Abstract
Background: Citicoline or CDP-choline is a neuroprotective/neurorestorative drug used in several countries for the treatment of traumatic brain injury (TBI). Since the publication of the controversial COBRIT, the use of citicoline has been questioned in this indication, so it was considered necessary to undertake a systematic review and meta-analysis to evaluate whether citicoline is effective in the treatment of patients with TBI. Methods: A systematic search was performed on OVID-Medline, EMBASE, Google Scholar, the Cochrane Central Register of Controlled Trials, ClinicalTrials.gov, and Ferrer databases, from inception to January 2021, to identify all published, unconfounded, comparative clinical trials of citicoline in the acute phase of head-injured patients— that is, treatment started during the first 24 h. We selected studies on complicated mild, moderate, and severe head-injured patients according to the score of the Glasgow Coma Scale (GCS). The primary efficacy measure was independence at the end of the scheduled clinical trial follow-up. Results: In total, 11 clinical studies enrolling 2771 patients were identified by the end. Under the random-effects model, treatment with citicoline was associated with a significantly higher rate of independence (RR, 1.18; 95% CI = 1.05–1.33; I2, 42.6%). The dose of citicoline or the administration route had no effect on outcomes. Additionally, no significant effects on mortality were found, and no safety concerns were noticed. Conclusions: This meta-analysis indicates some beneficial effects of citicoline’s increasing the number of independent patients with TBI. The most important limitation of our meta-analysis was the presumed heterogeneity of the studies included. Registration: PROSPERO CRD42021238998
1. Introduction
Traumatic brain injury (TBI) is a acquired insult to the brain from an external mechanical force that can result in a temporary or permanent impairment or death, especially in moderate and severe cases [1], which is considered a major worldwide neurological disorder of epidemic proportions [2]. To date, there are still no FDA-approved therapies to treat any forms of TBI [2]. In 2016, there were 27.08 million (24.30–30.30 million) new cases of TBI in the world, with an age-standardized incidence rate of 369 (331–412) per 100,000 persons [3]. In the same year, the number of prevalent cases of TBI was 55.50 million (53.40–57.62 million). From 1990 to 2016, the age-standardized prevalence of TBI increased by 8.4% (7.7 to 9.2) worldwide [3]. TBI affects both elderly persons, mainly due to falls, and young individuals, because of road injuries, assault, or sports- and work-related accidents [4,5]. A high proportion of survivors from moderate or severe head injuries exhibit some grade of disability, with reduced independence [4,6]. For this reason, TBI is associated with a vast health and economic burden [7]. Permanent cost estimates ranged from $279 million to $1.22 billion, depending on the diagnostic criteria used to define TBI [7]. Therefore, it is imperative to treat TBI, with the aims of reducing severity and improving recovery [5].
It is considered that a better comprehension of the complex pathophysiology of TBI will help to decrease the frequency of TBI-associated complications. Alterations in cell membrane integrity, as well as impairments of the lipid metabolism, can lead to cell death after TBI, as in ischemic brain injuries [8,9,10]. In this sense, the importance of the role of lipids in cell signaling and tissue physiology has been demonstrated in many central nervous system (CNS) disorders and injuries related to dysregulated metabolism, as a key step in the pathophysiology of the ischemic/traumatic brain injury [8]. Choline-based phospholipids are involved in the maintenance of the structural integrity of the neuronal and glial cell membranes and are simultaneously the essential component of various biochemical pathways, such as cholinergic neurotransmission in the brain [9]. Consequently, a therapeutic approach based on the protection and the regeneration of cell membranes and on the normalization of lipid metabolism could prove beneficial [5,8] as a neuroprotective strategy. As a treatment approach, neuroprotection could be considered beneficial in patients with TBI [5,11,12] among other areas of improvement, such as intracranial pressure management, neuromonitoring, and the management of paroxysmal sympathetic hyperactivity [12].
Among the neuroprotective drugs used for the management of brain ischemia is citicoline [8]. Citicoline (cytidine 5′-diphosphocholine, or CDP-choline) is an endogenous compound involved in the biosynthesis of phosphatidylcholine, the major neuronal membrane lipid. Exogenous administration of citicoline has been shown to generate phospholipids, thus making it a neuroprotective/neurorestorative agent with an appropriate benefit–risk profile in the management of brain-ischemia-related disorders [5,13,14]. Citicoline has a pleiotropic effect on the molecular events involved in the pathophysiology of ischemic/traumatic brain injury, and it is widely used as a neuroprotective treatment of stroke and head injuries and the sequelae of both diseases [14]. In 2012, the Citicoline Brain Injury Treatment Trial (COBRIT) was published. This trial did not reveal an improvement in functional or cognitive status compared with placebo [15]. In any case, COBRIT was subject to a number of limitations, such as the extremely low adherence and the atypical oro-enteral administration of citicoline. The latter is not approved for TBI in any country, has not yet been scientifically studied, and, among other things, is not suitable for many of the patients included in the study [5,16]. Additionally, a meta-analysis performed in 2014 by Secades [17] showed that citicoline could have a positive impact on the rates of independence among patients following TBI, whereas the meta-analysis of El Sayed et al. [18] reported a neutral effect of citicoline on the Glasgow Outcome Scale/Glasgow Outcome Scale extended (GOS/GOSe) score, cognitive performance, and survival. Recently, an exhaustive narrative review on the role of citicoline in TBI has been published [5].
Therefore, given the available evidence, the role of citicoline in patients with TBI should be further investigated and clarified, especially after the controversy arising from the neutral results of the COBRIT. The aim of this study was to assess the benefits and hazards of therapy with citicoline in patients with TBI through a systematic review and meta-analysis of comparative trials.
2. Materials and Methods
This systematic review was conducted according to the methodological standards of the Cochrane Collaboration [19] and was based on a protocol (CRD42021238998) registered at https://www.crd.york.ac.uk/PROSPERO (accessed on 17 August 2022) [20]. The report followed the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) statement guidelines [21].
2.1. Study Selection Criteria
Eligible studies included randomized controlled trials (RCTs), comparative studies, and cohort studies of male and female patients of any age with a diagnosis of TBI and treatment starting in the first 24 h after injury and included the reporting results for independence or functional outcome. The trials included analyses of patients with complicated mild (defined as patients with a baseline Glasgow Coma Scale (GCS) score of 13–15 with some lesions on the CT scan), moderate (GCS 9–12), and severe TBI (GCS 3–8), but not mild TBI. The main reason for including complicated mild patients was because independence results for this category of patients were included in the COBRIT publication [15]. This systematic review comprised all studies in which the active agent was citicoline, irrespective of whether it was compared with another active treatment.
2.2. Search Methods
We searched OVID-Medline, EMBASE (access through OVID), Google Scholar, the Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library, latest issue), and the US National Institutes of Health’s ClinicalTrials.gov website, from inception to week three of January 2021, using appropriate controlled vocabulary and free search terms. Search strategies for OVID, MEDLINE, and EMBASE are detailed in Appendix A. For the other searches, the keywords CDP-choline, citicoline, traumatic brain injury, head injury, and craniocerebral trauma were used. Additionally, the reference lists from eligible studies, other reviews on this topic, and the Ferrer bibliographic database were screened to identify relevant studies. No restrictions on language, publication date, or publication status were applied. We did not search the gray literature.
2.3. Study Selection and Data Extraction
One author (J.J.S.) reviewed the abstracts of the articles retrieved in the search. Full-length papers for any abstract that we thought met the inclusion criteria were obtained. Three authors (J.J.S., H.T., B.S.) reviewed all the retrieved papers to identify those trials meeting the inclusion criteria for the review. One review author (J.J.S.) initially extracted the efficacy data from eligible trials that were then independently confirmed by H.T. and B.S. Discrepancies were resolved by discussion with the other authors (H.T., B.S., J.A.G.) and by referencing the original report. J.A.G. performed all the statistical analyses.
2.4. Risk of Bias Assessment
We used the Cochrane Collaboration’s tool to assess the risk of bias in the included studies [19]. We scored the risk of bias by using the RoB2 tool v.7 [22] for randomized trials and the ROBINS-I [23] for nonrandomized studies.
2.5. Data Analysis
The primary efficacy measure was independence at the end of the scheduled clinical trial follow-up. If available, we used the GOS/GOSe for this measure. In studies lacking the GOS/GOSe measurement, we used the most comprehensive measure of disability or handicap available from the study for the classification of patients as dependent or independent, defining independence as the ability to perform almost all the activities of daily life without the assistance of another.
We applied dichotomous outcomes in the statistical meta-analytic methods, and a weighted estimation (with inverse-variance weights) was used. For the GOS, scores of 4 or 5 were considered as good outcomes (independence), and for GOSe, 7 or 8. The risk ratio (RR) was used to test for the proportional treatment effects of citicoline. The odds ratio (OR) and risk difference (RD) were used in complementary analysis.
The formal meta-analysis was performed by using metafor (version 2.4-0), a meta-analysis package for R [24]. Given that we assumed heterogeneity with respect to the studies performed over 4 decades, the main analysis used the random-effects model to obtain a 95% confidence interval (CI) estimate for the effects of citicoline compared with controls. We chose the REML (restricted maximum likelihood estimator) method, that is, the default in the package; confidence intervals are based on a standard normal distribution. The strength of the body of evidence was assessed by using the grading of recommendations assessment, development, and evaluation (GRADE) score [25]. The results of the meta-analysis were presented as forest plots, with studies listed in order of age, oldest first. In addition to unadjusted random- and fixed-effects models, as subgroups data were analyzed according to the dose (trials with doses higher than 2 g/day vs. 2 g/day or lower) and the administration route (parenteral vs. only oral/enteral) because these study-level variables included in the model might account for part of the heterogeneity in the effects. We also performed a sensitivity analysis restricted to RCT studies.
3. Results
3.1. Study Selection
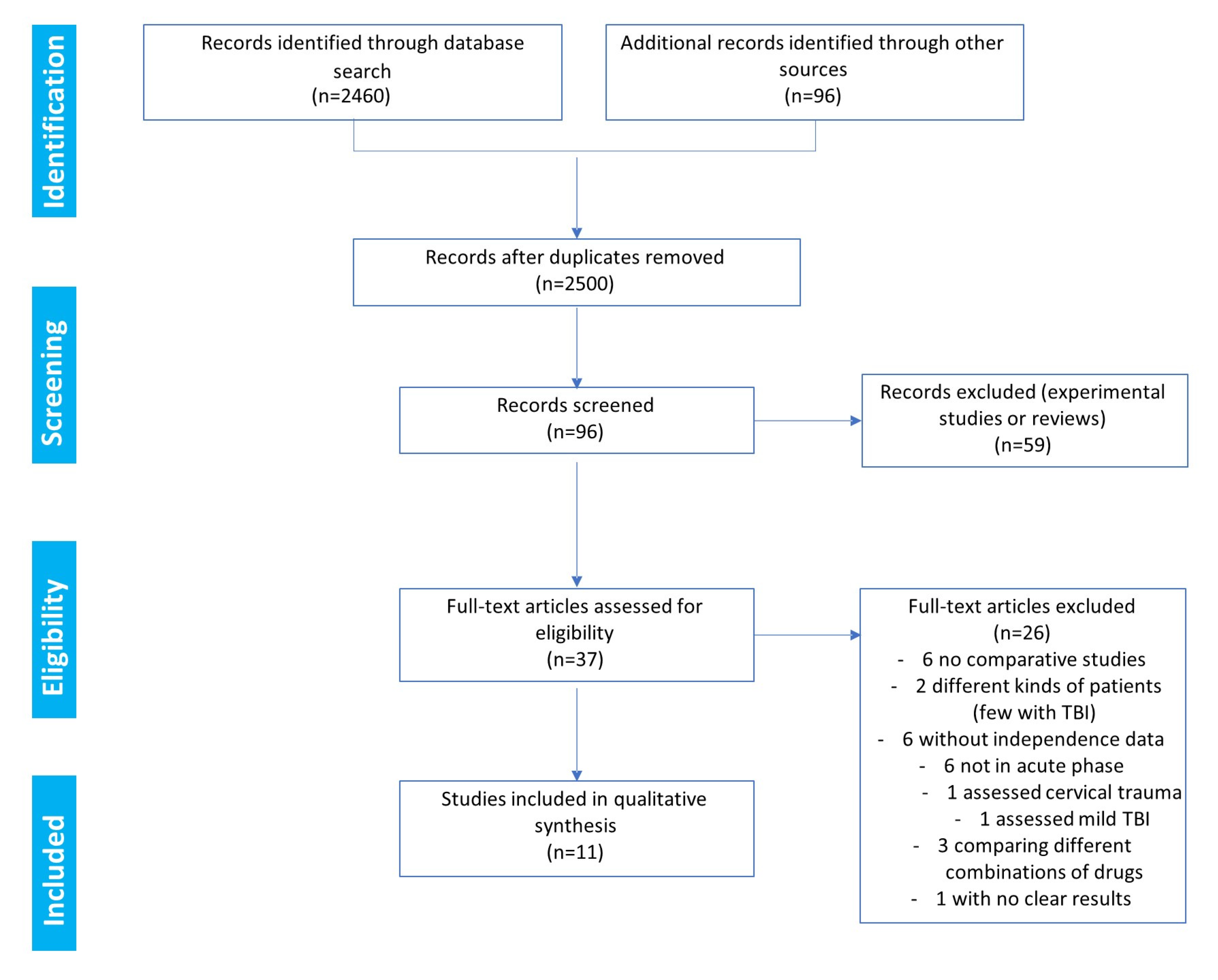
The search results and the decisions made during the eligibility process are displayed in the PRISMA flowchart (Figure 1). The search provided 2460 records. Another 96 records were identified through the search of the Ferrer bibliographic database. The removal of duplicates left a total of 2500 references. After a review of the citations, the abstracts, and the full papers (when available), 96 records were initially screened. In total, 59 references were excluded as they corresponded to animal studies or reviews. Consequently, in the end, 37 full-text clinical studies were assessed for eligibility (Figure 1). Among these clinical studies, only 11 fulfilled the criteria to be included in the qualitative synthesis [15,26,27,28,29,30,31,32,33,34,35]. Among the 26 studies not selected for the meta-analysis, six were noncomparative studies [36,37,38,39,40,41], six were not in the acute phase of TBI [42,43,44,45,46,47], six had unavailable independence data [48,49,50,51,52,53], three compared different combinations of drugs [54,55,56], two analyzed different types of patients (including few with TBI) [57,58], one assessed cervical trauma [59], one assessed mild TBI [60], and one study reported no clear results, with only 10 patients per arm [61].
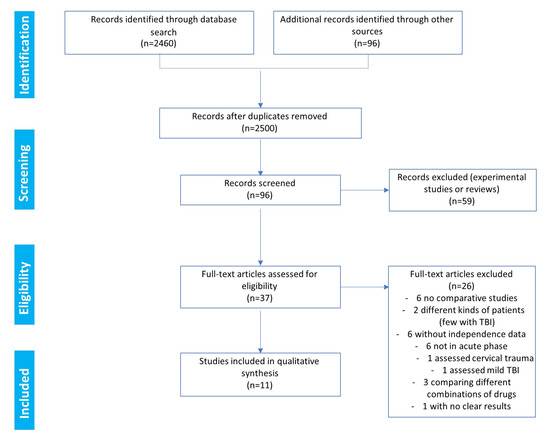
Figure 1.
Eligibility: PRISMA flowchart.
3.2. Study Characteristics
The 11 included studies comprised two cohort studies [31,35], seven RCTs comparing citicoline with placebo or a control group [15,26,27,28,32,33,34], one RCT comparing citicoline with meclofenoxate [29], and another RCT comparing citicoline with piracetam [30]. All the studies assessed the effect of citicoline on the recovery of patients with complicated mild, moderate, or severe head injury. The oldest study was published in 1978, and the most recent study was published in 2018; thus, there was a gap of 40 years between the first and last studies. As stated above, this may be a source of heterogeneity owing to improvements in the management of TBI during this period, and it justifies an analysis based on the random-effects model. The studies included a total of 2771 patients, in whom citicoline was administered at doses ranging from 300 mg to 6 g. The duration of treatment varied from 10 to 90 days. The drug was administered intravenously in six studies [26,29,30,31,33,35], intravenously or intramuscularly in two studies [27,28], intravenously followed by oral administration in one study [32], and orally in two studies [15,34]. All the studies included the rate of independence, albeit at different times of evaluation (Table 1). The PEDro score [62] of the trials ranged from 7 to 11, with an average of 9.6 (Table 2).

Table 1.
Summary of the studies evaluating the efficacy of citicoline in TBI included in the present analysis.

Table 2.
PEDro scores of the studies.
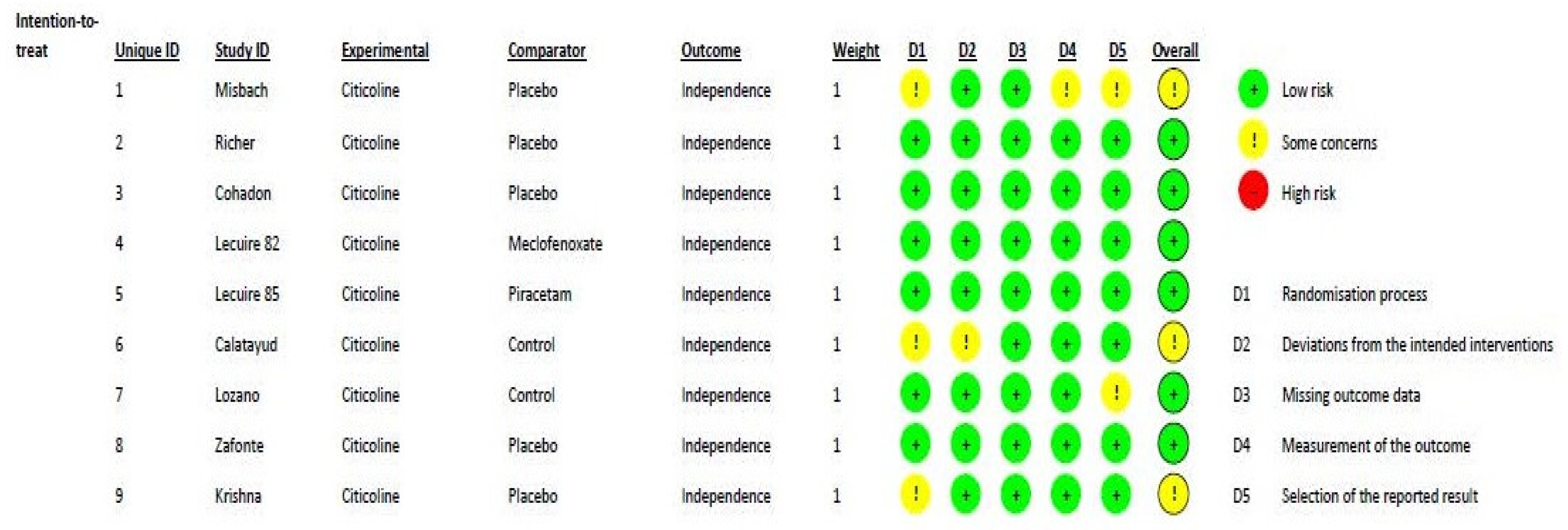
Figure 2 shows the methodological quality of the included randomized studies, based on the RoB2 tool, with a low risk of bias. According to the ROBINS-I assessment tool for nonrandomized studies, the two studies [31,35] included in this meta-analysis can be considered to have a low or moderate risk of bias for all domains. One major difference between the trials was the standard of care applied, which changed over time.
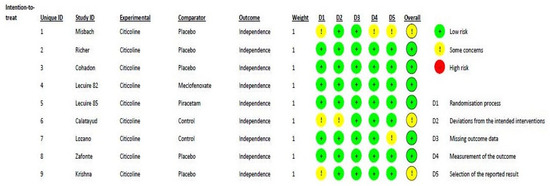
Figure 2.
Risk of bias distribution diagram.
3.3. Synthesis of the Results
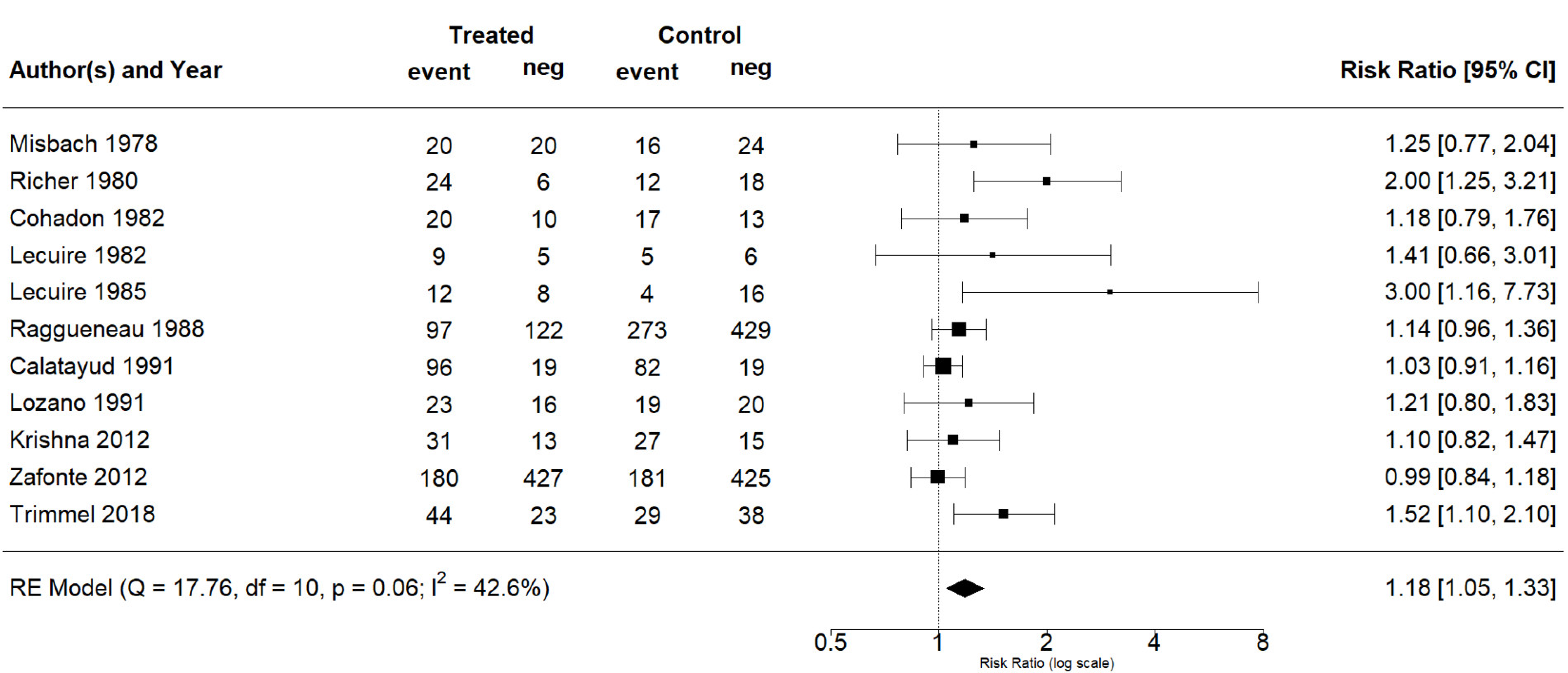
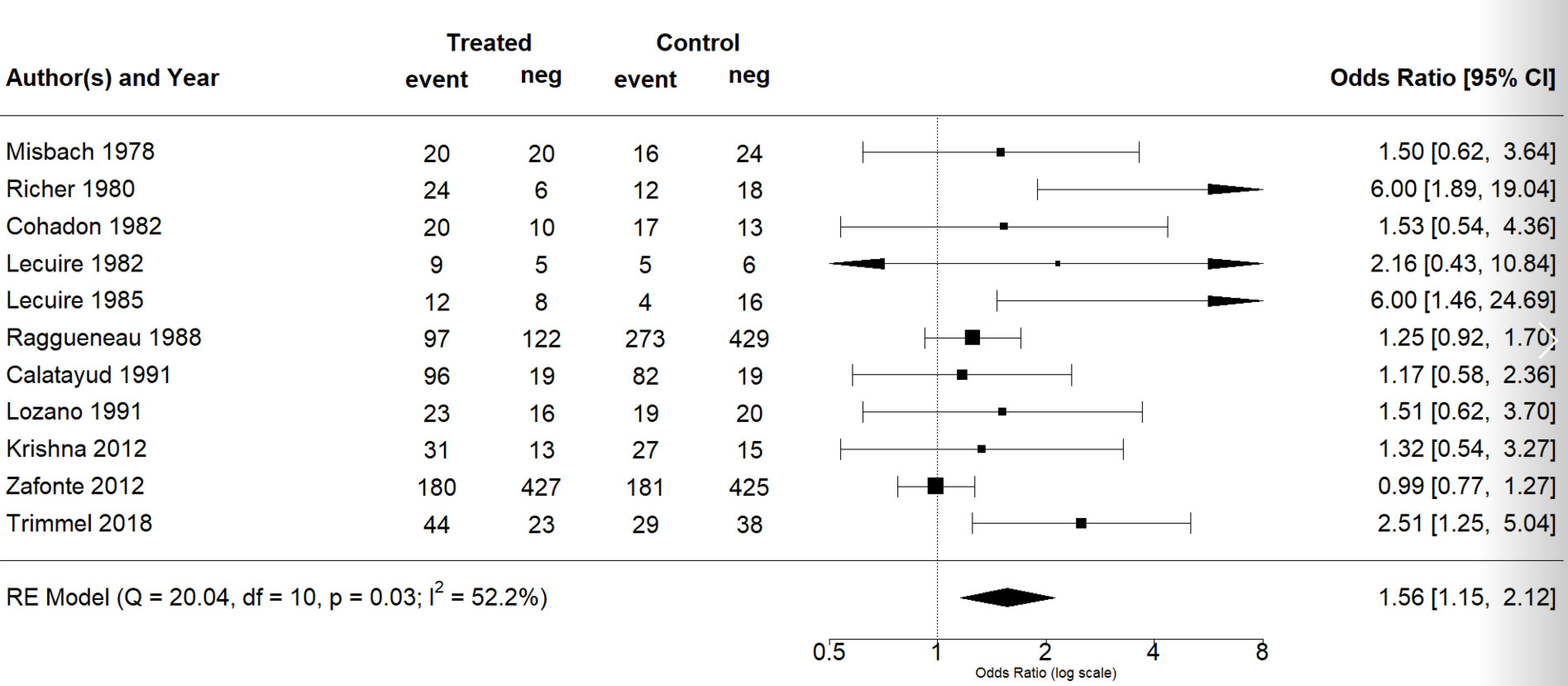
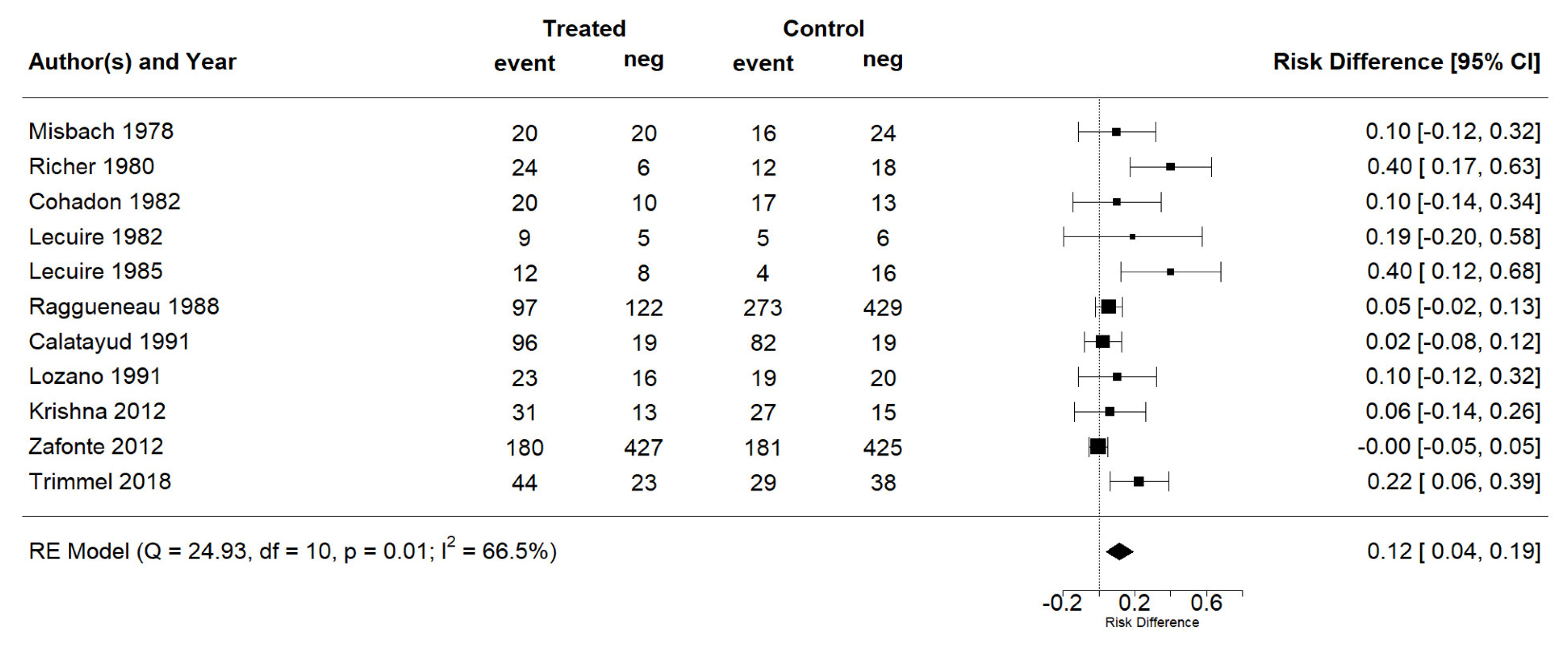
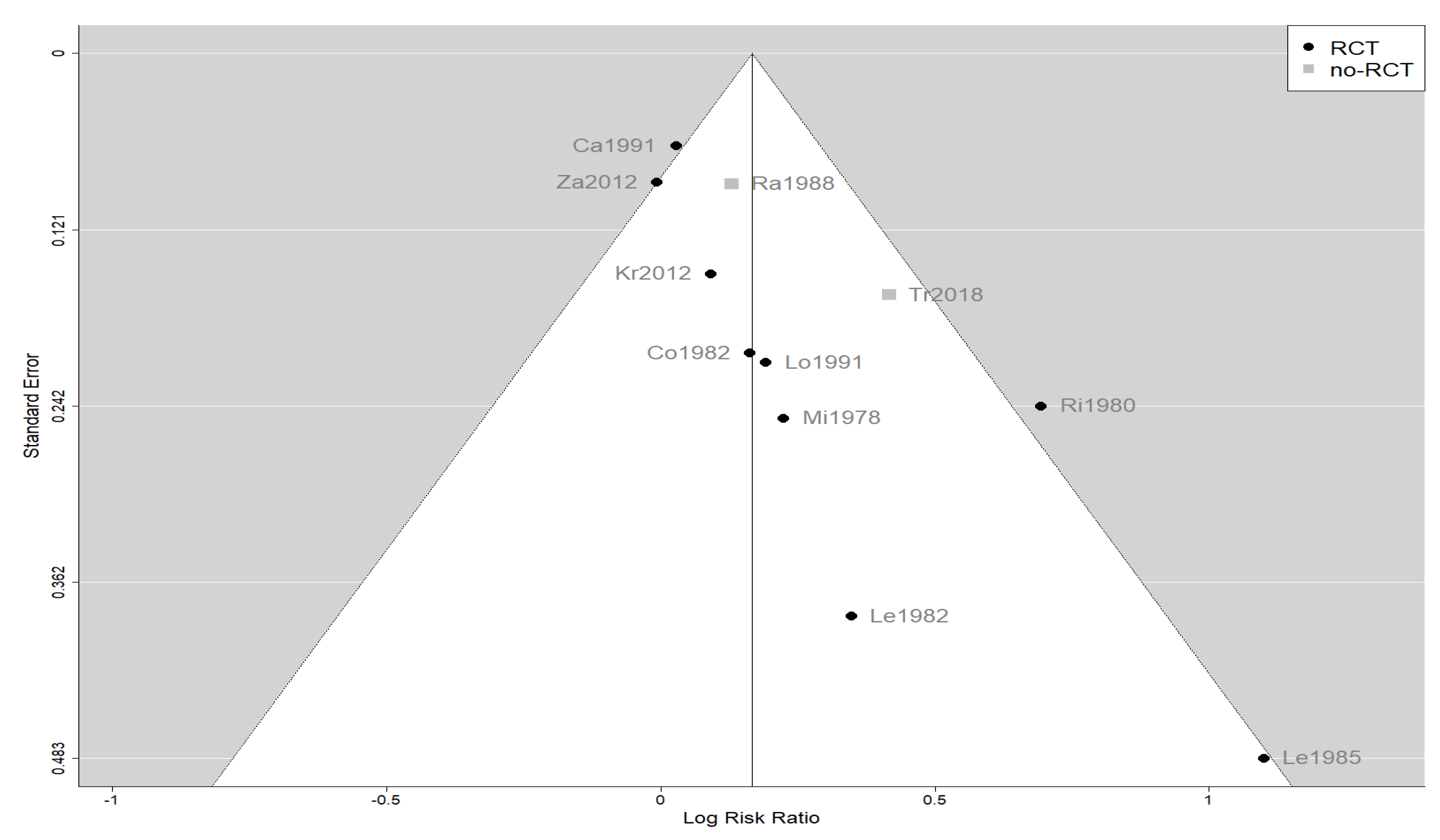
The effect estimates and the confidence intervals (CIs) are presented as a forest plot. All the results were directly reported, as obtained from the original publication. The administration of citicoline was associated with a significantly higher rate of independence (RR = 1.18; 95% CI = 1.05–1.33; I2 = 42.6%) (Figure 3). Complementarily, we performed OR and RD analyses, and in both cases, the results were congruent with the RR obtained (OR = 1.56; 95% CI = 1.15–2.12 (Figure 4); RD = 0.12; 95% CI = 0.04–0.19 (Figure 5)). Thus, the probability of presenting the favorable event was 18% higher with the intervention; alternatively, citicoline increased by 0.12 points with respect to that of a favorable event without the intervention. The I2 heterogeneity indicator obtained (42.6%) was not large but was considerable, as described in the funnel plot (Figure 6); nonetheless, we also estimated the effect of citicoline under the fixed-effects model, with similar results (Table 3).
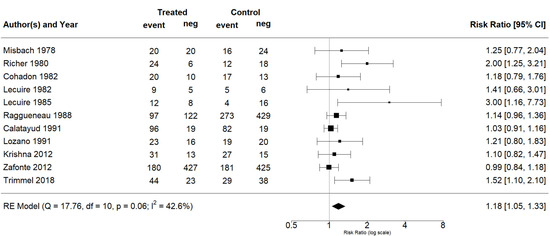
Figure 3.
Forest plot of the meta-analysis, based on the random-effects model (risk ratio).
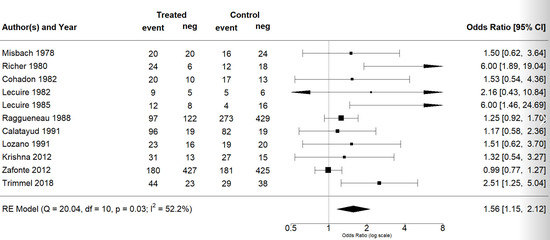
Figure 4.
Forest plot of the meta-analysis: complementary analysis (odds ratio).
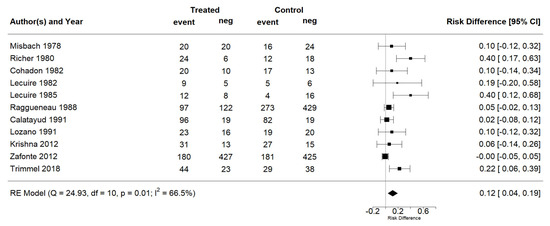
Figure 5.
Forest plot of the meta-analysis: complementary analysis (risk difference).
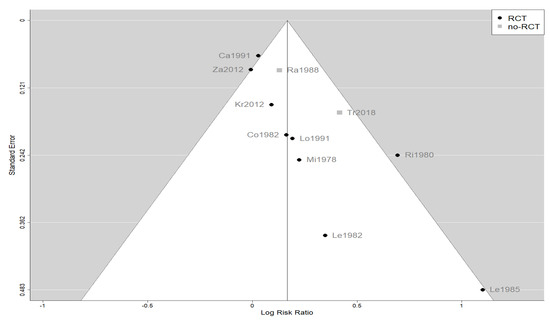
Figure 6.
Funnel plot of the meta-analysis showing the heterogeneity among the studies included in the meta-analysis.

Table 3.
Summary of results *.
None of the adjusted analyses yielded a significant difference, and thus, we were unable to find a relationship between the outcome and either the dose of citicoline or the route of administration. Table 3summarizes the results obtained. Note that the significant effect was maintained when we analyzed only the RCTs (RR = 1.16; 95% CI = 1.01–1.33; I2 = 39.4%). According to the results, we can categorize the evidence as moderate certainty (GRADE). There was no difference in mortality rates in the randomized studies. Only in the study of Trimmel et al. [35] was a significant reduction in mortality for patients with severe TBI treated with citicoline described. Additionally, none of the included studies reported any serious safety problem associated with citicoline.
4. Discussion
This meta-analysis showed that in nearly 2800 patients with acute-phase TBI, treatment with citicoline was associated with a significant improvement in the level of independence, with a moderate level of evidence according to GRADE.
Given the considerable disease burden associated with moderate to severe TBI, there is a need to improve recovery in affected patients. Although the treatment for TBI has improved in recent years, mortality and disability rates remain high [3,4,9,10]. Inflammation, alterations in cell membrane integrity, and the impairment of phospholipid metabolism have all been implicated in the pathophysiology of TBI [5,8,9,10,14]. Importantly, many studies have shown that citicoline has neuroprotective and neurorestorative properties, including the following: the normalization or stabilization of damaged neuronal cell membranes (i.e., phospholipid content and function, ion exchange); the restoration of some enzymatic activities; a reduction in the generation of damaging free fatty acids and free radicals; the improvement of neurotransmission and cerebral metabolism; anti-inflammatory and antioxidant properties; the enhanced integrity of the blood–brain barrier; the accelerated absorption of brain edema and the decreased volume of ischemic lesions; the inhibition of apoptosis; and the enhancement of neurorepair and neuroplasticity properties [5,8,9,10,14,63,64,65,66]. Thus, given its biochemical, pharmacological, and pharmacokinetic characteristics, citicoline should considered as a potentially useful drug for the treatment of patients with TBI [5,14,17].
Nevertheless, the COBRIT did not show any improvement in outcomes in this population [15]. The COBRIT has been the largest study performed with citicoline in TBI patients, but there are relevant methodological issues that question the validity and applicability of the results obtained in the study. The first point to consider is the financing of this study; the study was an independent study, financed by the US National Institute of Health, with a limited budget. A relevant point to consider is the sample size calculation. The authors chose an OR of 1.4 as the effect of the treatment, when in the most recent publications, the size of the effect of citicoline was 1.26 in acute ischemic stroke patients, a less heterogeneous pathology than TBI. The sample size was likely calculated on the basis of the number of patients that could be afforded, and then the OR of the treatment was established accordingly, instead of basing it on the real effects of the drug. With a more conservative and realistic OR of 1.2 or less, the sample size should be much higher and would likely have been unaffordable for the authors. Another questionable point to consider is that the authors mixed different populations, confusing mild, moderate, and severe TBI. The pathophysiology, localization, and trajectory for recovery can be quite different among these different groups. To avoid this issue, the authors should have used a randomized, matched sample design. This mixing of lesion severity is a clear source of heterogeneity and would have to be considered an important confounding factor in the analysis and interpretation of the data. Another point is the atypical oro-enteral administration of citicoline used in this trial, which is not approved in any country, has not previously been scientifically tested, and is not appropriate for many of the patients enrolled in the study, particularly in moderate and severe cases. However, the most controversial point is the extremely poor compliance with the treatment. Only 44.4% compliance for patients having taken more than 75% of the medication expected is clearly insufficient and needs further elaboration in the interpretation of the results. Not receiving the active treatment is not the same as not receiving the placebo, in terms of the standard of care being received. This means that fewer than half of the patients received something close to a therapeutic dose of citicoline. Thus, the COBRIT is not the definitive study on citicoline, especially when the methodological confounds just described are taken into consideration [5,14,16]). As a result, the evidence provided by the study must be considered controversial [5,16].
In this context, meta-analyses could prove to be very helpful in clarifying the role of citicoline in clinical practice. In 2014, a previous meta-analysis based on 12 clinical studies enrolling 2706 patients with mild to severe TBI treated in the acute phase with/without citicoline showed a significant increase in the rates of independence with citicoline (OR, 1.815 (95% CI, 1.302–2.530) under the random-effects model vs. 1.451 (95% CI, 1.224–1.721) under the fixed-effects model) [17]. These results are in line with those in this study. In contrast, another meta-analysis found neutral effects for citicoline in the treatment of patients with TBI, although it is noteworthy that this meta-analysis was restricted to studies published in English, with only 1355 patients for the GOS outcome, 1,291 patients for the assessment of cognitive performance, and 1037 patients for the assessment of survival [18]. Therefore, the sample size in this meta-analysis was too limited to find a beneficial impact of citicoline in the study population, owing to the English language restriction, which is a well-known source of heterogeneity [5].
While our study analyzed only the neuroprotective efficacy of citicoline among patients following TBI, clinical trials and real-life studies have confirmed its excellent safety profile [5,14,17]. The neuroprotective effects of citicoline should not be limited to patients with TBI but instead could also be extended to patients with other neurological diseases [14], as some studies have suggested, including COVID-19-related cognitive complications, multiple sclerosis, and dementia [67,68,69].
The main limitation of our meta-analysis was the presumed heterogeneity of the included studies. This was the result of marked improvements in the management of patients with TBI over 4 decades. As shown in Figure 6, a funnel plot suggests that the old, small studies may be overestimating the effect size, although the number of these studies is too few to be conclusive. In addition, our meta-analysis was the largest performed to date and analyzed the level of independence, an outcome that was well defined in the included studies. In any case, our results are quite relevant because no new large-scale clinical trials with citicoline for this indication are expected.
The initial extraction of the data was performed by only one author (J.J.S.) because he was the author of a recent narrative review on the effects of citicoline on TBI [5]. Despite that, the initially extracted data were confirmed by the other clinical authors (H.T. and B.S.).
5. Conclusions
In summary, with regard to the level of independence, our meta-analysis provided some evidence of the benefits of citicoline in combination with the standard of care in the management of patients with complicated mild, moderate, and severe TBI. This benefit could be independent of the dose used and of the administration route (oral or parenteral).
Author Contributions
Draft the protocol: J.J.S.; approve the protocol: H.T., B.S. and J.A.G.; develop a search strategy: J.J.S.; search for trials: J.J.S.; obtain copies of trials: J.J.S.; select which trials to include: J.J.S., H.T. and B.S.; initial extract data from trials: J.J.S.; confirm data extracted from trials: H.T. and B.S.; carry out formal meta-analysis: J.A.G.; interpret the analysis: J.J.S., H.T., B.S. and J.A.G.; draft the final review: J.J.S.; approve the final review: H.T., B.S. and J.A.G.; guarantee the review: H.T., B.S. and J.A.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Ferrer, who performed the bibliographic searches, retrieved the necessary articles and provided the writing assistance by Content Ed Net.
Institutional Review Board Statement
Ethical approval is not required, as this study is only a systematic review and does not involve confidential or private data.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the papers included in this meta-analysis are available upon request.
Acknowledgments
Writing assistance was provided by Content Ed Net (Madrid, Spain), with funding from Ferrer.
Conflicts of Interest
Julio J. Secades is a full-time employee of Ferrer. Helmut Trimmel and Byron Salazar received lecture fees for a webinar sponsored by Ferrer. José Antonio González declares that he has no conflicts of interest associated with Ferrer.
Appendix A. Search Algorithms Used to Identify Studies in the Databases
| Database | Search Algorithm |
| OVID-Medline (PubMed) Up to January Week 3, 2021 | #1. exp CRANIOCEREBRAL TRAUMA/ #2. exp Cerebrovascular Trauma/ #3. exp BRAIN EDEMA/ #4. ((brain or cerebral or intracranial) adj3 (oedema or edema or swell$)).ab,ti. #5. exp GLASGOW COMA SCALE/ #6. exp GLASGOW OUTCOME SCALE/ #7. exp UNCONSCIOUSNESS/ #8. (Glasgow adj3 (coma or outcome) adj3 (scale$ or score$)).ab,ti. #9. (Unconscious$ or coma$ or concuss$ or ‘persistent vegetative state’).ab,ti. #10. “Rancho Los Amigos Scale”.ab,ti. #11. ((head or crani$ or cerebr$ or capitis or brain$ or forebrain$ or skull$ or hemispher$ or intra-cran$ or inter-cran$) adj3 (injur$ or trauma$ or damag$ or wound$ or fracture$ or contusion$)).ab,ti. #12. “Diffuse axonal injur$”.ab,ti. #13. ((head or crani$ or cerebr$ or brain$ or intra-cran$ or inter-cran$) adj3 (haematoma$ or hematoma$ or haemorrhag$ or hemorrhag$ or bleed$ or pressure)).ab,ti. #14. or/#1–13 #15. randomized.ab,ti. #16. randomized controlled trial.pt. #17. controlled clinical trial.pt. #18. placebo.ab. #19. clinical trials as topic.sh. #20. randomly.ab. #21. trial.ti. #22. Comparative Study/ #23. #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 #24. (animals not (humans and animals)).sh. #25. #23 not #24 #26. #14 and #25 #27. Cytidine Diphosphate Choline/ #28. citicoline.ab,ti. #29. cdp-choline.ab,ti. #30. cytidine-5-diphosphocholine.ab,ti. #31. (“cdp choline” or cdpcholine).ab,ti. #32. #27 or #28 or #29 or #30 or #31 #33. #26 and #32 |
| EMBASE Up to January Week 3, 2021 | (‘acute brain injury’/exp OR ‘acute brain injury’ OR ‘brain injuries’/exp OR ‘brain injuries’ OR ‘brain injury’/exp OR ‘brain injury’ OR ‘brain injury, chronic’/exp OR ‘brain injury, chronic’ OR ‘cerebral injury’/exp OR ‘cerebral injury’ OR ‘cerebrum lesion’/exp OR ‘cerebrum lesion’ OR ‘chronic brain injury’/exp OR ‘chronic brain injury’ OR ‘injury, brain’/exp OR ‘injury, brain’ OR ‘left hemisphere injury’/exp OR ‘left hemisphere injury’ OR ‘right hemisphere injury’/exp OR ‘right hemisphere injury’ OR ‘brain injuries, traumatic’/exp OR ‘brain injuries, traumatic’ OR ‘brain lesion, traumatic’/exp OR ‘brain lesion, traumatic’ OR ‘brain system trauma’/exp OR ‘brain system trauma’ OR ‘brain trauma’/exp OR ‘brain trauma’ OR ‘cerebral trauma’/exp OR ‘cerebral trauma’ OR ‘cerebrovascular trauma’/exp OR ‘cerebrovascular trauma’ OR ‘encephalopathy, traumatic’/exp OR ‘encephalopathy, traumatic’ OR ‘mild traumatic brain injury’/exp OR ‘mild traumatic brain injury’ OR ‘organic cerebral trauma’/exp OR ‘organic cerebral trauma’ OR ‘posttraumatic encephalopathy’/exp OR ‘posttraumatic encephalopathy’ OR ‘traumatic brain injuries’/exp OR ‘traumatic brain injuries’ OR ‘traumatic brain injury’/exp OR ‘traumatic brain injury’ OR ‘traumatic brain lesion’/exp OR ‘traumatic brain lesion’ OR ‘traumatic cerebral lesion’/exp OR ‘traumatic cerebral lesion’ OR ‘traumatic encephalopathy’/exp OR ‘traumatic encephalopathy’ OR ‘brain edema’/exp OR ‘brain edema’ OR ‘brain oedema’/exp OR ‘brain oedema’ OR ‘cerebral edema’/exp OR ‘cerebral edema’ OR ‘cerebral oedema’/exp OR ‘cerebral oedema’ OR ‘edema, brain’/exp OR ‘edema, brain’ OR ‘oedema, brain’/exp OR ‘oedema, brain’ OR ‘cerebrocranial injury’/exp OR ‘cerebrocranial injury’ OR ‘cerebrocranial trauma’/exp OR ‘cerebrocranial trauma’ OR ‘closed head injuries’/exp OR ‘closed head injuries’ OR ‘cranial injury’/exp OR ‘cranial injury’ OR ‘cranial trauma’/exp OR ‘cranial trauma’ OR ‘craniocerebral injury’/exp OR ‘craniocerebral injury’ OR ‘craniocerebral lesion’/exp OR ‘craniocerebral lesion’ OR ‘craniocerebral trauma’/exp OR ‘craniocerebral trauma’ OR ‘craniocerebral wound’/exp OR ‘craniocerebral wound’ OR ‘head injuries’/exp OR ‘head injuries’ OR ‘head injuries, closed’/exp OR ‘head injuries, closed’ OR ‘head injuries, penetrating’/exp OR ‘head injuries, penetrating’ OR ‘head injury’/exp OR ‘head injury’ OR ‘head trauma’/exp OR ‘head trauma’ OR ‘head wound’/exp OR ‘head wound’ OR ‘injury, head’/exp OR ‘injury, head’ OR ‘penetrating head injuries’/exp OR ‘penetrating head injuries’ OR ‘trauma capitis’/exp OR ‘trauma capitis’ OR ‘trauma, cranial’/exp OR ‘trauma, cranial’ OR ‘trauma, head’/exp OR ‘trauma, head’) AND (‘cdp choline’/exp OR ‘cdp choline’ OR ‘brassel 1000’/exp OR ‘brassel 1000’ OR ‘ceraxon’/exp OR ‘ceraxon’ OR ‘cidifos’/exp OR ‘cidifos’ OR ‘cidiphos’/exp OR ‘cidiphos’ OR ‘citicholine’/exp OR ‘citicholine’ OR ‘citicolin’/exp OR ‘citicolin’ OR ‘citicolina’/exp OR ‘citicolina’ OR ‘citicoline’/exp OR ‘citicoline’ OR ‘cyticholine’/exp OR ‘cyticholine’ OR ‘cytidine 5 diphosphocholine’/exp OR ‘cytidine 5 diphosphocholine’ OR ‘cytidine 5‘ diphosphocholine’/exp OR ‘cytidine 5‘ diphosphocholine’ OR ‘cytidine 5‘ diphosphorylcholine’/exp OR ‘cytidine 5‘ diphosphorylcholine’ OR ‘cytidine diphosphate choline ester’/exp OR ‘cytidine diphosphate choline ester’ OR ‘cytidine diphosphocholine’/exp OR ‘cytidine diphosphocholine’ OR ‘cytocholine’/exp OR ‘cytocholine’ OR ‘nicholin’/exp OR ‘nicholin’ OR ‘rexort’/exp OR ‘rexort’ OR ‘sauran’/exp OR ‘sauran’ OR ‘sinkron’/exp OR ‘sinkron’ OR ‘sintoclar’/exp OR ‘sintoclar’ OR ‘somazina’/exp OR ‘somazina’ OR ‘cdp’/exp OR ‘cdp’ OR ‘cytidine 5‘ diphosphate’/exp OR ‘cytidine 5‘ diphosphate’ OR ‘cytidine diphosphate’/exp OR ‘cytidine diphosphate’ OR ‘cytosine diphosphate’/exp OR ‘cytosine diphosphate’ OR ‘cytidine diphosphate choline’/exp OR ‘cytidine diphosphate choline’) AND (‘clinical trial, controlled’/exp OR ‘clinical trial, controlled’ OR ‘controlled clinical comparison’/exp OR ‘controlled clinical comparison’ OR ‘controlled clinical drug trial’/exp OR ‘controlled clinical drug trial’ OR ‘controlled clinical experiment’/exp OR ‘controlled clinical experiment’ OR ‘controlled clinical study’/exp OR ‘controlled clinical study’ OR ‘controlled clinical test’/exp OR ‘controlled clinical test’ OR ‘controlled clinical trial’/exp OR ‘controlled clinical trial’ OR ‘review, systematic’/exp OR ‘review, systematic’ OR ‘systematic review’/exp OR ‘systematic review’ OR ‘meta analysis’/exp OR ‘meta analysis’) |
References
- Capizzi, A.; Woo, J.; Verduzco-Gutierrez, M. Traumatic Brain Injury: An Overview of Epidemiology, Pathophysiology, and Medical Management. Med. Clin. N. Am. 2020, 104, 213–238. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.K.; Yang, Z.; Zhu, T.; Shi, Y.; Rubenstein, R.; Tyndall, J.A.; Manley, G.T. An update on diagnostic and prognostic biomarkers for traumatic brain injury. Expert Rev. Mol. Diagn. 2018, 18, 165–180. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Traumatic Brain Injury and Spinal Cord Injury Collaborators. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 56–87. [Google Scholar] [CrossRef] [PubMed]
- James, S.L.; Lucchesi, L.R.; Bisignano, C.; Castle, C.D.; Dingels, Z.V.; Fox, J.T.; Hamilton, E.B.; Liu, Z.; McCracken, D.; Nixon, M.R.; et al. Morbidity and mortality from road injuries: Results from the Global Burden of Disease Study 2017. Inj. Prev. 2020, 26 (Suppl. S1), i46–i56. [Google Scholar] [CrossRef] [PubMed]
- Secades, J.J. Role of Citicoline in the Management of Traumatic Brain Injury. Pharmaceuticals 2021, 14, 410. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, L.S.; Doonan, M. Value and Cost Savings from Access to Multi-disciplinary Rehabilitation Services After Severe Acquired Brain Injury. Front. Public Health 2021, 9, 753447. [Google Scholar] [CrossRef]
- Fu, T.S.; Jing, R.; McFaull, S.R.; Cusimano, M.D. Health & Economic Burden of Traumatic Brain Injury in the Emergency Department. Can. J. Neurol. Sci. 2016, 43, 238–247. [Google Scholar] [CrossRef]
- Adibhatla, R.M.; Hatcher, J.F. Role of Lipids in Brain Injury and Diseases. Future Lipidol. 2007, 2, 403–422. [Google Scholar] [CrossRef]
- Javaid, S.; Farooq, T.; Rehman, Z.; Afzal, A.; Ashraf, W.; Rasool, M.F.; Alqahtani, F.; Alsanea, S.; Alasmari, F.; Alanazi, M.M.; et al. Dynamics of Choline-Containing Phospholipids in Traumatic Brain Injury and Associated Comorbidities. Int. J. Mol. Sci. 2021, 22, 11313. [Google Scholar] [CrossRef]
- Maas, A.I.; Stocchetti, N.; Bullock, R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008, 7, 728–741. [Google Scholar] [CrossRef]
- Carney, N.; Totten, A.M.; O’Reilly, C.; Ullman, J.S.; Hawryluk, G.W.; Bell, M.J.; Bratton, S.L.; Chesnut, R.; Harris, O.A.; Kissoon, N.; et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 2017, 80, 6–15. [Google Scholar] [CrossRef]
- Marehbian, J.; Muehlschlegel, S.; Edlow, B.L.; Hinson, H.E.; Hwang, D.Y. Medical Management of the Severe Traumatic Brain Injury Patient. Neurocrit. Care 2017, 27, 430–446. [Google Scholar] [CrossRef] [PubMed]
- Grieb, P. Neuroprotective properties of citicoline: Facts, doubts and unresolved issues. CNS Drugs 2014, 28, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Secades, J.J.; Gareri, P. Citicoline: Pharmacological and clinical review, 2022 update. Rev. Neurol. 2022, 75, S1–S89. [Google Scholar] [CrossRef]
- Zafonte, R.D.; Bagiella, E.; Ansel, B.M.; Novack, T.A.; Friedewald, W.T.; Hesdorffer, D.C.; Timmons, S.D.; Jallo, J.; Eisenberg, H.; Hart, T.; et al. Effect of citicoline on functional and cognitive status among patients with traumatic brain injury: Citicoline Brain Injury Treatment Trial (COBRIT). JAMA 2012, 308, 1993–2000. [Google Scholar] [CrossRef]
- Adibhatla, R.M. Citicoline in stroke and TBI clinical trials. Nat. Rev. Neurol. 2013, 9, 173. [Google Scholar] [CrossRef] [PubMed]
- Secades, J.J. Citicoline for the treatment of head injury: A systematic review and meta-analysis of controlled clinical trials. J. Trauma Treat. 2014, 4, 227. [Google Scholar] [CrossRef]
- El Sayed, I.; Zaki, A.; Fayed, A.M.; Shehata, G.M.; Abdelmonem, S. A meta-analysis of the effect of different neuroprotective drugs in management of patients with traumatic brain injury. Neurosurg. Rev. 2018, 41, 427–438. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Lasserson, T.; Chandler, J.; Tovey, D.; Thomas, J.; Flemyng, E.; Churchill, R. Methodological Expectations of Cochrane Intervention Reviews; Cochrane: London, UK, 2022. [Google Scholar]
- Booth, A.; Clarke, M.; Dooley, G.; Ghersi, D.; Moher, D.; Petticrew, M.; Stewart, L. The nuts and bolts of PROSPERO: An international prospective register of systematic reviews. Syst. Rev. 2012, 1, 2. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Savović, J.; Page, M.J.; Sterne, J.A.C.; on behalf of the RoB2 Development Group. Revised Cochrane Risk-of-Bias Tool for Randomized Trials (RoB 2). Available online: https://www.riskofbias.info/welcome/rob-2-0-tool/current-version-of-rob-2 (accessed on 26 January 2022).
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010, 36, 1–48. Available online: https://wwwjstatsoftorg/v36/i03/ (accessed on 20 July 2021). [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J.; GRADE Working Group. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef] [PubMed]
- Misbach, J.; Andradi, S.; Harahap, T.P.; Soemargo, S.; Markam, S. Double blind trial of Nicholin (CDP-choline) on the patients with severe head injury. In Proceedings of the Biannual Meeting of Neurology, Psychiatry and Neurosurgery, Surabaya, Indonesia, 6–8 November 1978. [Google Scholar]
- Richer, E.; Cohadon, F. Therapeutic trial of a precursor phospholipids on the treatment of severe traumatic comas. In Proceedings of the International Symposium: Suffering and Cerebral Phospholipid Precursors; Laboratoires Cassenne-Takeda: Paris, France, 1980. [Google Scholar]
- Cohadon, F.; Richer, E.; Poletto, B. Etude d’un precurseur des phospholipides dans le traitement des comas traumatiques graves [A precursor of phospholipids in the treatment of severe traumatic comas]. Neurochirurgie 1982, 28, 287–290. [Google Scholar]
- Lecuire, J.; Duplay, J. Double-blind trial of citicoline versus meclofenossato in patients with head injury. G. Ital. Ric. Clin Ter. 1982, 3, 51–55. [Google Scholar]
- Lecuire, J. Traumatismes craniens: Etude comparative Piracetam- CDPcholine. CR Ther. Pharmacol. Clin. 1985, 3, 3–7. [Google Scholar]
- Raggueneau, J.L.; Jarrige, B. National Survey result in serious head injuries: Analysis of 219 injuries treated with CDP-choline [National inquiry on the outcome of severe head injuries: Analysis of 921 Injured Patients Treated with CDP-choline]. Agressologie 1988, 29, 439–443. [Google Scholar]
- Calatayud Maldonado, V.; Calatayud Pérez, J.B.; Aso Escario, J. Effects of CDP-choline on the recovery of patients with head injury. J. Neurol. Sci. 1991, 103 Suppl, S15–S18. [Google Scholar] [CrossRef]
- Lozano, R. CDP-choline in the treatment of cranio-encephalic traumata. J. Neurol. Sci. 1991, 103, S43–S47. [Google Scholar] [CrossRef]
- Krishna, D.; Chaurasia, I.D.; Jethwani, U. Role of citicoline in traumatic brain injury: A randomized controlled study. IJPMR 2012, 2, 1–5. [Google Scholar]
- Trimmel, H.; Majdan, M.; Wodak, A.; Herzer, G.; Csomor, D.; Brazinova, A. Citicoline in severe traumatic brain injury: Indications for improved outcome: A retrospective matched pair analysis from 14 Austrian trauma centers. Wien. Klin. Wochenschr. 2018, 130, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, M.; Tsukumo, T.; Nakagawa, Y. Effects of CDP-choline on head trauma. Gendai Rinsho 1967, 1, 114–120. [Google Scholar]
- Oka, M.; Oku, N.; Kujiraoka, Y.; Yoshida, S.; Higashiguchi, H. The effects of cytidine diphosphate choline (CDP-choline) on patients with head injury. Shinryo Shinyaku 1968, 3, 1101–1104. [Google Scholar]
- Miyazaki, M. Evaluation of the effectiveness of citicoline (CDP-choline) on motor paralysis as a sequela of cerebral apoplexy. Jpn. J. Geriatr. 1969, 6, 245–252. [Google Scholar] [CrossRef]
- Miyake, H.; Hayakawa, I.; Takakura, K. Treatment of head injuries with intermediate substances of the metabolic cycle of the brain. 1. The use of CDP-choline]. No To Shinkei 1964, 16, 873–878. [Google Scholar] [PubMed]
- Lecuire, J.; Duplay, J. Sperimentazione della citicolina in un campione di 154 traumatizzati cranici [Trial of citicoline in a sample of 154 head injuries]. G. Ital. Ric. Clin Ter. 1982, 3, 61–67. [Google Scholar]
- Hinev, S.; Tzoneva, D.; Ljubenova, K.; Tzvetkov, V.; Dimitrov, G.; Stefanov, I. Neuroprotection as component of complex therapy in patients with severe head trauma and cerebrovascular diseases. Anaesthesiol. Intensive Care 2007, 34, 19–24. [Google Scholar]
- Kumoi, T.; Hosomi, H.; Amatsu, M.; Shita, E.; Inokuchi, J.; Kokan, T.; et al. Recognition of the pharmacological effect of cdp-choline (nicholin) on vertigo caused by the sequelae of head trauma, whiplash injury and by alterations in cerebral circulation. A double blind trial. Data on file (Ferrer database).
- Miyashita, E. Clinical treatment with Audes (CDP-choline). Data on file (Ferrer database).
- Levin, H.S. Treatment of postconcussional symptoms with CDP-choline. J. Neurol. Sci. 1991, 103, S39–S42. [Google Scholar] [CrossRef]
- Carrion, J.L.; Dominguez-Roldán, J.M.; Cabezas, F.M.; Dominguez-Morales, M.R.; Munoz-Sánchez, M.A. Normalization of memory-related cerebral blood flow in severe traumatic brain injury patients and improvements of memory induced by citicholine (CDP-choline): The role of a pro-cognitive drug. In Proceedings of the ICRAN’99, Taipei, Taiwan, 20–23 November 1999. [Google Scholar]
- Leon-Carrion, J.; Dominguez-Roldan, J.M.; Murillo-Cabezas, F.; del Rosario Dominguez-Morales, M.; Munoz-Sanchez, M.A. The role of citicholine in neuropsychological training after traumatic brain injury. NeuroRehabilitation 2000, 14, 33–40. [Google Scholar] [CrossRef]
- Arenth, P. CDP-Choline and Working Memory After TBI: A Neuroimaging Study. Available online: https://clinicaltrials.gov/ct2/show/NCT00727246 (accessed on 26 January 2022).
- De Blas, A.; Martinez Cubells, J.; Hernando, C. Assessment of the effectiveness of citicoline in the treatment of traumatic brain injury. Med. Clin. 1986, 87, 41–44. [Google Scholar]
- El Reweny, E.M.; Okasha, A.; Hafez, A. The neuroprotective effect of citicholine (CDP choline) in patients with traumatic brain injury. In Proceedings of the 25th ESICM Annual Congress, Lisbon, Portugal, 13–17 October 2012. [Google Scholar]
- Salehpour, F.; Aghazade, J.; Mirzaee, F.; Mahdkhah, A. Citicoline in patients with traumatic brain injuries. EC Neurol. 2015, 2, 87–93. [Google Scholar]
- Shokouhi, G.; Haghjoo, A.G.; Sattarnezhad, N.; Asghari, M.; Sattarnezhad, A.; Asghari, A.; Pezeshki, A. Effects of citicoline on level of consciousness, serum level of fetuin-A and matrix Gla-protein (MGP) in trauma patients with diffuse axonal injury (DAI) and GCS ≤ 8. Ulus. Travma Acil Cerrahi Derg. 2014, 20, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Espagno, J.; Trémoulet, M.; Gigaud, M.; Espagno, C. Étude de l’action de la CDP-choline dans les troubles de la vigilance posttraumatique. Vie Méd. 1979, 3, 95–196. [Google Scholar]
- Carcassonne, M.; Letourneau, J. Double-blind study of Rexort in childhood neurotrauma. Life Med. 1979, 12, 1007. [Google Scholar]
- Varadaraju, D.N.; Ananthakishan, A. Effect of cerebroprotein hydrolysate with citicoline versus citicoline alone in the initial management of head injury and its clinical outcome a prospective randomised comparative study. J. Evid. Based Med. Healthc. 2017, 4, 2835–2837. [Google Scholar]
- Titov, I.I.; Voloshinsky, O.V.; Martin, A.Y.; Vintonyak, I.V.; Nestor, I.I. Evaluation of neuroprotectoral therapy efficiency in TBI. Pain Anesth. Intensive Care 2018, 3, 61–68. [Google Scholar]
- Geng, H.J.; Xie, Y.M.; Zhang, M. Tabu search algorithm analysis on effect of Xingnaojing Injection in treatment of craniocerebral injury and complications in real world. Zhongguo Zhong Yao Za Zhi 2020, 45, 3324–3330. [Google Scholar] [CrossRef]
- Araki, O.; Ishii, S.; Kondo, Y. Biochemical studies of head injury and brain edema. Jpn. J. Med. Prog. 1961, 48, 519–539. [Google Scholar]
- Ogashiwa, M.; Sano, K.; Manaka, S.; Kitamura, K.; Kagawa, M.; Takeuchi, K. Effectiveness of CDP-choline on disturbance of consciousness (DOC): 1. An experimental study of concussive head injury in mice. 2. A controlled trial in patients with DOC. In Novel Biochemical, Pharmacological and Clinical Aspects of Cytidinediphosphocholine; Zappia, V., Kennedy, E.P., Nilsson, B.I., Galletti, P., Eds.; Elsevier Science Publishing: Amsterdam, The Netherlands, 1985; pp. 317–327. [Google Scholar]
- Uyama, T.; Shigemoto, K.; Yanagisawa, T. Therapeutic effect of CDP-choline on sequelae of head injury on cervical injury in particular. Comparison with analgesics by the doble blind method. In Proceedings of the Symposium on the Chemotherapies on the Sequelae of Head Injuries-—The 27th Annual Meeting of Japan Neurosurgical Society, Tokyo, Japan; 1968. [Google Scholar]
- Aniruddha, T.J.; Pillai, S.; Devi, B.I.; Sampath, S.; Chandramouli, B.A. Role of citicoline in the management of mild head injury. Indian J. Neurotrauma 2009, 6, 49–52. [Google Scholar]
- Ahmadi, J.; Hoseinzadeh-Chahkandak, F.; Roobiyat, M.Y.; Pourbagher-Shahri, A.M.; Irankhah, S.; Rajabpour-Sanati, A. Comparison of two different doses of citicoline in patients with traumatic brain injury. J. Surg. Trauma 2020, 8, 8–15. [Google Scholar]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Wurtman, R.J.; Ulus, I.H.; Cansev, M.; Watkins, C.J.; Wang, L.; Marzloff, G. Synaptic proteins and phospholipids are increased in gerbil brain by administering uridine plus docosahexaenoic acid orally. Brain Res. 2006, 1088, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, O.; Cardenas, A.; Pradillo, J.M.; Morales, J.R.; Ortego, F.; Sobrino, T.; Castillo, J.; Moro, M.A.; Lizasoain, I. A chronic treatment with CDP-choline improves functional recovery and increases neuronal plasticity after experimental stroke. Neurobiol. Dis. 2007, 26, 105–111. [Google Scholar] [CrossRef]
- Diederich, K.; Frauenknecht, K.; Minnerup, J.; Schneider, B.K.; Schmidt, A.; Altach, E.; Eggert, V.; Sommer, C.J.; Schäbitz, W.R. Citicoline Enhances Neuroregenerative Processes After Experimental Stroke in Rats. Stroke 2012, 43, 1931–1940. [Google Scholar] [CrossRef]
- Krupinski, J.; Abudawood, M.; Matou-Nasri, S.; Al-Baradie, R.; Petcu, E.; Justicia, C.; Planas, A.; Liu, D.; Rovira, N.; Grau-Slevin, M.; et al. Citicoline induces angiogenesis improving survival of vascular/human brain microvessel endothelial cells through pathways involving ERK1/2 and insulin receptor substrate-1. Vasc. Cell 2012, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Turana, Y.; Nathaniel, M.; Shen, R.; Ali, S.; Aparasu, R.R. Citicoline and COVID-19-Related Cognitive and Other Neurologic Complications. Brain Sci. 2021, 12, 59. [Google Scholar] [CrossRef]
- Grieb, P.; Świątkiewicz, M.; Kamińska, A.; Jünemann, A.; Rejdak, R.; Rejdak, K. Citicoline: A Candidate for Adjunct Treatment of Multiple Sclerosis. Pharmaceuticals 2021, 14, 326. [Google Scholar] [CrossRef]
- Gareri, P.; Veronese, N.; Cotroneo, A.M. An Overview of Combination Treatment with Citicoline in Dementia. Rev. Recent Clin. Trials 2022, 17, 4–8. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).