Synergic Effect of Robot-Assisted Rehabilitation and Antispasticity Therapy: A Narrative Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
2.1.1. Types of Studies
2.1.2. Types of Participants
2.1.3. Types of Interventions
2.1.4. Types of Outcome Measures
2.2. Search Methods for Identification of Studies
2.3. Data Collection and Analysis
2.3.1. Selection of Studies
2.3.2. Data Extraction and Management
2.3.3. Methodological Quality Assessment
2.3.4. Assessment of Reporting Biases
2.3.5. Qualitative Analysis
2.3.6. Reaching Conclusions
3. Results
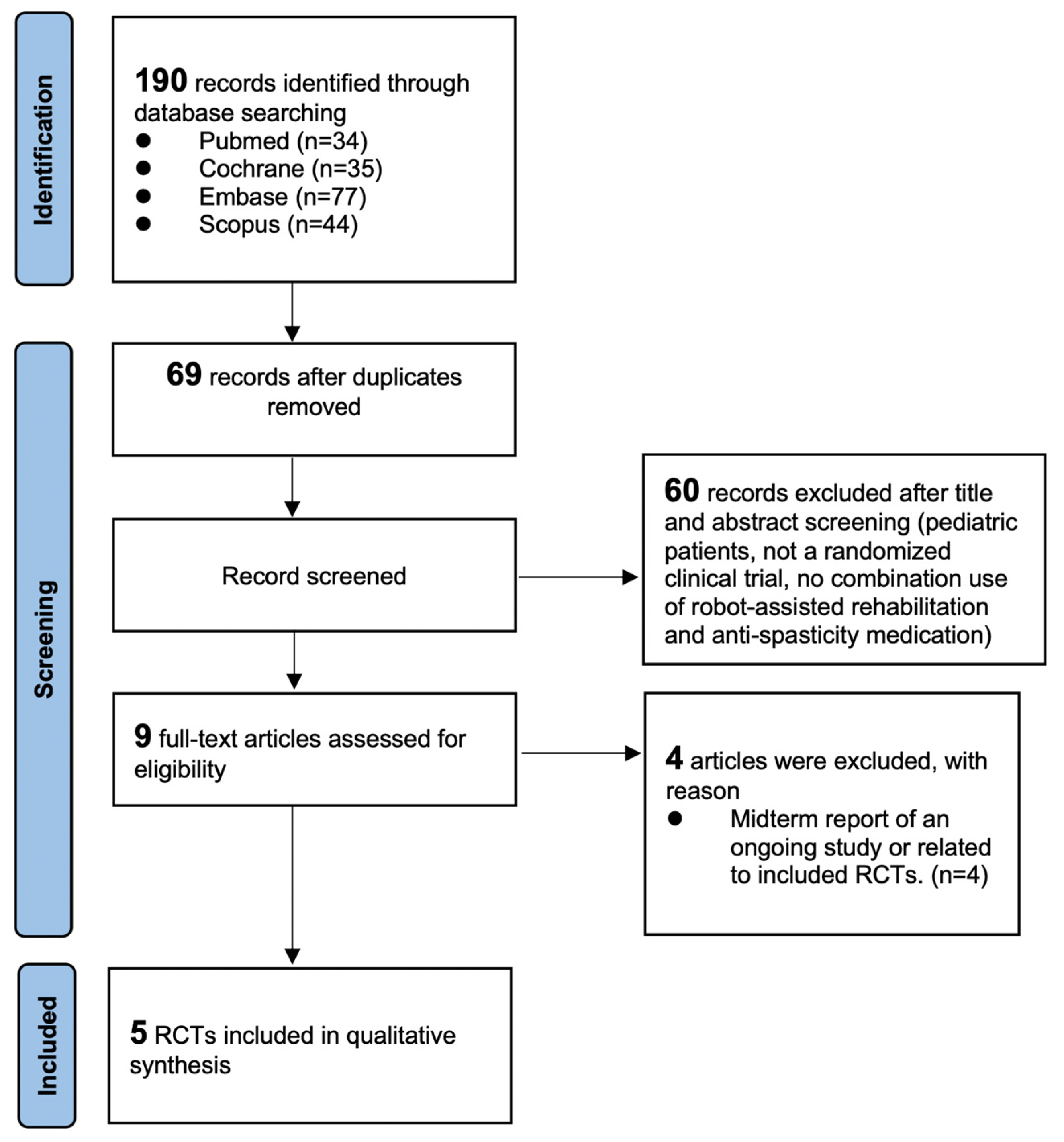
3.1. Literature Search
3.2. Risk-of-Bias Assessment
3.3. Features of Included Studies
3.4. Intervention Protocols
3.5. Primary Outcome: Functional Recovery
3.6. Secondary Outcome: Spasticity
3.7. Power Analysis and Effect Size
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
| 6MWT | 6-min walk test |
| 10MWT | 10-m walk test |
| B&B | Box and Block Test |
| BBS | Berg Balance Scale |
| BoNT | Botulinum toxin A |
| FMA | Fugl–Meyer Assessment |
| MRC | Medical Research Council Scale |
| PT | Physical therapy |
| RAT | Robot-assisted training |
| RAGT | Robot-assisted gait training |
| RCTs | Randomized controlled trials |
| RVGA | Rivermead Visual Gait Assessment |
| SCI | Spinal cord injury |
| TUG | Timed up-and-go test |
| MA | Modified Ashworth Scale |
References
- Emos, M.C.; Agarwal, S. Neuroanatomy, Upper Motor Neuron Lesion. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021. [Google Scholar]
- Harris, J.E.; Eng, J.J. Strength training improves upper-limb function in individuals with stroke: A meta-analysis. Stroke 2010, 41, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Hatem, S.M.; Saussez, G.; Della Faille, M.; Prist, V.; Zhang, X.; Dispa, D.; Bleyenheuft, Y. Rehabilitation of Motor Function after Stroke: A Multiple Systematic Review Focused on Techniques to Stimulate Upper Extremity Recovery. Front. Hum. Neurosci. 2016, 10, 442. [Google Scholar] [CrossRef] [PubMed]
- Li, S. Spasticity, Motor Recovery, and Neural Plasticity after Stroke. Front. Neurol. 2017, 8, 120. [Google Scholar] [CrossRef]
- Lance, J.W. The control of muscle tone, reflexes, and movement: Robert Wartenberg Lecture. Neurology 1980, 30, 1303–1313. [Google Scholar] [CrossRef]
- Lundström, E.; Smits, A.; Terént, A.; Borg, J. Time-course and determinants of spasticity during the first six months following first-ever stroke. J. Rehabil. Med. 2010, 42, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Moura Rde, C.; Fukujima, M.M.; Aguiar, A.S.; Fontes, S.V.; Dauar, R.F.; Prado, G.F. Predictive factors for spasticity among ischemic stroke patients. Arq. Neuropsiquiatr. 2009, 67, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Urban, P.P.; Wolf, T.; Uebele, M.; Marx, J.J.; Vogt, T.; Stoeter, P.; Bauermann, T.; Weibrich, C.; Vucurevic, G.D.; Schneider, A.; et al. Occurence and clinical predictors of spasticity after ischemic stroke. Stroke 2010, 41, 2016–2020. [Google Scholar] [CrossRef]
- Wissel, J.; Schelosky, L.D.; Scott, J.; Christe, W.; Faiss, J.H.; Mueller, J. Early development of spasticity following stroke: A prospective, observational trial. J. Neurol. 2010, 257, 1067–1072. [Google Scholar] [CrossRef]
- Schinwelski, M.; Sławek, J. Prevalence of spasticity following stroke and its impact on quality of life with emphasis on disability in activities of daily living. Systematic review. Neurol. I Neurochir. Pol. 2010, 44, 404–411. [Google Scholar] [CrossRef]
- Adams, M.M.; Hicks, A.L. Spasticity after spinal cord injury. Spinal Cord 2005, 43, 577–586. [Google Scholar] [CrossRef]
- Thibaut, A.; Chatelle, C.; Ziegler, E.; Bruno, M.A.; Laureys, S.; Gosseries, O. Spasticity after stroke: Physiology, assessment and treatment. Brain Inj. 2013, 27, 1093–1105. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wang, G.; Wang, A.; Cheng, L.J.; Lau, Y. Robot-assisted distal training improves upper limb dexterity and function after stroke: A systematic review and meta-regression. Neurol. Sci. 2022, 43, 1641–1657. [Google Scholar] [CrossRef] [PubMed]
- Mehrholz, J.; Pohl, M.; Platz, T.; Kugler, J.; Elsner, B. Electromechanical and robot-assisted arm training for improving activities of daily living, arm function, and arm muscle strength after stroke. Cochrane Database Syst. Rev. 2015, 2015, Cd006876. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Shin, J.H.; Yang, S.P.; Shin, M.A.; Lee, S.H. Robot-assisted gait training for balance and lower extremity function in patients with infratentorial stroke: A single-blinded randomized controlled trial. J. Neuroeng. Rehabil. 2019, 16, 99. [Google Scholar] [CrossRef] [PubMed]
- Dobkin, B.H. Strategies for stroke rehabilitation. Lancet Neurol. 2004, 3, 528–536. [Google Scholar] [CrossRef]
- Hara, T.; Momosaki, R.; Niimi, M.; Yamada, N.; Hara, H.; Abo, M. Botulinum Toxin Therapy Combined with Rehabilitation for Stroke: A Systematic Review of Effect on Motor Function. Toxins 2019, 11, 707. [Google Scholar] [CrossRef]
- Erbil, D.; Tugba, G.; Murat, T.H.; Melike, A.; Merve, A.; Cagla, K.; Mehmetali, Ç.C.; Akay, Ö.; Nigar, D. Effects of robot-assisted gait training in chronic stroke patients treated by botulinum toxin-a: A pivotal study. Physiother. Res. Int. 2018, 23, e1718. [Google Scholar] [CrossRef]
- Gandolfi, M.; Valè, N.; Dimitrova, E.K.; Mazzoleni, S.; Battini, E.; Filippetti, M.; Picelli, A.; Santamato, A.; Gravina, M.; Saltuari, L.; et al. Effectiveness of Robot-Assisted Upper Limb Training on Spasticity, Function and Muscle Activity in Chronic Stroke Patients Treated With Botulinum Toxin: A Randomized Single-Blinded Controlled Trial. Front. Neurol. 2019, 10, 41. [Google Scholar] [CrossRef]
- Pennati, G.V.; Da Re, C.; Messineo, I.; Bonaiuti, D. How could robotic training and botolinum toxin be combined in chronic post stroke upper limb spasticity? A pilot study. Eur. J. Phys. Rehabil. Med. 2015, 51, 381–387. [Google Scholar]
- Picelli, A.; Bacciga, M.; Melotti, C.; Elisabetta, L.A.M.; Verzini, E.; Ferrari, F.; Pontillo, A.; Corradi, J.; Tamburin, S.; Saltuari, L.; et al. Combined effects of robot-assisted gait training and botulinum toxin type A on spastic equinus foot in patients with chronic stroke: A pilot, single blind, randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2016, 52, 759–766. [Google Scholar]
- Duffell, L.D.; Brown, G.L.; Mirbagheri, M.M. Facilitatory effects of anti-spastic medication on robotic locomotor training in people with chronic incomplete spinal cord injury. J. Neuroeng. Rehabil. 2015, 12, 29. [Google Scholar] [CrossRef] [PubMed]
- Kamper, D.; Rymer, W.Z. Impairment of voluntary control of finger motion following stroke: Role of inappropriate muscle coactivation. Muscle Nerve Off. J. Am. Assoc. Electrodiagn. Med. 2001, 24, 673–681. [Google Scholar] [CrossRef]
- Hayashi, H.; Shimizu, H.; Okumura, S.; Miwa, K. Necessary metacarpophalangeal joints range of motion to maintain hand function. Hong Kong J. Occup. Ther. 2014, 24, 51–55. [Google Scholar] [CrossRef]
- Pu, S.-W.; Pei, Y.-C.; Chang, J.-Y. Decoupling finger joint motion in an exoskeletal hand: A design for robot-assisted rehabilitation. IEEE Trans. Ind. Electron. 2019, 67, 686–697. [Google Scholar] [CrossRef]
- Zhou, M.; Ben-Tzvi, P. RML glove—An exoskeleton glove mechanism with haptics feedback. IEEE/ASME Trans. Mechatron. 2014, 20, 641–652. [Google Scholar]
- Iqbal, J.; Khan, H.; Tsagarakis, N.G.; Caldwell, D.G. A novel exoskeleton robotic system for hand rehabilitation–conceptualization to prototyping. Biocybern. Biomed. Eng. 2014, 34, 79–89. [Google Scholar] [CrossRef]
- Cempini, M.; De Rossi, S.M.M.; Lenzi, T.; Cortese, M.; Giovacchini, F.; Vitiello, N.; Carrozza, M.C. Kinematics and design of a portable and wearable exoskeleton for hand rehabilitation. In Proceedings of the 2013 IEEE 13th International Conference on Rehabilitation Robotics (ICORR), Seattle, WA, USA, 24–26 June 2013; pp. 1–6. [Google Scholar]
- Zhang, F.; Wang, X.; Fu, Y.; Agrawal, S.K. A human-robot interaction modeling approach for hand rehabilitation exoskeleton using biomechanical technique. In Proceedings of the 2015 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Hamburg, Germany, 28 September–2 October 2015; pp. 5593–5598. [Google Scholar]
- Lee, K.W.; Kim, S.B.; Lee, J.H.; Lee, S.J.; Yoo, S.W. Effect of upper extremity robot-assisted exercise on spasticity in stroke patients. Ann. Rehabil. Med. 2016, 40, 961–971. [Google Scholar] [CrossRef]
- Sun, L.-C.; Chen, R.; Fu, C.; Chen, Y.; Wu, Q.; Chen, R.; Lin, X.; Luo, S. Efficacy and safety of botulinum toxin type A for limb spasticity after stroke: A meta-analysis of randomized controlled trials. BioMed Res. Int. 2019, 2019, 8329306. [Google Scholar] [CrossRef]
- Elia, A.E.; Filippini, G.; Calandrella, D.; Albanese, A. Botulinum neurotoxins for post-stroke spasticity in adults: A systematic review. Mov. Disord. Off. J. Mov. Disord. Soc. 2009, 24, 801–812. [Google Scholar] [CrossRef]
- Rosales, R.L.; Chua-Yap, A. Evidence-based systematic review on the efficacy and safety of botulinum toxin-A therapy in post-stroke spasticity. J. Neural Transm. 2008, 115, 617–623. [Google Scholar] [CrossRef]
- Teasell, R.; Salbach, N.M.; Foley, N.; Mountain, A.; Cameron, J.I.; Jong, A.d.; Acerra, N.E.; Bastasi, D.; Carter, S.L.; Fung, J. Canadian stroke best practice recommendations: Rehabilitation, recovery, and community participation following stroke. Part one: Rehabilitation and recovery following stroke; Update 2019. Int. J. Stroke 2020, 15, 763–788. [Google Scholar] [CrossRef] [PubMed]
- Mehrholz, J.; Pohl, M. Electromechanical-assisted gait training after stroke: A systematic review comparing end-effector and exoskeleton devices. J. Rehabil. Med. 2012, 44, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Zinn, S.; Bosworth, H.B.; Hoenig, H.M.; Swartzwelder, H.S. Executive function deficits in acute stroke. Arch. Phys. Med. Rehabil. 2007, 88, 173–180. [Google Scholar] [PubMed]
- Desrosiers, J.; Malouin, F.; Richards, C.; Bourbonnais, D.; Rochette, A.; Bravo, G. Comparison of changes in upper and lower extremity impairments and disabilities after stroke. Int. J. Rehabil. Res. 2003, 26, 109–116. [Google Scholar]
- Kelly-Hayes, M.; Wolf, P.A.; Kase, C.S.; Gresham, G.E.; Kannel, W.B.; D’Agostino, R.B. Time Course of Functional Recovery After Stroke: The Framingham Study. J. Neurol. Rehabil. 1989, 3, 65–70. [Google Scholar] [CrossRef]
- Bhalla, A.; Wang, Y.; Rudd, A.; Wolfe, C.D. Differences in outcome and predictors between ischemic and intracerebral hemorrhage: The South London Stroke Register. Stroke 2013, 44, 2174–2181. [Google Scholar] [CrossRef] [PubMed]
| Question | Gandolfi et al., 2019 [19] | Pennati et al., 2015 [20] | Picelli et al., 2016 [21] | Erbil et al., 2018 [18] | Duffell et al., 2015 [22] |
|---|---|---|---|---|---|
| Was the study described as randomized? | Yes | Yes | Yes | Yes | Yes |
| Was the method of randomization appropriate? | Yes | Yes | Yes | Not described | Not described |
| Was the study described as blinding? | Single blind | Single blind | Single blind | No | No |
| Was the method of blinding appropriate? | No | No | No | No | No |
| Was there a description of withdrawals and dropouts? | Yes | Yes | Yes | Yes | Yes |
| Was there a clear description of the inclusion/exclusion criteria? | Yes | Yes | Yes | Yes | Yes |
| Was the method used to assess adverse effects described? | No | No | Yes | No | No |
| Was the methods of statistical analysis described? | Yes | No | Yes | Yes | Yes |
| Score | 4.5 | 3.5 | 5.5 | 3 | 3 |
| Author, Year | Disease | Limbs of Evaluation | Total No. of Patients (F/M) | Group | No. of Patients (F/M) | Mean Age, Year (SD) | Time since Injury (SD) |
|---|---|---|---|---|---|---|---|
| Gandolfi et al., 2019 [19] | Stroke | UL | 32 (10/22) | Control | 16 (6/10) | 59.13 (14.97) | 5.1 yr (2.2 yr) |
| Intervention | 16 (4/12) | 59.31 (14.40) | 6.0 yr (3.1 yr) | ||||
| Pennati et al., 2015 [20] | Stroke | UL | 15 (6/9) | Control | 8 (NA) | NA | >6 mo, (10 mo to 20 yr) |
| Intervention | 7 (NA) | NA | >6 mo, (10 mo to 20 yr) | ||||
| Picelli et al., 2016 [21] | Stroke | LL | 22 (6/16) | Control | 11 (4/7) | 65.1 (3.4) | 6.1 yr (3.8 yr) |
| Intervention | 11 (2/9) | 62.4 (9.5) | 6.2 yr (4.2 yr) | ||||
| Erbil et al., 2018 [18] | Stroke | LL | 43 (16/27) | Control | 14 (3/11) | 48.7 (10.4) | 25.9 mo (24.6 mo) |
| Intervention | 29 (13/16) | 50.1 (11.8) | 39 mo (34.3 mo) | ||||
| Duffell et al., 2015 [22] | SCI | LL | 48 (14/34) | Control | 26 (7/19) | 46.6 (12.6) | 9.3 yr (8.9 yr) |
| Intervention | 22 (7/15) | 46.5 (11.9) | 10.2 yr (10.47 yr) |
| Author, Year | Group | Intervention Details | Functional Measurement | Spasticity Measurement | Follow Up |
|---|---|---|---|---|---|
| Gandolfi et al., 2019 [19] | Control | BoNT + PT (Individualized dosage and targe muscle of BoNT) (Mobilization and stretching (10 min) followed by UL exercises of PT, 10 sessions within 5 weeks) | FMA *, MRC § | MAS * | Post-treatment |
| Intervention | BoNT + RAT (BoNT as above) (End-effector device of RAT with Armotion [Reha Technology, Olten, Switzerland], 10 sessions within 5 weeks) | FMA *, MRC *§ | MAS * | ||
| Pennati et al., 2015 [20] | Control | RAT (End-effector device of RAT with ReoGo System [Mo-torika Medical Ltd.; Caesarea, Israel], 10 sessions within 3–5 weeks) | FMA *§, B&B *§ | MAS *§ | End of RAT |
| Intervention | BoNT + RAT (Individualized dosage and targe muscle of BoNT) (RAT as above) | FMA *§, B&B*§ | MAS *§ | ||
| Picelli et al., 2016 [21] | Control | BoNT (GM, GL and soleus muscle, 250U each muscle) | 6MWT § | MAS *, Tardieu * | 4 weeks |
| Intervention | BoNT + RAGT (BoNT as above) (End-effector static robot of RAGT with G-EO System Evolution [Reha Technology, Olten, Switzerland], 5 sessions within 1 week) | 6MWT *§ | MAS *, Tardieu * | ||
| Erbil et al., 2018 [18] | Control | BoNT + PT (Individualized dosage and targe muscle of BoNT) (15 sessions within 3 weeks: 90 min of PT with stretch-ing and strengthening exercises, proprioception, weight bearing, balance, coordination and ambulatory training) | BBS *§, TUG *§, RVGA *§ | MAS *, Tardieu * | 6, 12 weeks |
| Intervention | BoNT + PT + RAGT (BoNT and PT as above) (End-effector static robot of RAGT with RoboGait [Bama Teknoloji, Ankara, Turkey], 15 sessions within 3 weeks: 30 min of RAGT + 60 min of PT) | BBS *§, TUG *§, RVGA *§ | MAS *, Tardieu * | ||
| Duffell et al., 2015 [22] | Control | RAGT (Treadmill-base exoskeletal static robot of RAGT with Lokomat [Hocoma AG, Switzerland], 12 sessions within 4 weeks) | 6MWT, 10MWT *, TUG | NA | 0, 1, 2, 4 weeks (from the start of RAGT) |
| Intervention | Tizanidine + RAGT (0.03 mg/kg QID, initiated 4 weeks prior to RAGT) (RAGT at above) | 6MWT, 10MWT *※, TUG | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.-C.; Yeh, C.-Y.; Huang, J.-J.; Chang, S.-C.; Pei, Y.-C. Synergic Effect of Robot-Assisted Rehabilitation and Antispasticity Therapy: A Narrative Review. Life 2023, 13, 252. https://doi.org/10.3390/life13020252
Wang W-C, Yeh C-Y, Huang J-J, Chang S-C, Pei Y-C. Synergic Effect of Robot-Assisted Rehabilitation and Antispasticity Therapy: A Narrative Review. Life. 2023; 13(2):252. https://doi.org/10.3390/life13020252
Chicago/Turabian StyleWang, Wei-Cheng, Chia-Yi Yeh, Jian-Jia Huang, Shih-Chieh Chang, and Yu-Cheng Pei. 2023. "Synergic Effect of Robot-Assisted Rehabilitation and Antispasticity Therapy: A Narrative Review" Life 13, no. 2: 252. https://doi.org/10.3390/life13020252
APA StyleWang, W.-C., Yeh, C.-Y., Huang, J.-J., Chang, S.-C., & Pei, Y.-C. (2023). Synergic Effect of Robot-Assisted Rehabilitation and Antispasticity Therapy: A Narrative Review. Life, 13(2), 252. https://doi.org/10.3390/life13020252









