Transspinal Direct Current Electrical Stimulation Selectively Affects the Excitability of the Corticospinal System, Depending on the Intensity but Not Motor Skills
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Design
- Conducting transcranial magnetic stimulation (TMS) to record 20 MEPs before stimulation (Tbefore);
- Applying tsDCS (two distinct groups—anodal stimulation and sham) for 11 min;
- Recording 20 MEPs immediately after the tsDCS session (T0) using TMS;
- Conducting TMS to record 20 MEPs at a 15 min interval after the tsDCS session (T15).
2.2.1. Transcranial Magnetic Stimulation
2.2.2. Transspinal Direct Current Stimulation
2.2.3. Electromyogram (EMG)
2.2.4. Motor Skill Tests
2.2.5. Serial Reaction Time Task
2.2.6. Nine-Hole Peg Test
2.3. Statistical Analysis
3. Results
3.1. Effects of 1.5 mA
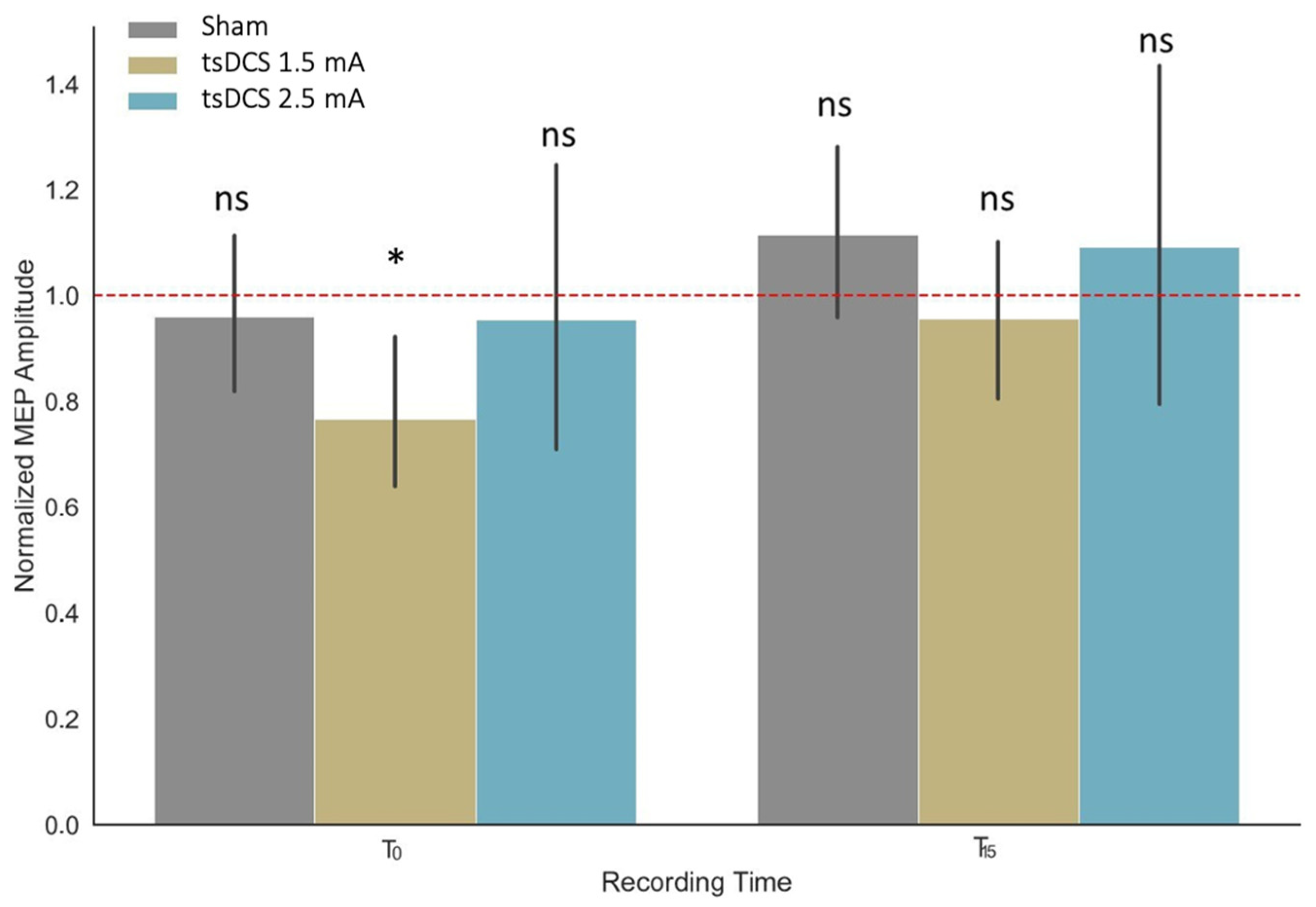
3.2. Effects of tsDCS on MEP Amplitudes
3.3. Effect of tsDCS on the Development of New Motor Skills in Healthy Subjects
4. Discussion
Limitations of the Study
5. Conclusions
- (1)
- The use of 11 min anode tsDCS at the level of the cervical spine C7-Th1 with a current value of 2.5 mA does not cause a change in the amplitude of the MEP of the muscle of the upper limbs, in contrast to the current value of 1.5 mA, which affects the muscle of the upper limbs, first decreasing the amplitude of the MEP induced by TMS immediately after stimulation. Fifteen minutes after stimulation, the MEP amplitude increases.
- (2)
- The application of tsDCS at the level of the upper spinal cord segments (C7-Th1) for 11 min at 2.5 mA and with a current strength of 1.5 mA does not affect the development of new motor skills in healthy people in the 9-HPT and SRT.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martin, J.H. The Corticospinal System: From Development to Motor Control. Neuroscientist 2005, 11, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Cogiamanian, F.; Vergari, M.; Pulecchi, F.; Marceglia, S.; Priori, A. Effect of Spinal Transcutaneous Direct Current Stimulation on Somatosensory Evoked Potentials in Humans. Clin. Neurophysiol. 2008, 119, 2636–2640. [Google Scholar] [CrossRef] [PubMed]
- Cogiamanian, F.; Ardolino, G.; Vergari, M.; Ferrucci, R.; Ciocca, M.; Scelzo, E.; Barbieri, S.; Priori, A. Transcutaneous Spinal Direct Current Stimulation. Front. Psychiatry 2012, 3, 63. [Google Scholar] [CrossRef] [PubMed]
- Jack, A.S.; Hurd, C.; Martin, J.; Fouad, K. Electrical Stimulation as a Tool to Promote Plasticity of the Injured Spinal Cord. J. Neurotrauma 2020, 37, 1933–1953. [Google Scholar] [CrossRef] [PubMed]
- Batsikadze, G.; Moliadze, V.; Paulus, W.; Kuo, M.F.; Nitsche, M.A. Partially Non-Linear Stimulation Intensity-Dependent Effects of Direct Current Stimulation on Motor Cortex Excitability in Humans. J. Physiol. 2013, 591, 1987–2000. [Google Scholar] [CrossRef] [PubMed]
- Jamil, A.; Batsikadze, G.; Kuo, H.I.; Labruna, L.; Hasan, A.; Paulus, W.; Nitsche, M.A. Systematic Evaluation of the Impact of Stimulation Intensity on Neuroplastic After-Effects Induced by Transcranial Direct Current Stimulation. J. Physiol. 2017, 595, 1273–1288. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Paulus, W. Sustained Excitability Elevations Induced by Transcranial DC Motor Cortex Stimulation in Humans. Neurology 2001, 57, 1899–1901. [Google Scholar] [CrossRef]
- Monte-Silva, K.; Kuo, M.F.; Hessenthaler, S.; Fresnoza, S.; Liebetanz, D.; Paulus, W.; Nitsche, M.A. Induction of Late LTP-like Plasticity in the Human Motor Cortex by Repeated Non-Invasive Brain Stimulation. Brain Stimul. 2013, 6, 424–432. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Excitability Changes Induced in the Human Motor Cortex by Weak Transcranial Direct Current Stimulation. J. Physiol. 2000, 527 Pt 3, 633–639. [Google Scholar] [CrossRef]
- Tremblay, S.; Larochelle-Brunet, F.; Lafleur, L.P.; El Mouderrib, S.; Lepage, J.F.; Théoret, H. Systematic Assessment of Duration and Intensity of Anodal Transcranial Direct Current Stimulation on Primary Motor Cortex Excitability. Eur. J. Neurosci. 2016, 44, 2184–2190. [Google Scholar] [CrossRef]
- Horvath, J.C.; Forte, J.D.; Carter, O. Evidence That Transcranial Direct Current Stimulation (TDCS) Generates Little-to-No Reliable Neurophysiologic Effect beyond MEP Amplitude Modulation in Healthy Human Subjects: A Systematic Review. Neuropsychologia 2015, 66, 213–236. [Google Scholar] [CrossRef] [PubMed]
- Hendy, A.M.; Kidgell, D.J. Anodal-TDCS Applied during Unilateral Strength Training Increases Strength and Corticospinal Excitability in the Untrained Homologous Muscle. Exp. Brain Res. 2014, 232, 3243–3252. [Google Scholar] [CrossRef] [PubMed]
- Esmaeilpour, Z.; Marangolo, P.; Hampstead, B.M.; Bestmann, S.; Galletta, E.; Knotkova, H.; Bikson, M. Incomplete Evidence That Increasing Current Intensity of TDCS Boosts Outcomes. Brain Stimul. 2018, 11, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.Y.; Shin, H.I. Noninvasive DC Stimulation on Neck Changes MEP. Neuroreport 2011, 22, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Dongés, S.C.; D’Amico, J.M.; Butler, J.E.; Taylor, J.L. The Effects of Cervical Transcutaneous Spinal Direct Current Stimulation on Motor Pathways Supplying the Upper Limb in Humans. PLoS ONE 2017, 12, e0172333. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.R.; Pereira, M.; Salvador, R.; Miranda, P.C.; De Carvalho, M. Cervical Trans-Spinal Direct Current Stimulation: A Modelling-Experimental Approach. J. Neuroeng. Rehabil. 2019, 16, 123. [Google Scholar] [CrossRef]
- Pomelova, E.; Popyvanova, A.; Iiyukina, N.; Bredikhin, D.; Koriakina, M.; Shestakova, A.N.; Blagovechtchenski, E. Effects of Transspinal Electrical Stimulation Estimated by Transcranial Magnetic Stimulation. In Proceedings of the 4th International Conference “Neurotechnologies Neurointerfaces”, CNN, Kaliningrad, Russia, 14–16 September 2022; pp. 121–124. [Google Scholar] [CrossRef]
- Lerner, O.; Friedman, J.; Frenkel-Toledo, S. The Effect of High-Definition Transcranial Direct Current Stimulation Intensity on Motor Performance in Healthy Adults: A Randomized Controlled Trial. J. Neuroeng. Rehabil. 2021, 18, 103. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. LmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Luke, S.G. Evaluating Significance in Linear Mixed-Effects Models in R. Behav. Res. Methods 2017, 49, 1494–1502. [Google Scholar] [CrossRef]
- Searle, S.R.; Speed, F.M.; Milliken, G.A. Population Marginal Means in the Linear Model: An Alternative to Least Squares Means. Am. Stat. 1980, 34, 216–221. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Roche, N.; Lackmy, A.; Achache, V.; Bussel, B.; Katz, R. Effects of anodal transcranial direct current stimulation over the leg motor area on lumbar spinal network excitability in healthy subjects. J. Physiol. 2011, 589 Pt 11, 2813. [Google Scholar] [CrossRef] [PubMed]
- Conley, A.C.; Marquez, J.; Parsons, M.W.; Fulham, W.R.; Heathcote, A.; Karayanidis, F. Anodal tDCS over the Motor Cortex on Prepared and Unprepared Responses in Young Adults. PLoS ONE 2015, 10, e0124509. [Google Scholar] [CrossRef] [PubMed]
- Nuzum, N.D.; Hendy, A.M.; Russell, A.P.; Teo, W.P. Measures to Predict The Individual Variability of Corticospinal Responses Following Transcranial Direct Current Stimulation. Front. Hum. Neurosci. 2016, 10, 487. [Google Scholar] [CrossRef] [PubMed]
- Schabrun, S.M.; Chipchase, L.S.; Zipf, N.; Thickbroom, G.W.; Hodges, P.W. Interaction between simultaneously applied neuromodulatory interventions in humans. Brain Stimul. 2013, 6, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Bortoletto, M.; Pellicciari, M.C.; Rodella, C.; Miniussi, C. The interaction with task-induced activity is more important than polarization: A tDCS study. Brain Stimul. 2015, 8, 269–276. [Google Scholar] [CrossRef]
- López-Alonso, V.; Cheeran, B.; Río-Rodríguez, D.; Fernández-Del-Olmo, M. Inter-individual Variability in Response to Non-invasive Brain Stimulation Paradigms. Brain Stimul. 2014, 7, 372–380. [Google Scholar] [CrossRef]
- Vosskuhl, J.; Strüber, D.; Herrmann, C.S. Non-invasive Brain Stimulation: A Paradigm Shift in Understanding Brain Oscillations. Front. Hum. Neurosci. 2018, 12, 211. [Google Scholar] [CrossRef]
- Agboada, D.; Mosayebi Samani, M.; Jamil, A.; Kuo, M.F.; Nitsche, M.A. Expanding the Parameter Space of Anodal Transcranial Direct Current Stimulation of the Primary Motor Cortex. Sci. Rep. 2019, 9, 18185. [Google Scholar] [CrossRef]
- Hassanzahraee, M.; Nitsche, M.A.; Zoghi, M.; Jaberzadeh, S. Determination of Anodal TDCS Intensity Threshold for Reversal of Corticospinal Excitability: An Investigation for Induction of Counter-Regulatory Mechanisms. Sci. Rep. 2020, 10, 16108. [Google Scholar] [CrossRef]
- Pereira, M.; Fernandes, S.R.; Miranda, P.C.; de Carvalho, M. Neuromodulation of Lower Limb Motor Responses with Transcutaneous Lumbar Spinal Cord Direct Current Stimulation. Clin. Neurophysiol. 2018, 129, 1999–2009. [Google Scholar] [CrossRef] [PubMed]
- Ranck, J.B. Which Elements Are Excited in Electrical Stimulation of Mammalian Central Nervous System: A Review. Brain Res. 1975, 98, 417–440. [Google Scholar] [CrossRef] [PubMed]
- Kuck, A.; Stegeman, D.F.; Van Asseldonk, E.H.F. Modeling Trans-Spinal Direct Current Stimulation for the Modulation of the Lumbar Spinal Motor Pathways. J. Neural Eng. 2017, 14, 056014. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.R.; Salvador, R.; Wenger, C.; De Carvalho, M.; Miranda, P.C. Transcutaneous Spinal Direct Current Stimulation of the Lumbar and Sacral Spinal Cord: A Modelling Study. J. Neural Eng. 2018, 15, 036008. [Google Scholar] [CrossRef] [PubMed]
- Berry, H.R.; Tate, R.J.; Conway, B.A. Transcutaneous Spinal Direct Current Stimulation Induces Lasting Fatigue Resistance and Enhances Explosive Vertical Jump Performance. PLoS ONE 2017, 12, e0173846. [Google Scholar] [CrossRef] [PubMed]
- Awosika, O.O.; Sandrini, M.; Volochayev, R.; Thompson, R.M.; Fishman, N.; Wu, T.; Floeter, M.K.; Hallett, M.; Cohen, L.G. Transcutaneous Spinal Direct Current Stimulation Improves Locomotor Learning in Healthy Humans. Brain Stimul. 2019, 12, 628. [Google Scholar] [CrossRef] [PubMed]
- El Basiouny, S.M.; Mushahwar, V.K. Suppressing the Excitability of Spinal Motoneurons by Extracellularly Applied Electrical Fields: Insights from Computer Simulations. J. Appl. Physiol. 2007, 103, 1824–1836. [Google Scholar] [CrossRef]
- Hernández-Labrado, G.R.; Polo, J.L.; López-Dolado, E.; Collazos-Castro, J.E. Spinal Cord Direct Current Stimulation: Finite Element Analysis of the Electric Field and Current Density. Med. Biol. Eng. Comput. 2011, 49, 417–429. [Google Scholar] [CrossRef]
- Jankowska, E. Spinal Control of Motor Outputs by Intrinsic and Externally Induced Electric Field Potentials. J. Neurophysiol. 2017, 118, 1221–1234. [Google Scholar] [CrossRef]
- Niérat, M.C.; Similowski, T.; Lamy, J.C. Does Trans-Spinal Direct Current Stimulation Alter Phrenic Motoneurons and Respiratory Neuromechanical Outputs in Humans? A Double-Blind, Sham-Controlled, Randomized, Crossover Study. J. Neurosci. 2014, 34, 14420–14429. [Google Scholar] [CrossRef]
- Bocci, T.; Marceglia, S.; Vergari, M.; Cognetto, V.; Cogiamanian, F.; Sartucci, F.; Priori, A. Transcutaneous Spinal Direct Current Stimulation Modulates Human Corticospinal System Excitability. J. Neurophysiol. 2015, 114, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Cogiamanian, F.; Vergari, M.; Schiaffi, E.; Marceglia, S.; Ardolino, G.; Barbieri, S.; Priori, A. Transcutaneous Spinal Cord Direct Current Stimulation Inhibits the Lower Limb Nociceptive Flexion Reflex in Human Beings. Pain 2011, 152, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Kuck, A.; Stegeman, D.F.; Van Asseldonk, E.H.F. Modeling Trans-Spinal Direct Current Stimulation in the Presence of Spinal Implants. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 790–797. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popyvanova, A.; Pomelova, E.; Bredikhin, D.; Koriakina, M.; Shestakova, A.; Blagovechtchenski, E. Transspinal Direct Current Electrical Stimulation Selectively Affects the Excitability of the Corticospinal System, Depending on the Intensity but Not Motor Skills. Life 2023, 13, 2353. https://doi.org/10.3390/life13122353
Popyvanova A, Pomelova E, Bredikhin D, Koriakina M, Shestakova A, Blagovechtchenski E. Transspinal Direct Current Electrical Stimulation Selectively Affects the Excitability of the Corticospinal System, Depending on the Intensity but Not Motor Skills. Life. 2023; 13(12):2353. https://doi.org/10.3390/life13122353
Chicago/Turabian StylePopyvanova, Alena, Ekaterina Pomelova, Dmitry Bredikhin, Maria Koriakina, Anna Shestakova, and Evgeny Blagovechtchenski. 2023. "Transspinal Direct Current Electrical Stimulation Selectively Affects the Excitability of the Corticospinal System, Depending on the Intensity but Not Motor Skills" Life 13, no. 12: 2353. https://doi.org/10.3390/life13122353
APA StylePopyvanova, A., Pomelova, E., Bredikhin, D., Koriakina, M., Shestakova, A., & Blagovechtchenski, E. (2023). Transspinal Direct Current Electrical Stimulation Selectively Affects the Excitability of the Corticospinal System, Depending on the Intensity but Not Motor Skills. Life, 13(12), 2353. https://doi.org/10.3390/life13122353





