The Cavernous Nerve Injury Rat Model: A Pictorial Essay on Post-Radical Prostatectomy Erectile Dysfunction Research
Abstract
1. Introduction
2. Comparative Pelvic Anatomy and Experimental Design Considerations

3. Ethical, Methodological, and Technical Prerequisites
4. Surgical Strategy Protocols
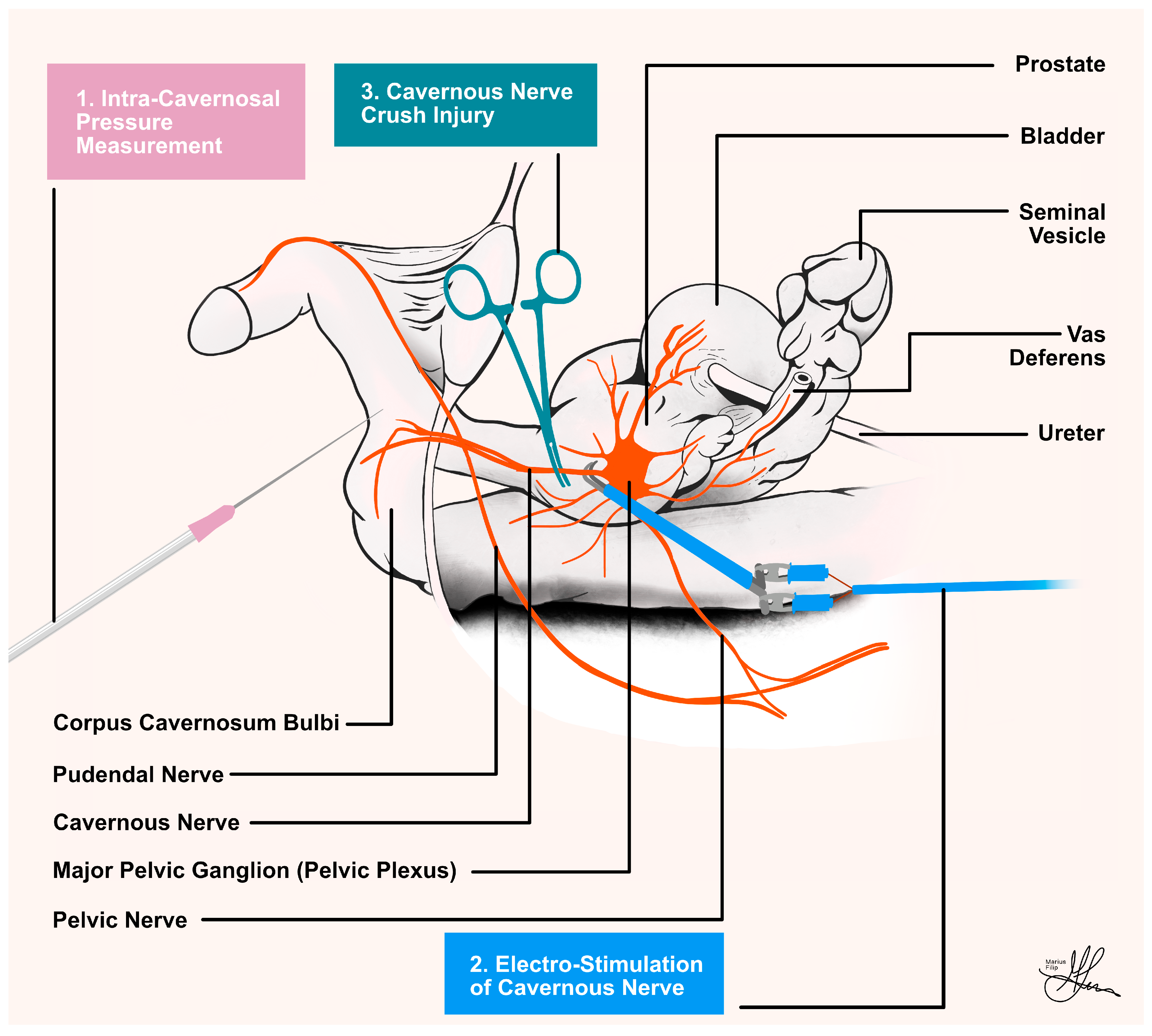
4.1. Mean Arterial Pressure Measurements
4.2. Intra-Cavernosal Pressure Measurements
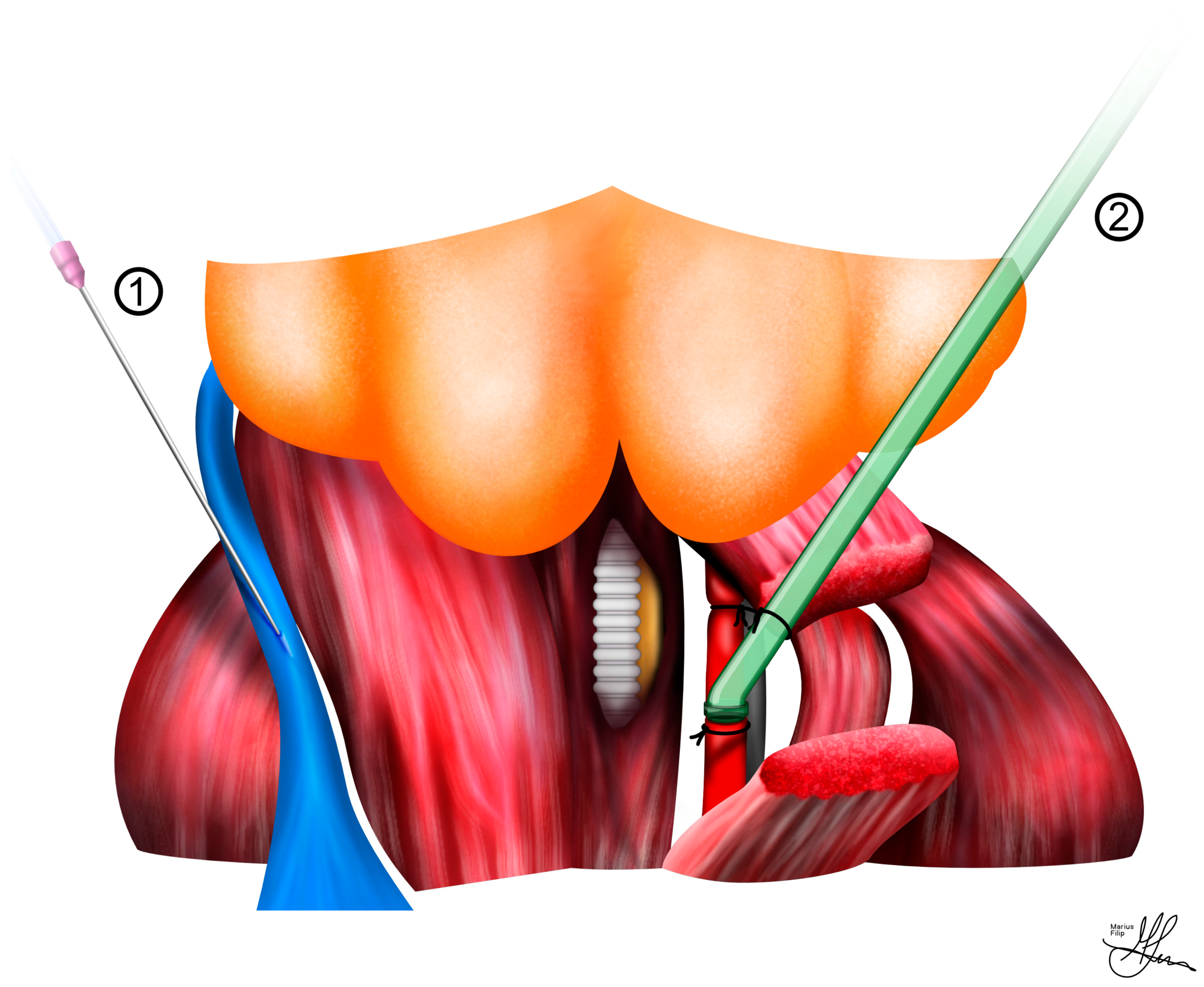
4.3. Cavernous Nerve Preparation
4.4. Standardized Cavernous Nerve Injury
4.5. Standardized Electrostimulation Parameters
5. Promising Results and Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Yafi, F.A.; Jenkins, L.; Albersen, M.; Corona, G.; Isidori, A.M.; Goldfarb, S.; Maggi, M.; Nelson, C.J.; Parish, S.; Salonia, A.; et al. Erectile Dysfunction. Nat. Rev. Dis. Primers 2016, 2, 16003. [Google Scholar] [CrossRef] [PubMed]
- Ayta, I.A.; McKinlay, J.B.; Krane, R.J. The Likely Worldwide Increase in Erectile Dysfunction between 1995 and 2025 and Some Possible Policy Consequences. BJU Int. 1999, 84, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Gratzke, C.; Angulo, J.; Chitaley, K.; Dai, Y.-T.; Kim, N.N.; Paick, J.-S.; Simonsen, U.; Uckert, S.; Wespes, E.; Andersson, K.E.; et al. Anatomy, Physiology, and Pathophysiology of Erectile Dysfunction. J. Sex. Med. 2010, 7 Pt 2, 445–475. [Google Scholar] [CrossRef]
- Bratu, O.; Oprea, I.; Marcu, D.; Spinu, D.; Niculae, A.; Geavlete, B.; Mischianu, D. Erectile Dysfunction Post-Radical Prostatectomy—A Challenge for Both Patient and Physician. J. Med. Life 2017, 10, 13–18. [Google Scholar] [PubMed]
- Secasan, C.; Onchis, D.; Bardan, R.; Cumpanas, A.; Novacescu, D.; Botoca, C.; Dema, A.; Sporea, I. Artificial Intelligence System for Predicting Prostate Cancer Lesions from Shear Wave Elastography Measurements. Curr. Oncol. 2022, 29, 4212–4223. [Google Scholar] [CrossRef]
- Haglind, E.; Carlsson, S.; Stranne, J.; Wallerstedt, A.; Wilderäng, U.; Thorsteinsdottir, T.; Lagerkvist, M.; Damber, J.-E.; Bjartell, A.; Hugosson, J.; et al. Urinary Incontinence and Erectile Dysfunction After Robotic Versus Open Radical Prostatectomy: A Prospective, Controlled, Nonrandomised Trial. Eur. Urol. 2015, 68, 216–225. [Google Scholar] [CrossRef]
- Castiglione, F.; Ralph, D.J.; Muneer, A. Surgical Techniques for Managing Post-Prostatectomy Erectile Dysfunction. Curr. Urol. Rep. 2017, 18, 90. [Google Scholar] [CrossRef]
- Fode, M.; Ohl, D.A.; Ralph, D.; Sønksen, J. Penile Rehabilitation after Radical Prostatectomy: What the Evidence Really Says. BJU Int. 2013, 112, 998–1008. [Google Scholar] [CrossRef]
- Milenkovic, U.; Albersen, M.; Castiglione, F. The Mechanisms and Potential of Stem Cell Therapy for Penile Fibrosis. Nat. Rev. Urol. 2019, 16, 79–97. [Google Scholar] [CrossRef]
- Briganti, A.; Di Trapani, E.; Abdollah, F.; Gallina, A.; Suardi, N.; Capitanio, U.; Tutolo, M.; Passoni, N.; Salonia, A.; DiGirolamo, V.; et al. Choosing the Best Candidates for Penile Rehabilitation after Bilateral Nerve-Sparing Radical Prostatectomy. J. Sex. Med. 2012, 9, 608–617. [Google Scholar] [CrossRef]
- Karakus, S.; Musicki, B.; Burnett, A.L. Phosphodiesterase Type 5 in Men with Vasculogenic and Post-Radical Prostatectomy Erectile Dysfunction: Is There a Molecular Difference? BJU Int. 2018, 122, 1066–1074. [Google Scholar] [CrossRef]
- Shamloul, R.; Ghanem, H. Erectile Dysfunction. Lancet 2013, 381, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Haahr, M.K.; Jensen, C.H.; Toyserkani, N.M.; Andersen, D.C.; Damkier, P.; Sørensen, J.A.; Lund, L.; Sheikh, S.P. Safety and Potential Effect of a Single Intracavernous Injection of Autologous Adipose-Derived Regenerative Cells in Patients with Erectile Dysfunction Following Radical Prostatectomy: An Open-Label Phase I Clinical Trial. EBioMedicine 2016, 5, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, D.M.; Nelson, R.J.; Partin, A.W.; Mostwin, J.L.; Walsh, P.C. The Rat as a Model for the Study of Penile Erection. J. Urol. 1989, 141, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Yoham, A.L.; Bordoni, B. Anatomy, Abdomen and Pelvis, Inferior Hypogastric Plexus. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Schaumburg, H.H.; Zotova, E.; Cannella, B.; Raine, C.S.; Arezzo, J.; Tar, M.; Melman, A. Structural and Functional Investigations of the Murine Cavernosal Nerve: A Model System for Serial Spatio-Temporal Study of Autonomic Neuropathy. BJU Int. 2007, 99, 916–924. [Google Scholar] [CrossRef]
- Burnett, A.L.; Lowenstein, C.J.; Bredt, D.S.; Chang, T.S.; Snyder, S.H. Nitric Oxide: A Physiologic Mediator of Penile Erection. Science 1992, 257, 401–403. [Google Scholar] [CrossRef] [PubMed]
- Panchatsharam, P.K.; Durland, J.; Zito, P.M. Physiology, Erection. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Montorsi, F.; Briganti, A.; Salonia, A.; Rigatti, P.; Burnett, A.L. Current and Future Strategies for Preventing and Managing Erectile Dysfunction Following Radical Prostatectomy. Eur. Urol. 2004, 45, 123–133. [Google Scholar] [CrossRef]
- Burnett, A.L. Rationale for Cavernous Nerve Restorative Therapy to Preserve Erectile Function after Radical Prostatectomy. Urology 2003, 61, 491–497. [Google Scholar] [CrossRef]
- Walsh, P.C. The Discovery of the Cavernous Nerves and Development of Nerve Sparing Radical Retropubic Prostatectomy. J. Urol. 2007, 177, 1632–1635. [Google Scholar] [CrossRef]
- Corbin, J.D. Mechanisms of Action of PDE5 Inhibition in Erectile Dysfunction. Int. J. Impot. Res. 2004, 16 (Suppl. S1), S4–S7. [Google Scholar] [CrossRef]
- Novacescu, D.; Nesiu, A.; Bardan, R.; Latcu, S.; Dema, V.; Croitor, A.; Raica, M.; Cut, T.G.; Walter, J.; Cumpanas, A. Rats, Neuregulins and Radical Prostatectomy: A Conceptual Overview. J. Clin. Med. 2023, 12, 2208. [Google Scholar] [CrossRef]
- Andersson, K.-E. Mechanisms of Penile Erection and Basis for Pharmacological Treatment of Erectile Dysfunction. Pharmacol. Rev. 2011, 63, 811–859. [Google Scholar] [CrossRef]
- Okamura, T.; Ayajiki, K.; Toda, N. Monkey Corpus Cavernosum Relaxation Mediated by NO and Other Relaxing Factor Derived from Nerves. Am. J. Physiol. 1998, 274, H1075–H1081. [Google Scholar] [CrossRef]
- Hedlund, P.; Larsson, B.; Alm, P.; Andersson, K.E. Distribution and Function of Nitric Oxide-Containing Nerves in Canine Corpus Cavernosum and Spongiosum. Acta Physiol. Scand. 1995, 155, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Mills, T.M.; Lewis, R.W.; Stopper, V.S. Androgenic Maintenance of Inflow and Veno-Occlusion during Erection in the Rat. Biol. Reprod. 1998, 59, 1413–1418. [Google Scholar] [CrossRef] [PubMed]
- Haney, N.M.; Nguyen, H.M.T.; Honda, M.; Abdel-Mageed, A.B.; Hellstrom, W.J.G. Bilateral Cavernous Nerve Crush Injury in the Rat Model: A Comparative Review of Pharmacologic Interventions. Sex. Med. Rev. 2018, 6, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, F.; Pfaus, J.; Balasubramanian, S.; Hedlund, P.; Hisasue, S.; Marson, L.; Wallen, K. Experimental Models for the Study of Female and Male Sexual Function. J. Sex. Med. 2010, 7, 2970–2995. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Rehman, J.; Santizo, C.; Melman, A.; Christ, G.J. Significant Physiological Roles of Ancillary Penile Nerves on Increase in Intracavernous Pressure in Rats: Experiments Using Electrical Stimulation of the Medial Preoptic Area. Int. J. Impot. Res. 2001, 13, 82–88. [Google Scholar] [CrossRef][Green Version]
- Kim, H.J.; Kim, H.Y.; Kim, S.Y.; Lee, S.H.; Lee, W.K.; Yang, D.Y. Spontaneous Recovery of Cavernous Nerve Crush Injury. Korean J. Urol. 2011, 52, 560–565. [Google Scholar] [CrossRef]
- Taylor, K.; Alvarez, L.R. An Estimate of the Number of Animals Used for Scientific Purposes Worldwide in 2015. Altern. Lab. Anim. 2019, 47, 196–213. [Google Scholar] [CrossRef]
- Del Pace, L.; Viviani, L.; Straccia, M. Researchers and Their Experimental Models: A Pilot Survey in the Context of the European Union Health and Life Science Research. Animals 2022, 12, 2778. [Google Scholar] [CrossRef] [PubMed]
- ARRIVE Guidelines|NC3Rs. Available online: https://www.nc3rs.org.uk/arrive-guidelines (accessed on 31 October 2023).
- Jin, H.-R.; Chung, Y.G.; Kim, W.J.; Zhang, L.W.; Piao, S.; Tuvshintur, B.; Yin, G.N.; Shin, S.H.; Tumurbaatar, M.; Han, J.-Y.; et al. A Mouse Model of Cavernous Nerve Injury-Induced Erectile Dysfunction: Functional and Morphological Characterization of the Corpus Cavernosum. J. Sex. Med. 2010, 7, 3351–3364. [Google Scholar] [CrossRef]
- Gibb, R.L. Environment. In The Behavior of the Laboratory Rat: A Handbook with Tests; Whishaw, I.Q., Kolb, B., Eds.; Oxford University Press: Oxford, UK, 2004. [Google Scholar] [CrossRef]
- Flecknell, P.A. Chapter 2—Anaesthesia. In Laboratory Animal Anaesthesia, 3rd ed.; Flecknell, P.A., Ed.; Academic Press: San Diego, CA, USA, 2009; pp. 19–78. [Google Scholar] [CrossRef]
- Quinlan, A.; Hansen, S.; Zvara, P.; Lund, L. Validation of a Modified Rat Model for Erectile Function Evaluation. In Larner College of Medicine Fourth Year Advanced Integration Teaching/Scholarly Projects; University of Vermont: Burlington, VT, USA, 2022. [Google Scholar]
- Clarkson, J.M.; Martin, J.E.; McKeegan, D.E.F. A Review of Methods Used to Kill Laboratory Rodents: Issues and Opportunities. Lab. Anim. 2022, 56, 419–436. [Google Scholar] [CrossRef]
- Feng, J.; Fitz, Y.; Li, Y.; Fernandez, M.; Cortes Puch, I.; Wang, D.; Pazniokas, S.; Bucher, B.; Cui, X.; Solomon, S.B. Catheterization of the Carotid Artery and Jugular Vein to Perform Hemodynamic Measures, Infusions and Blood Sampling in a Conscious Rat Model. J. Vis. Exp. 2015, 95, 51881. [Google Scholar] [CrossRef]
- ter Horst, E.N.; Krijnen, P.A.J.; Flecknell, P.; Meyer, K.W.; Kramer, K.; van der Laan, A.M.; Piek, J.J.; Niessen, H.W.M. Sufentanil–Medetomidine Anaesthesia Compared with Fentanyl/Fluanisone–Midazolam Is Associated with Fewer Ventricular Arrhythmias and Death during Experimental Myocardial Infarction in Rats and Limits Infarct Size Following Reperfusion. Lab. Anim. 2018, 52, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Lapin, K.; Ryzhkov, I.; Maltseva, V.; Udut, E. Vascular Catheterization in Small Laboratory Animals in Biomedical Research: Technological Aspects of the Method (Review Article). Bull. Sib. Med. 2021, 20, 168–181. [Google Scholar] [CrossRef]
- Uhlig, C.; Krause, H.; Koch, T.; Gama de Abreu, M.; Spieth, P.M. Anesthesia and Monitoring in Small Laboratory Mammals Used in Anesthesiology, Respiratory and Critical Care Research: A Systematic Review on the Current Reporting in Top-10 Impact Factor Ranked Journals. PLoS ONE 2015, 10, e0134205. [Google Scholar] [CrossRef]
- Tong, C.; Peng, X.; Hu, H.; Wang, Z.; Zhou, H. The Effect of Different Flushing Methods in a Short Peripheral Catheter 1. Acta Cir. Bras. 2019, 34, e201900804. [Google Scholar] [CrossRef]
- Mullerad, M.; Donohue, J.F.; Li, P.S.; Scardino, P.T.; Mulhall, J.P. Functional Sequelae of Cavernous Nerve Injury in the Rat: Is There Model Dependency. J. Sex. Med. 2006, 3, 77–83. [Google Scholar] [CrossRef]
- Chung, E.; De Young, L.; Brock, G.B. Investigative Models in Erectile Dysfunction: A State-of-the-Art Review of Current Animal Models. J. Sex. Med. 2011, 8, 3291–3305. [Google Scholar] [CrossRef]
- Mizusawa, H.; Hedlund, P.; Håkansson, A.; Alm, P.; Andersson, K.-E. Morphological and Functional in Vitro and in Vivo Characterization of the Mouse Corpus Cavernosum. Br. J. Pharmacol. 2001, 132, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Escrig, A.; Gonzalez-Mora, J.L.; Mas, M. Nitric Oxide Release in Penile Corpora Cavernosa in a Rat Model of Erection. J. Physiol. 1999, 516 Pt 1, 261–269. [Google Scholar] [CrossRef]
- Pederzoli, F.; Campbell, J.D.; Matsui, H.; Sopko, N.A.; Bivalacqua, T.J. Surgical Factors Associated with Male and Female Sexual Dysfunction After Radical Cystectomy: What Do We Know and How Can We Improve Outcomes? Sex. Med. Rev. 2018, 6, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Celentano, V.; Cohen, R.; Warusavitarne, J.; Faiz, O.; Chand, M. Sexual Dysfunction Following Rectal Cancer Surgery. Int. J. Color. Dis. 2017, 32, 1523–1530. [Google Scholar] [CrossRef] [PubMed]
- Walz, J.; Burnett, A.L.; Costello, A.J.; Eastham, J.A.; Graefen, M.; Guillonneau, B.; Menon, M.; Montorsi, F.; Myers, R.P.; Rocco, B.; et al. A Critical Analysis of the Current Knowledge of Surgical Anatomy Related to Optimization of Cancer Control and Preservation of Continence and Erection in Candidates for Radical Prostatectomy. Eur. Urol. 2010, 57, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Hlaing, S.M.; Garcia, L.A.; Kovanecz, I.; Martinez, R.A.; Shah, S.; Artaza, J.N.; Ferrini, M.G. Sildenafil Promotes Neuroprotection of the Pelvic Ganglia Neurones after Bilateral Cavernosal Nerve Resection in the Rat. BJU Int. 2013, 111, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Mulhall, J.P.; Müller, A.; Donohue, J.F.; Mullerad, M.; Kobylarz, K.; Paduch, D.A.; Tal, R.; Li, P.S.; Cohen-Gould, L.; Scardino, P.T. The Functional and Structural Consequences of Cavernous Nerve Injury Are Ameliorated by Sildenafil Citrate. J. Sex. Med. 2008, 5, 1126–1136. [Google Scholar] [CrossRef]
- Pavlovich, C.P.; Levinson, A.W.; Su, L.-M.; Mettee, L.Z.; Feng, Z.; Bivalacqua, T.J.; Trock, B.J. Nightly vs On-Demand Sildenafil for Penile Rehabilitation after Minimally Invasive Nerve-Sparing Radical Prostatectomy: Results of a Randomized Double-Blind Trial with Placebo. BJU Int. 2013, 112, 844–851. [Google Scholar] [CrossRef]
- Montorsi, F.; Brock, G.; Lee, J.; Shapiro, J.; Van Poppel, H.; Graefen, M.; Stief, C. Effect of Nightly versus On-Demand Vardenafil on Recovery of Erectile Function in Men Following Bilateral Nerve-Sparing Radical Prostatectomy. Eur. Urol. 2008, 54, 924–931. [Google Scholar] [CrossRef]
- Montorsi, F.; Brock, G.; Stolzenburg, J.-U.; Mulhall, J.; Moncada, I.; Patel, H.R.H.; Chevallier, D.; Krajka, K.; Henneges, C.; Dickson, R.; et al. Effects of Tadalafil Treatment on Erectile Function Recovery Following Bilateral Nerve-Sparing Radical Prostatectomy: A Randomised Placebo-Controlled Study (REACTT). Eur. Urol. 2014, 65, 587–596. [Google Scholar] [CrossRef]
- Fang, J.-F.; Jia, C.-C.; Zheng, Z.-H.; Ye, X.-L.; Wei, B.; Huang, L.-J.; Wei, H.-B. Periprostatic Implantation of Neural Differentiated Mesenchymal Stem Cells Restores Cavernous Nerve Injury-Mediated Erectile Dysfunction. Am. J. Transl. Res. 2016, 8, 2549–2561. [Google Scholar] [PubMed]
- Albersen, M.; Fandel, T.M.; Lin, G.; Wang, G.; Banie, L.; Lin, C.-S.; Lue, T.F. Injections of Adipose Tissue-Derived Stem Cells and Stem Cell Lysate Improve Recovery of Erectile Function in a Rat Model of Cavernous Nerve Injury. J. Sex. Med. 2010, 7, 3331–3340. [Google Scholar] [CrossRef]
- Baraniak, P.R.; McDevitt, T.C. Stem Cell Paracrine Actions and Tissue Regeneration. Regen. Med. 2010, 5, 121–143. [Google Scholar] [CrossRef] [PubMed]
- Tkach, M.; Théry, C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Li, Y.; Cui, Y.; Yang, J.J.; Zhang, Z.G.; Chopp, M. Systemic Administration of Exosomes Released from Mesenchymal Stromal Cells Promote Functional Recovery and Neurovascular Plasticity after Stroke in Rats. J. Cereb. Blood Flow. Metab. 2013, 33, 1711–1715. [Google Scholar] [CrossRef]
- Hu, G.; Li, Q.; Niu, X.; Hu, B.; Liu, J.; Zhou, S.; Guo, S.; Lang, H.; Zhang, C.; Wang, Y.; et al. Exosomes Secreted by Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Attenuate Limb Ischemia by Promoting Angiogenesis in Mice. Stem Cell Res. Ther. 2015, 6, 10. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, M.; Gong, A.; Zhang, X.; Wu, X.; Zhu, Y.; Shi, H.; Wu, L.; Zhu, W.; Qian, H.; et al. HucMSC-Exosome Mediated-Wnt4 Signaling Is Required for Cutaneous Wound Healing. Stem Cells 2015, 33, 2158–2168. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, H.; Xu, W.; Wang, B.; Wu, H.; Tao, Y.; Zhang, B.; Wang, M.; Mao, F.; Yan, Y.; et al. Exosomes Released by Human Umbilical Cord Mesenchymal Stem Cells Protect against Cisplatin-Induced Renal Oxidative Stress and Apoptosis in Vivo and in Vitro. Stem Cell Res. Ther. 2013, 4, 34. [Google Scholar] [CrossRef]
- Ouyang, X.; Han, X.; Chen, Z.; Fang, J.; Huang, X.; Wei, H. MSC-Derived Exosomes Ameliorate Erectile Dysfunction by Alleviation of Corpus Cavernosum Smooth Muscle Apoptosis in a Rat Model of Cavernous Nerve Injury. Stem Cell Res. Ther. 2018, 9, 246. [Google Scholar] [CrossRef]
- Britsch, S. The Neuregulin-I/ErbB Signaling System in Development and Disease. Adv. Anat. Embryol. Cell Biol. 2007, 190, 1–65. [Google Scholar]
- Falls, D.L. Neuregulins: Functions, Forms, and Signaling Strategies. Exp. Cell Res. 2003, 284, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Burnett, A.L.; Sezen, S.F.; Hoke, A.; Caggiano, A.O.; Iaci, J.; Lagoda, G.; Musicki, B.; Bella, A.J. GGF2 Is Neuroprotective in a Rat Model of Cavernous Nerve Injury-Induced Erectile Dysfunction. J. Sex. Med. 2015, 12, 897–905. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latcu, S.C.; Novacescu, D.; Buciu, V.-B.; Dumitru, C.-S.; Ceausu, R.A.; Raica, M.; Cut, T.G.; Ilina, R.; Malita, D.C.; Tarta, C.; et al. The Cavernous Nerve Injury Rat Model: A Pictorial Essay on Post-Radical Prostatectomy Erectile Dysfunction Research. Life 2023, 13, 2337. https://doi.org/10.3390/life13122337
Latcu SC, Novacescu D, Buciu V-B, Dumitru C-S, Ceausu RA, Raica M, Cut TG, Ilina R, Malita DC, Tarta C, et al. The Cavernous Nerve Injury Rat Model: A Pictorial Essay on Post-Radical Prostatectomy Erectile Dysfunction Research. Life. 2023; 13(12):2337. https://doi.org/10.3390/life13122337
Chicago/Turabian StyleLatcu, Silviu Constantin, Dorin Novacescu, Victor-Bogdan Buciu, Cristina-Stefania Dumitru, Raluca Amalia Ceausu, Marius Raica, Talida Georgiana Cut, Razvan Ilina, Daniel Claudiu Malita, Cristi Tarta, and et al. 2023. "The Cavernous Nerve Injury Rat Model: A Pictorial Essay on Post-Radical Prostatectomy Erectile Dysfunction Research" Life 13, no. 12: 2337. https://doi.org/10.3390/life13122337
APA StyleLatcu, S. C., Novacescu, D., Buciu, V.-B., Dumitru, C.-S., Ceausu, R. A., Raica, M., Cut, T. G., Ilina, R., Malita, D. C., Tarta, C., & Cumpanas, A. A. (2023). The Cavernous Nerve Injury Rat Model: A Pictorial Essay on Post-Radical Prostatectomy Erectile Dysfunction Research. Life, 13(12), 2337. https://doi.org/10.3390/life13122337










