia, M.A.; Mehra, R.; Pack, A.I.; Polotsky, V.Y.; et al. Sleep Apnea: Types, Mechanisms, and Clinical Cardiovascular Consequences. J. Am. Coll. Cardiol. 2017, 69, 841–858. [Google Scholar] [CrossRef]
- Tuleta, I.; França, C.N.; Wenzel, D.; Fleischmann, B.; Nickenig, G.; Werner, N.; Skowasch, D. Intermittent Hypoxia Impairs Endothelial Function in Early Preatherosclerosis. Adv. Exp. Med. Biol. 2015, 858, 1–7 . [Google Scholar] [CrossRef] [PubMed]
- Shamsuzzaman, A.S.; Gersh, B.J.; Somers, V.K. Obstructive sleep apnea: Implications for cardiac and vascular disease. JAMA 2003, 290, 1906–1914. [Google Scholar] [CrossRef]
- Orrù, G.; Storari, M.; Scano, A.; Piras, V.; Taibi, R.; Viscuso, D. Obstructive Sleep Apnea, oxidative stress, inflammation and endothelial dysfunction-An overview of predictive laboratory biomarkers. Eur. Rev. Med. Pharm. Sci. 2020, 24, 6939–6948. [Google Scholar]
- Stanek, A.; Brożyna-Tkaczyk, K.; Myśliński, W. Oxidative Stress Markers among Obstructive Sleep Apnea Patients. Oxid. Med. Cell Longev. 2021, 2021, 9681595 . [Google Scholar] [CrossRef]
- Trakada, G.; Lombardi, C.; Knechtle, B. Editorial: The Complex Interaction Between Biological, Metabolic and Neurologic Dysregulation in Obstructive Sleep Apnea. Front. Psychiatry. 2021, 12, 770930 . [Google Scholar] [CrossRef]
- Bhatt, S.P.; Guleria, R.; Kabra, S.K. Metabolic alterations and systemic inflammation in overweight/obese children with obstructive sleep apnea. PLoS ONE 2021, 16, e0252353. [Google Scholar] [CrossRef] [PubMed]
- Lévy, P.; Pépin, J.-L.; Arnaud, C.; Baguet, J.-P.; Dematteis, M.; Mach, F. Obstructive sleep apnea and atherosclerosis. Prog. Cardiovasc. Dis. 2009, 51, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Seiler, A.; Camilo, M.; Korostovtseva, L.; Haynes, A.G.; Brill, A.K.; Horvath, T.; Egger, M.; Bassetti, C.L. Prevalence of sleep-disordered breathing after stroke and TIA: A meta-analysis. Neurology 2019, 92, e648–e654. [Google Scholar] [CrossRef]
- Bonetti, P.O.; Lerman, L.O.; Lerman, A. Endothelial dysfunction: A marker of atherosclerotic risk. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 168–175. [Google Scholar] [CrossRef]
- Gimbrone, M.A., Jr.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef]
- Ip, M.S.; Tse, H.-F.; Lam, B.; Tsang, K.W.; Lam, W.-K. Endothelial function in obstructive sleep apnea and response to treatment. Am. J. Respir. Crit. Care Med. 2004, 169, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Helkin, A.; Stein, J.J.; Lin, S.; Siddiqui, S.; Maier, K.G.; Gahtan, V. Dyslipidemia Part 1--Review of Lipid Metabolism and Vascular Cell Physiology. Vasc Endovasc. Surg. 2016, 50, 107–118. [Google Scholar] [CrossRef]
- Oravec, S.; Dukat, A.; Gavornik, P.; Caprnda, M.; Kucera, M.; Ocadlik, I. Contribution of the atherogenic lipoprotein profile to the development of arterial hypertension. Bratisl. Lek. Listy 2011, 112, 4–7. [Google Scholar]
- Oravec, S.; Krivosikova, Z.; Krivosik, M.; Gruber, K.; Gruber, M.; Dukát, A.; Gavorník, P. Lipoprotein profile in patients who survive a stroke. Neuro Endocrinol. Lett. 2011, 32, 496–501. [Google Scholar]
- Oravec, S.; Gruber, K.; Dostal, E.; Mikl, J. Hyper-betalipoproteinemia LDL 1,2: A newly identified nonatherogenic hypercholesterolemia in a group of hypercholesterolemic subjects. Neuro Endocrinol. Lett. 2011, 32, 322–327. [Google Scholar] [PubMed]
- Šiarnik, P.; Čarnická, Z.; Krivošíková, Z.; Klobučníková, K.; Žitňanová, I.; Kollár, B.; Sýkora, M.; Turčáni, P. Association of lipoprotein subfractions with endothelial function and arterial stiffness in acute ischemic stroke. Scand. J. Clin. Lab. Invest. 2017, 77, 36–39. [Google Scholar] [CrossRef]
- Sopkova, Z.; Berneis, K.; Rizzo, M.; Spinas, G.A.; Dorkova, Z.; Tisko, R.; Tkacova, R. Size and subclasses of low-density lipoproteins in patients with obstructive sleep apnea. Angiology 2012, 63, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Meszaros, M.; Bikov, A. Obstructive Sleep Apnoea and Lipid Metabolism: The Summary of Evidence and Future Perspectives in the Pathophysiology of OSA-Associated Dyslipidaemia. Biomedicines 2022, 10, 2754. [Google Scholar] [CrossRef] [PubMed]
- Rizk, S.M.; Sabri, N.A. Evaluation of clinical activity and safety of Daflon 500 mg in type 2 diabetic female patients. Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc. 2009, 17, 199–207. [Google Scholar] [CrossRef]
- Ateya, A.M.; Sabri, N.A.; El Hakim, I.; Shaheen, S.M. Effect of Omega-3 Fatty Acids on Serum Lipid Profile and Oxidative Stress in Pediatric Patients on Regular Hemodialysis: A Randomized Placebo-Controlled Study. J. Ren. Nutr. Off. J. Counc. Ren. Nutr. Natl. Kidney Found. 2017, 27, 169–174. [Google Scholar] [CrossRef]
- Ateya, A.M.; El Hakim, I.; Shahin, S.M.; El Borolossy, R.; Kreutz, R.; Sabri, N.A. Effects of Ramipril on Biomarkers of Endothelial Dysfunction and Inflammation in Hypertensive Children on Maintenance Hemodialysis: The SEARCH Randomized Placebo-Controlled Trial. Hypertension 2022, 79, 1856–1865. [Google Scholar] [CrossRef]
- Alshahawey, M.; Shaheen, S.M.; Elsaid, T.; Sabri, N.A. Effect of febuxostat on oxidative stress in hemodialysis patients with endothelial dysfunction: A randomized, placebo-controlled, double-blinded study. Int. Urol. Nephrol. 2019, 51, 1649–1657. [Google Scholar] [CrossRef]
- Alshahawey, M.; Shahin, S.M.; Elsaid, T.W.; Sabri, N.A. Effect of Febuxostat on the Endothelial Dysfunction in Hemodialysis Patients: A Randomized, Placebo-Controlled, Double-Blinded Study. Am. J. Nephrol. 2017, 45, 452–459. [Google Scholar] [CrossRef]
- Alshahawey, M.; El Borolossy, R.; El Wakeel, L.; Elsaid, T.; Sabri, N.A. The impact of cholecalciferol on markers of vascular calcification in hemodialysis patients: A randomized placebo controlled study. Nutr. Metab. Cardiovasc. Dis. NMCD 2021, 31, 626–633. [Google Scholar] [CrossRef]
- Dutheil, F.; Walther, G.; Chapier, R.; Mnatzaganian, G.; Lesourd, B.; Naughton, G.; Verney, J.; Fogli, A.; Sapin, V.; Duclos, M.; et al. Atherogenic subfractions of lipoproteins in the treatment of metabolic syndrome by physical activity and diet-the RESOLVE trial. Lipids Health Dis. 2014, 13, 112. [Google Scholar] [CrossRef] [PubMed]
- Bajer, B.; Rádiková, Ž.; Havranová, A.; Žitňanová, I.; Vlček, M.; Imrich, R.; Sabaka, P.; Bendžala, M.; Penesová, A. Effect of 8-weeks intensive lifestyle intervention on LDL and HDL subfractions. Obes. Res. Clin. Pract. 2019, 13, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Habanova, M.; Holovicova, M.; Scepankova, H.; Lorkova, M.; Gazo, J.; Gazarova, M.; Pinto, C.A.; Saraiva, J.A.; Estevinho, L.M. Modulation of Lipid Profile and Lipoprotein Subfractions in Overweight/Obese Women at Risk of Cardiovascular Diseases through the Consumption of Apple/Berry Juice. Antioxidants 2022, 11, 2239. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yi, H.; Guan, J.; Yin, S. Effect of continuous positive airway pressure on lipid profile in patients with obstructive sleep apnea syndrome: A meta-analysis of randomized controlled trials. Atherosclerosis 2014, 234, 446–453. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).