Association of the Mannose-Binding Lectin 2 BB Genotype with COVID-19-Related Mortality
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Quantitation of Serum MBL
2.3. Determination of MBL2 Gene Variants
2.4. Determination of COVID-19 Severity
2.5. Statistical Analysis
3. Results
3.1. Number of Patients, Age, and Serum MBL Levels
3.2. Each MBL Genotype and Age
3.3. Different COVID-19 Severities, Each MBL Genotype and Serum MBL Levels
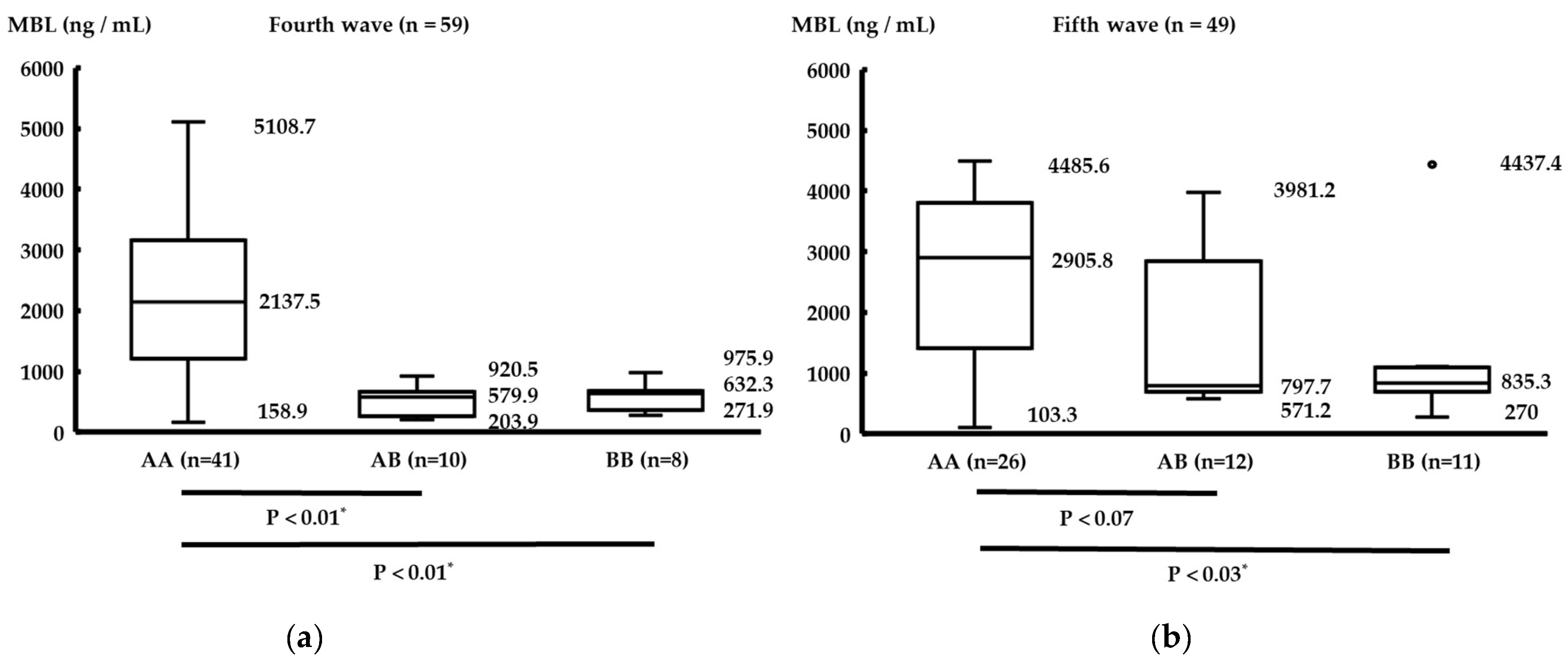
3.4. Comparison between Each MBL Genotype and Serum MBL Levels
3.5. Comparison between Allele Frequency in the Fourth and Fifth Waves, and the East Asian Database
3.6. Binary Logistic Regression Analysis to Identify Predisposing Factors for More Than a Mild Level of COVID-19 Severity and Death from COVID-19
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takahashi, K. Mannose-binding lectin and the balance between immune protection and complication. Expert Rev. Anti-Infect. Ther. 2011, 9, 1179–1190. [Google Scholar] [CrossRef]
- Boschmann, S.E.; Goeldner, I.; Tuon, F.F.; Schiel, W.; Aoyama, F.; de Messias-Reason, I.J. Mannose-binding lectin polymorphisms and rheumatoid arthritis: A short review and meta-analysis. Mol. Immunol. 2016, 69, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.F.; Ho, M.H.; Ip, W.K.; Fok, S.F.; Yung, T.C.; Lau, Y.L. Modulating effects of mannose binding lectin genotype on arterial stiffness in children after Kawasaki disease. Pediatr. Res. 2004, 56, 591–596. [Google Scholar] [CrossRef]
- Sato, S.; Kawashima, H.; Kashiwagi, Y.; Fujioka, T.; Takekuma, K.; Hoshika, A. Association of mannose-binding lectin gene polymorphisms with Kawasaki disease in the Japanese. Int. J. Rheum. Dis. 2009, 12, 307–310. [Google Scholar] [CrossRef]
- Hammad, N.M.; Badawy, N.E.E.; Nasr, A.M.; Ghramh, H.A.; Kady., L.M.A. Mannose-Binding Lectin Gene Polymorphism and Its Association with Susceptibility to Recurrent Vulvovaginal Candidiasis. Biomed. Res. Int. 2018, 2018, 7648152. [Google Scholar] [CrossRef] [PubMed]
- Dogan, P.; Ozkan, H.; Koksal, N.; Oral, H.B.; Bagci, O.; Varal, I.G. Mannose-binding lectin gene polymorphism and its effect on short term outcomes in preterm infants. J. Pediatr. (Rio J.) 2020, 96, 520–526. [Google Scholar] [CrossRef]
- Erdemir, G.; Ozkan, T.B.; Ozgur, T.; Budak, F.; Kilic, S.S.; Onay, H. Mannose-binding lectin gene polymorphism and chronic hepatitis B infection in children. Saudi J. Gastroenterol. 2015, 21, 84–89. [Google Scholar] [CrossRef]
- Ip, W.K.; Chan, K.H.; Law, H.K.; Tso, G.H.; Kong, E.K.; Wong, W.H.; To, Y.F.; Yung, R.W.; Chow, E.Y.; Au, K.L.; et al. Mannose-binding lectin in severe acute respiratory syndrome coronavirus infection. J. Infect. Dis. 2005, 191, 1697–1704. [Google Scholar] [CrossRef]
- Takahashi, K.; Ip, W.E.; Michelow, I.C.; Ezekowitz, R.A. The mannose-binding lectin: A prototypic pattern recognition molecule. Curr. Opin. Immunol. 2006, 18, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Medetalibeyoglu, A.; Bahat, G.; Senkal, N.; Kose, M.; Avci, K.; Sayin, G.Y.; Isoglu-Alkac, U.; Tukek, T.; Pehlivan, S. Mannose binding lectin gene 2 (rs1800450) missense variant may contribute to development and severity of COVID-19 infection. Infect. Genet. Evol. 2021, 89, 104717. [Google Scholar] [CrossRef] [PubMed]
- Poppelaars, F.; Faria, B.; Schwaeble, W.; Daha, M.R. The Contribution of Complement to the Pathogenesis of IgA Nephropathy: Are Complement-Targeted Therapies Moving from Rare Disorders to More Common Diseases? J. Clin. Med. 2021, 10, 4715. [Google Scholar] [CrossRef]
- Andrighetto, S.; Leventhal, J.; Zaza, G.; Cravedi, P. Complement and Complement Targeting Therapies in Glomerular Diseases. Int. J. Mol. Sci. 2019, 20, 6336. [Google Scholar] [CrossRef] [PubMed]
- Outbreak.info. SARS-CoV-2 Data Explorer. Available online: https://outbreak.info/location-reports?loc=JPN&dark=true&selected=Delta&selected=Alpha&selected=Beta&selected=Gamma (accessed on 2 May 2022).
- Japanese Multi Omics Reference Panel. jMorp. Available online: https://jmorp.megabank.tohoku.ac.jp (accessed on 2 May 2022).
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Terai, I.; Kobayashi, K.; Fujita, T.; Hagiwara, K. Human serum mannose binding protein (MBP): Development of an enzyme-linked immunosorbent assay (ELISA) and determination of levels in serum from 1085 normal Japanese and in some body fluids. Biochem. Med. Metab. Biol. 1993, 50, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Verdu, P.; Barreiro, L.B.; Patin, E.; Gessain, A.; Cassar, O.; Kidd, J.R.; Kidd, K.K.; Behar, D.M.; Froment, A.; Heyer, E.; et al. Evolutionary insights into the high worldwide prevalence of MBL2 deficiency alleles. Hum. Mol. Genet. 2006, 15, 2650–2658. [Google Scholar] [CrossRef]
- Boldt, A.B.; Messias-Reason, I.J.; Meyer, D.; Schrago, C.G.; Lang, F.; Lell, B.; Dietz, K.; Kremsner, P.G.; Petzl-Erler, M.L.; Kun, J.F. Phylogenetic nomenclature and evolution of mannose-binding lectin (MBL2) haplotypes. BMC Genet. 2010, 11, 38. [Google Scholar] [CrossRef]
- Tomaiuolo, R.; Ruocco, A.; Salapete, C.; Carru, C.; Baggio, G.; Franceschi, C.; Zinellu, A.; Vaupel, J.; Bellia, C.; Lo Sasso, B.; et al. Activity of mannose-binding lectin in centenarians. Aging. Cell 2012, 11, 394–400. [Google Scholar] [CrossRef]
- Su, C.; Lin, Y.; Cai, L.; Mao, Q.; Niu, J. Association between mannose-binding lectin variants, haplotypes and risk of hepatocellular carcinoma: A case-control study. Sci. Rep. 2016, 6, 32147. [Google Scholar] [CrossRef]
- Speletas, M.; Dadouli, K.; Syrakouli, A.; Gatselis, N.; Germanidis, G.; Mouchtouri, V.A.; Koulas, I.; Samakidou, A.; Nikolaidou, A.; Stefos, A.; et al. MBL deficiency-causing B allele (rs1800450) as a risk factor for severe COVID-19. Immunobiology 2021, 226, 152136. [Google Scholar] [CrossRef]
- Tukek, T.; Pehlivan, S.; Oyaci, Y.; Isoglu-Alkac, U. Mannose-Binding Lectin 2 Gene Polymorphism during Pandemic: COVID-19 Family. Glob. Med. Genet. 2022, 9, 185–188. [Google Scholar] [CrossRef]
- Chen, Y.; Klein, S.L.; Garibaldi, B.T.; Li, H.; Wu, C.; Osevala, N.M.; Li, T.; Margolick, J.B.; Pawelec, G.; Leng, S.X. Aging in COVID-19: Vulnerability, immunity and intervention. Ageing Res. Rev. 2021, 65, 101205. [Google Scholar] [CrossRef] [PubMed]
- Goronzy, J.J.; Weyand, C.M. Successful and maladaptive T cell aging. Immunity 2017, 46, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Rambaldi, A.; Gritti, G.; Micò, M.C.; Frigeni, M.; Borleri, G.; Salvi, A.; Landi, F.; Pavoni, C.; Sonzogni, A.; Gianatti, A.; et al. Endothelial injury and thrombotic microangiopathy in COVID-19: Treatment with the lectin-pathway inhibitor narsoplimab. Immunobiology 2020, 225, 152001. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Huang, S.; Yin, L. The cytokine storm and COVID-19. J. Med. Virol. 2021, 93, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Collard, C.D.; Vakeva, A.; Morrissey, M.A.; Agah, A.; Rollins, S.A.; Reenstra, W.R.; Buras, J.A.; Meri, S.; Stahl, G.L. Complement activation after oxidative stress: Role of the lectin complement pathway. Am. J. Pathol. 2000, 156, 1549–1556. [Google Scholar] [CrossRef]
- Asakura, H.; Ogawa, H. COVID-19-associated coagulopathy and disseminated intravascular coagulation. Int. J. Hematol. 2021, 113, 45–57. [Google Scholar] [CrossRef]
- Gupta, A.; Gupta, G.S. Status of mannose-binding lectin (MBL) and complement system in COVID-19 patients and therapeutic applications of antiviral plant MBLs. Mol. Cell. Biochem. 2021, 476, 2917–2942. [Google Scholar] [CrossRef]
- Ahmed, M.N.; Jahan, R.; Nissapatorn, V.; Wilairatana, P.; Rahmatullah, M. Plant lectins as prospective antiviral biomolecules in the search for COVID-19 eradication strategies. Biomed. Pharmacother. 2022, 146, 112507. [Google Scholar] [CrossRef]
- Takasumi, M.; Omori, T.; Machida, T.; Ishida, Y.; Hayashi, M.; Suzuki, T.; Homma, Y.; Endo, Y.; Takahashi, M.; Ohira, H.; et al. A novel complement inhibitor sMAP-FH targeting both the lectin and alternative complement pathways. FASEB J. 2020, 34, 6598–6612. [Google Scholar] [CrossRef]
- Flude, B.M.; Nannetti, G.; Mitchell, P.; Compton, N.; Richards, C.; Heurich, M.; Brancale, A.; Ferla, S.; Bassetto, M. Targeting the Complement Serine Protease MASP-2 as a Therapeutic Strategy for Coronavirus Infections. Viruses 2021, 13, 312. [Google Scholar] [CrossRef]
- Świerzko, A.S.; Cedzyński, M. The Influence of the Lectin Pathway of Complement Activation on Infections of the Respiratory System. Front. Immunol. 2020, 11, 585243. [Google Scholar] [CrossRef] [PubMed]
- Eisen, D.P.; Minchinton, R.M. Impact of mannose-binding lectin on susceptibility to infectious diseases. Clin. Infect. Dis. 2003, 37, 1496–1505. [Google Scholar] [CrossRef] [PubMed]
- Monticelli, M.; Mele, B.H.; Andreotti, G.; Cubellis, M.V.; Riccio, G. Why does SARS-CoV-2 hit in different ways? Host genetic factors can influence the acquisition or the course of COVID-19. Eur. J. Med. Genet. 2021, 64, 104227. [Google Scholar] [CrossRef] [PubMed]

| Age (y) | 10–19 | 20–29 | 30–39 | 40–49 | 50–59 | 60–69 | 70–79 | 80–89 | 90–99 | Total (Men/%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Fourth wave | 0 | 8 (7) | 5 (5) | 5 (2) | 15 (9) | 11 (9) | 9 (6) | 5 (2) | 1 (0) | 59 (40/67.8%) |
| Fifth wave | 1 (0) | 6 (3) | 3 (3) | 11 (6) | 13 (10) | 7 (6) | 3 (2) | 3 (2) | 2 (1) | 49 (33/67.3%) |
| Total (men) | 1 (0) | 14 (10) | 8 (8) | 16 (8) | 28 (19) | 18 (15) | 12 (8) | 8 (4) | 3 (1) | 108 (77/67.6 %) |
| (a) Fourth wave (n = 59) | |||||
| AA | AB | BB | Test of significance | p-value | |
| 41 | 10 | 8 | |||
| Age Median(range) | 54 (20–93) | 70.5 (30–86) | 62.5 (27–80) | Kruskal–Wallis | 0.11 |
| (b) Fifth wave (n = 49) | |||||
| AA | AB | BB | Test of significance | p-value | |
| 26 | 12 | 11 | |||
| Age Median(range) | 55 (21–99) | 45.5 (14–84) | 50 (26–80) | Kruskal–Wallis | 0.16 |
| (a) Fourth wave (n = 59) | |||||||
| Mild | Moderate | Severe | Critical | Death | Test of significance | p Value | |
| AA | 18 | 6 | 11 | 2 | 4 | ||
| AB | 3 | 1 | 4 | 0 | 2 | Fisher’s exact | 0.22 |
| BB | 2 | 2 | 0 | 1 | 3 | ||
| serum MBL levels (ng/mL) | |||||||
| Median(range) | 1254.6 (158.9–3988.7) | 975.5 (289.2–3680.9) | 3170.7 (641.9–5108.7) | 2106.0 (975.9–2264.4) | 686 (271.9–3132.2) | Kruskal–Wallis | 0.42 |
| (b) Fifth wave (n = 49) | |||||||
| Mild | Moderate | Severe | Critical | Death | Test of significance | p Value | |
| AA | 9 | 5 | 6 | 3 | 3 | ||
| AB | 7 | 1 | 0 | 1 | 3 | Fisher’s exact | 0.63 |
| BB | 6 | 1 | 1 | 1 | 2 | ||
| serum MBL levels (ng/mL) | |||||||
| Median(range) | 1521.9 (270–4437.4) | 2783.8 (1092.7–4407.9) | 1257.1 (103.3–3590.0) | 1462.8 (574.8–3840.7) | 1026.0 (537–4485.6) | Kruskal–Wallis | 0.83 |
| Allele | A | B | OR | 95% CI | p Value |
|---|---|---|---|---|---|
| Fourth wave | 0.78 | 0.22 | |||
| Fifth wave | 0.65 | 0.35 | 1.13 | 0.82–1.57 | 0.56 |
| East Asian Database | 0.82 | 0.18 | 2.13 | 1.69–2.69 | <0.001 *1 |
| p-Value | OR | 95% CI | |
|---|---|---|---|
| Sex | 0.16 | 0.51 | 0.20–1.23 |
| Age | <0.001 * | 1.06 | 1.03–1.08 |
| AA genotype | 0.89 | ||
| AB genotype | 0.69 | 0.80 | 0.26–2.44 |
| BB genotype | 0.72 | 0.82 | 0.27–2.51 |
| p-Value | OR | 95% CI | |
|---|---|---|---|
| Sex | 0.92 | 0.93 | 0.23–3.82 |
| Age | <0.001 * | 1.11 | 1.06–1.17 |
| AA genotype | 0.10 | ||
| AB genotype | 0.23 | 2.63 | 0.55–12.59 |
| BB genotype | 0.04 * | 5.66 | 1.10–29.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kashiwagi, Y.; Suzuki, S.; Takahashi, R.; Yamanaka, G.; Hirai, Y.; Kawashima, H. Association of the Mannose-Binding Lectin 2 BB Genotype with COVID-19-Related Mortality. Life 2023, 13, 382. https://doi.org/10.3390/life13020382
Kashiwagi Y, Suzuki S, Takahashi R, Yamanaka G, Hirai Y, Kawashima H. Association of the Mannose-Binding Lectin 2 BB Genotype with COVID-19-Related Mortality. Life. 2023; 13(2):382. https://doi.org/10.3390/life13020382
Chicago/Turabian StyleKashiwagi, Yasuyo, Shinji Suzuki, Ryo Takahashi, Gaku Yamanaka, Yuji Hirai, and Hisashi Kawashima. 2023. "Association of the Mannose-Binding Lectin 2 BB Genotype with COVID-19-Related Mortality" Life 13, no. 2: 382. https://doi.org/10.3390/life13020382
APA StyleKashiwagi, Y., Suzuki, S., Takahashi, R., Yamanaka, G., Hirai, Y., & Kawashima, H. (2023). Association of the Mannose-Binding Lectin 2 BB Genotype with COVID-19-Related Mortality. Life, 13(2), 382. https://doi.org/10.3390/life13020382





