Oxidation Products of Tryptophan and Proline in Adipokinetic Hormones—Artifacts or Post-Translational Modifications?
Abstract
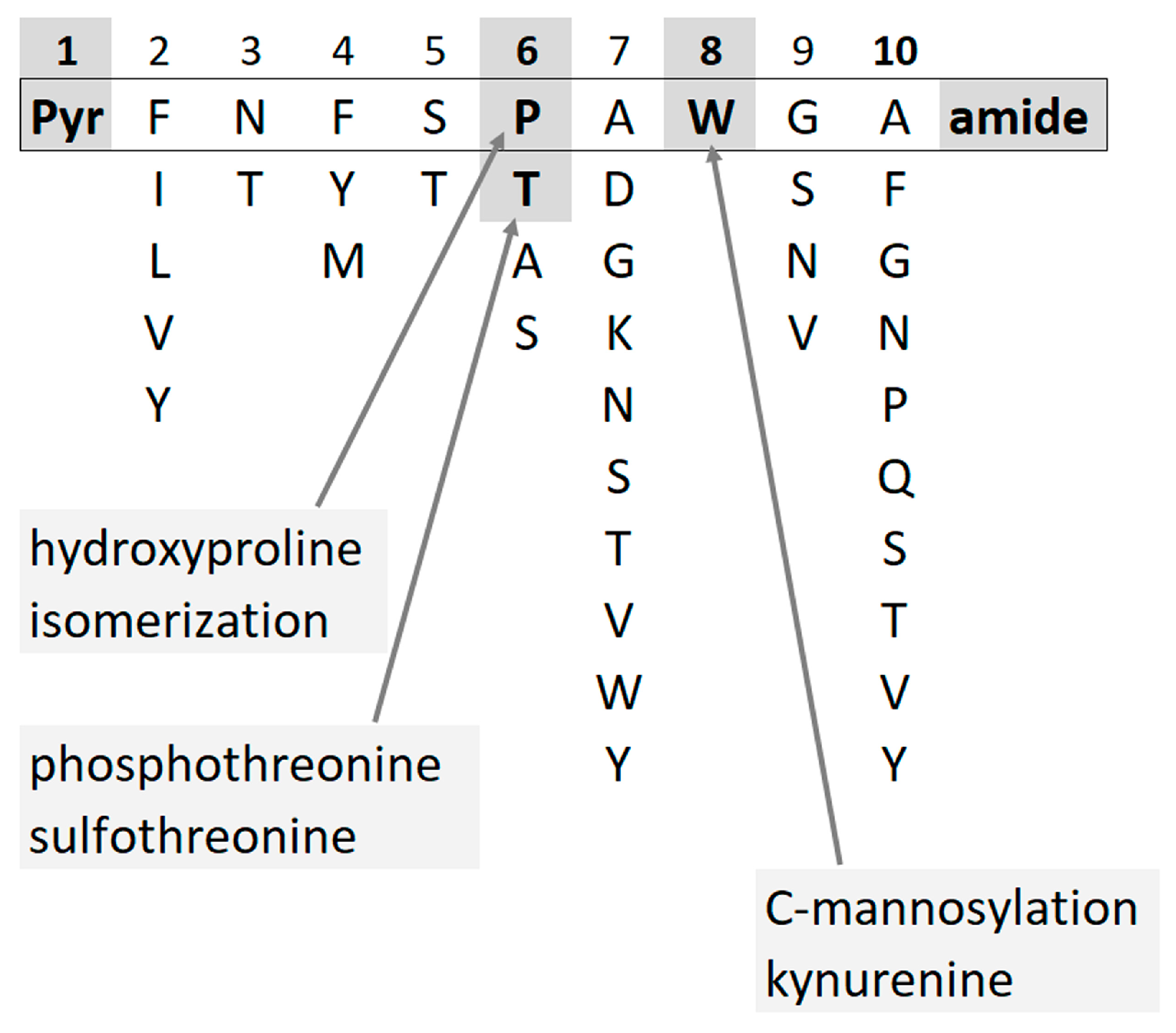
:1. Introduction
1.1. Hydroxyproline
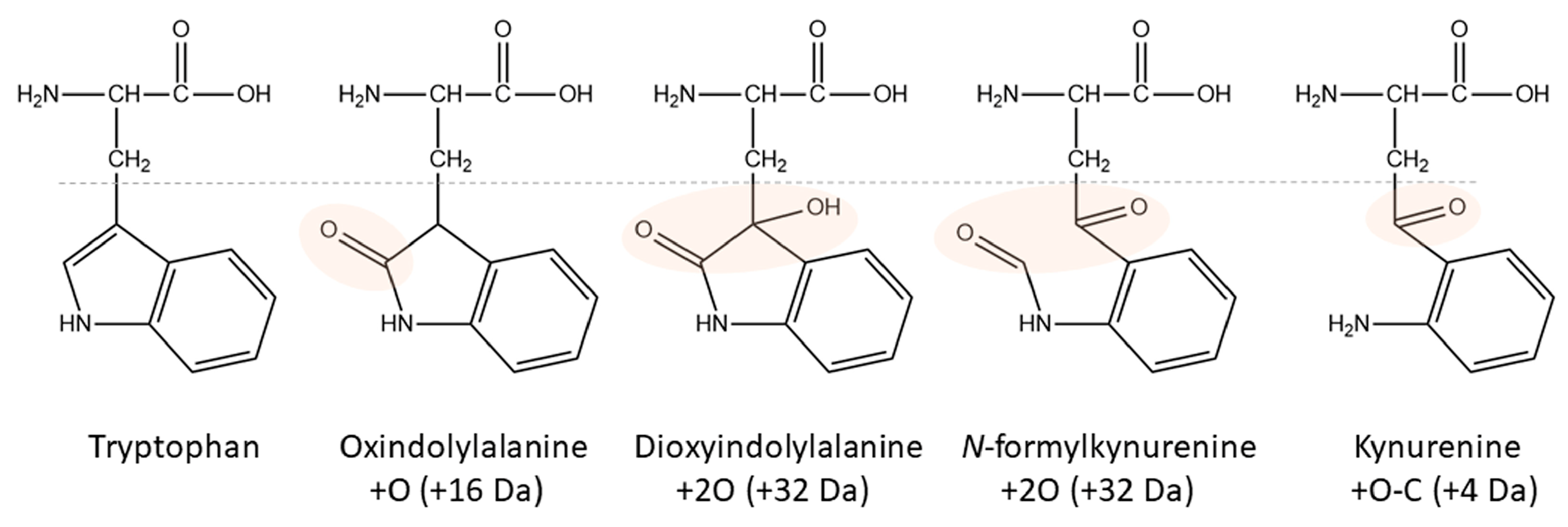
1.2. Tryptophan Oxidation
2. Results and Discussion
2.1. Hydroxyproline
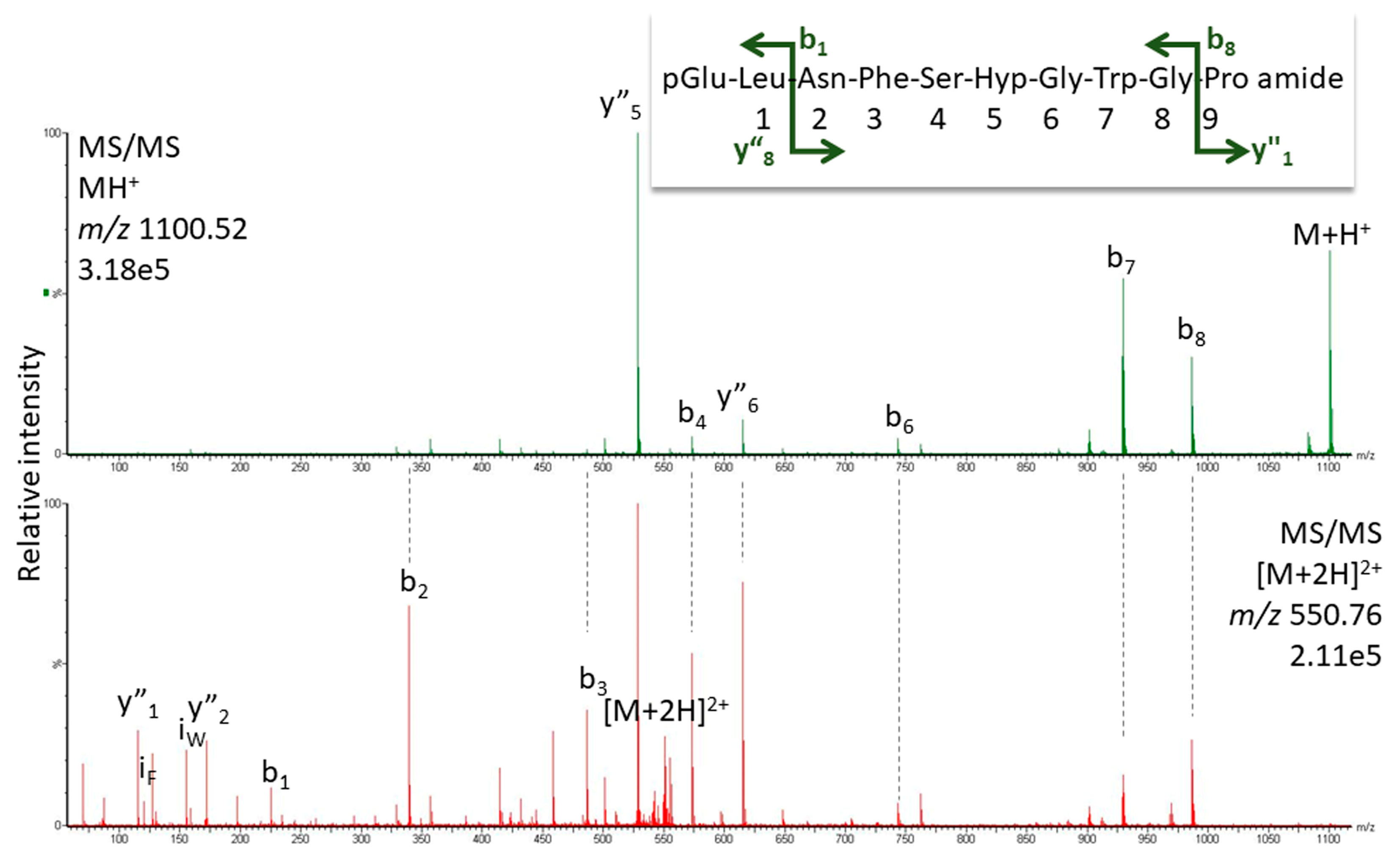
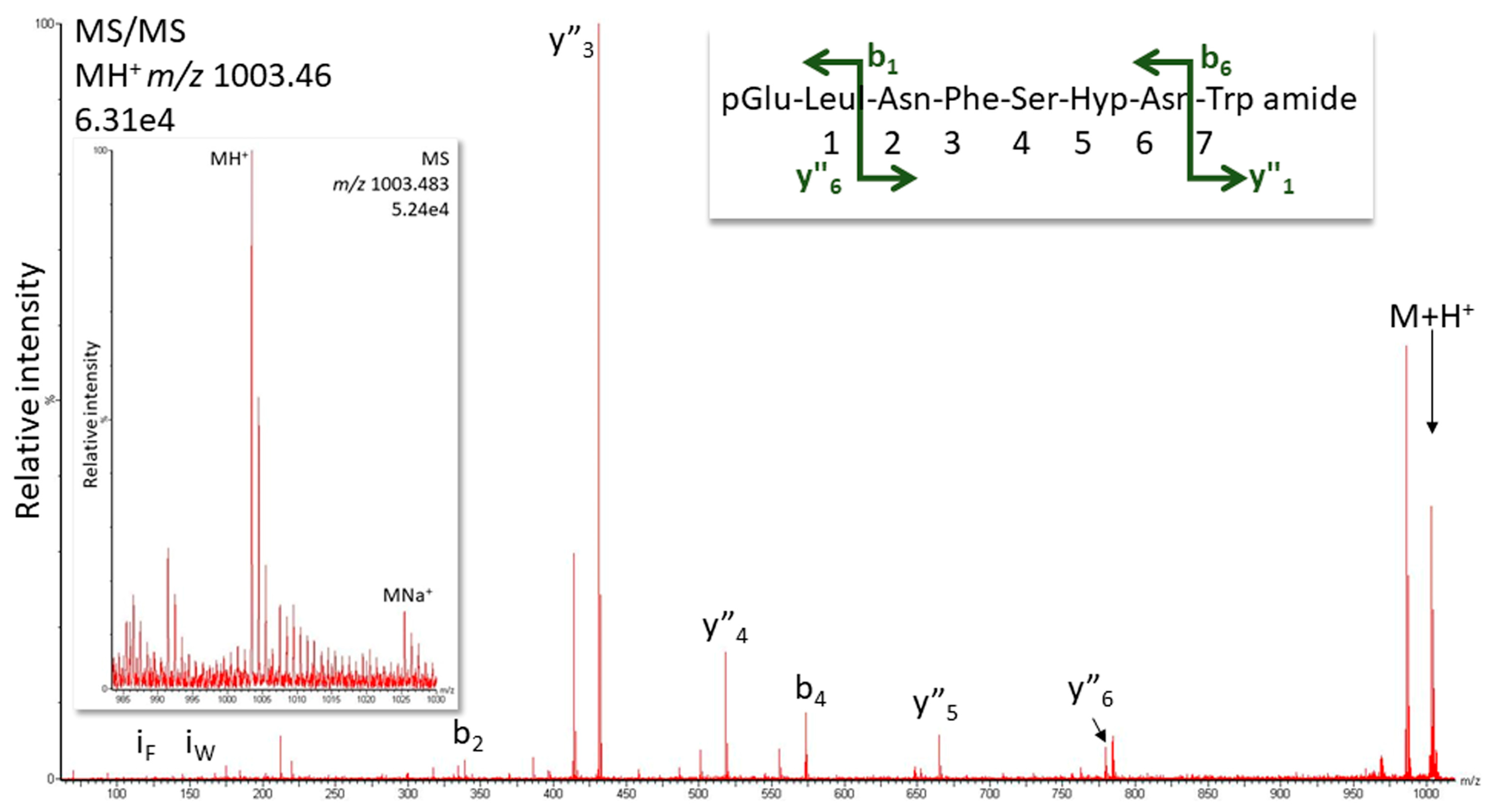
2.1.1. MS Analysis
2.1.2. Artifact or Natural Product?
2.1.3. Bioactivity
2.2. Tryptophan Oxidation
3. Materials and Methods
3.1. Insects and CC Preparation
3.2. Biological Assay
3.3. Release Experiment
3.4. Synthetic Peptides
3.5. Precautions against Oxidation
3.6. Structure Elucidation by LC-MS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Nässel, D.R.; Winther, Å. Drosophila neuropeptides in regulation of physiology and behavior. Prog. Neurobiol. 2010, 92, 42–104. [Google Scholar] [CrossRef] [PubMed]
- Gäde, G.; Hoffmann, K.H.; Spring, J.H. Hormonal regulation in insects: Facts, gaps, and future directions. Physiol. Rev. 1997, 77, 963–1032. [Google Scholar] [CrossRef] [PubMed]
- Gäde, G.; Marco, H.G. Structure, function and mode of action of select arthropod neuropeptides. In Bioactive Natural Products (Part M); Atta-Ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 33, pp. 69–139. [Google Scholar]
- Marco, H.G.; Gäde, G. Adipokinetic hormone: A hormone for all seasons? In Advances in Invertebrate (Neuro)Endocrinology: A Collection of Reviews in the Post-Genomic Era; Saleuddin, S., Lange, A.B., Orchard, I., Eds.; Apple Academic Press: Palm Bay, FL, USA, 2020; Volume 2, pp. 126–170. [Google Scholar]
- Gäde, G.; Šimek, P.; Clark, K.D.; Auerswald, L. Unique translational modification of an invertebrate neuropeptide: A phosphorylated member of the adipokinetic hormone peptide family. Biochem. J. 2006, 393, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Gäde, G.; Šimek, P.; Marco, H.G. A sulfothreonine adipokinetic peptide—A novel post-translational modification revealed in the twig wilter Holopterna alata (Hemiptera, Coreidae). In Proceedings of the 27th Conference of European Comparative Endocrinologists, Rennes, France, 25–29 August 2014; p. 68. [Google Scholar]
- Munte, C.E.; Gäde, G.; Domogalla, B.; Kremer, W.; Kellner, R.; Kalbitzer, H.R. C-mannosylation in the hypertrehalosaemic hormone from the stick insect Carausius morosus. FEBS J. 2008, 275, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Gäde, G.; Marco, H.G. The unique C-mannosylated hypertrehalosemic hormone of Carausius morosus: Identity, release, and biological activity. Arch. Insect Biochem. Physiol. 2023, 113, e22016. [Google Scholar] [CrossRef]
- König, S.; Bayer, M.; Marco, H.G.; Gäde, G. The hypertrehalosaemic neuropeptide conformational twins of cicadas consist of only L-amino acids: Are they cis–trans isomers? Amino Acids 2019, 51, 1023–1028. [Google Scholar] [CrossRef]
- Malik, A.; Gäde, G.; Lange, A.B. Sequencing and biological effects of an adipokinetic/hypertrehalosemic peptide in the stick insect, Baculum extradentatum. Peptides 2012, 34, 51–56. [Google Scholar] [CrossRef]
- Gäde, G.; Šimek, P.; Marco, H.G. An invertebrate [hydroxyproline]-modified neuropeptide: Further evidence for a close evolutionary relationship between insect adipokinetic hormone and mammalian gonadotropin hormone family. Biochem. Biophys. Res. Commun. 2011, 414, 592–597. [Google Scholar] [CrossRef]
- Marco, H.G.; König, S.; Gäde, G. Mass spectrometric proof of predicted peptides: Novel adipokinetic hormones in insects. Molecules 2022, 27, 6469. [Google Scholar] [CrossRef]
- Marco, H.G.; König, S.; Gäde, G. Predicted novel hypertrehalosaemic peptides of cockroaches are verified by mass spectrometry. Amino Acids 2023, 55, 1641–1654. [Google Scholar] [CrossRef]
- Ehrenshaft, M.; Deterding, L.J.; Mason, R.P. Tripping up Trp: Modification of protein tryptophan residues by reactive oxygen species, modes of detection, and biological consequences. Free Radic. Biol. Med. 2015, 89, 220–228. [Google Scholar] [CrossRef]
- Davies, M.J.; Truscott, R.J.W. Chapter 12—Photo-oxidation of proteins and its consequences. In Comprehensive Series in Photosciences; Giacomoni, P.U., Ed.; Elsevier: Amsterdam, The Netherlands, 2001; Volume 3, pp. 251–275. [Google Scholar]
- Chen, M.; Cook, K.D. Oxidation artifacts in the electrospray mass spectrometry of Aβ peptide. Anal Chem. 2007, 79, 2031–2036. [Google Scholar] [CrossRef]
- Morand, K.; Talbo, G.; Mann, M. Oxidation of peptides during electrospray ionization. Rapid Commun. Mass Spectrom. 1993, 7, 738–743. [Google Scholar] [CrossRef]
- Schweikart, F.; Hulthe, G. HPLC-UV-MS analysis: A source for severe oxidation artifacts. Anal. Chem. 2019, 91, 1748–1751. [Google Scholar] [CrossRef]
- Hu, S.; He, W.; Wu, G. Hydroxyproline in animal metabolism, nutrition, and cell signaling. Amino Acids 2022, 54, 513–528. [Google Scholar] [CrossRef]
- Gorres, K.L.; Raines, R.T. Prolyl 4-hydroxylase. Crit. Rev. Mol. Biol. 2010, 45, 106–124. [Google Scholar] [CrossRef]
- Zahradníčková, H.; Opekar, S.; Řimnáčová, L.; Šimek, P.; Moos, M. Chiral secondary amino acids, their importance, and methods of analysis. Amino Acids 2022, 54, 687–719. [Google Scholar] [CrossRef]
- Rapaka, R.S.; Renugopalakrishnan, V.; Urry, D.W.; Bhatnagar, R.S. Hydroxylation of proline in polytripeptide models of collagen: Stereochemistry of polytripeptide-prolyl hydroxylase interaction. Biochemistry 1978, 17, 2892–2898. [Google Scholar] [CrossRef]
- McGee, J.O.; Rhoads, R.E.; Udenfriend, S. The substrate recognition site of collagen proline hydroxylase: The hydroxylation of -X-Pro-Gly- sequences in bradykinin analogs and other peptides. Arch. Biochem. Biophys. 1971, 144, 343–351. [Google Scholar] [CrossRef]
- Gautron, J.P.; Pattou, E.; Bauer, K.; Kordon, C. (Hydroxyproline(9)) luteinizing hormone-releasing hormone: A novel peptide in mammalian and frog hypothalamus. Neurochem. Int. 1991, 18, 221–235. [Google Scholar] [CrossRef]
- Arsenault, P.R.; Heaton-Johnson, K.J.; Li, L.S.; Song, D.; Ferreira, V.S.; Patel, N.; Master, S.R.; Lee, F.S. Identification of prolyl hydroxylation modifications in mammalian cell proteins. Proteomics 2015, 15, 1259–1267. [Google Scholar] [CrossRef]
- Cruz, L.J.; Gray, W.R.; Olivera, B.M.; Zeikus, R.D.; Kerr, L.; Yoshikami, D.; Moczydlowski, E. Conus geographus toxins that discriminate between neuronal and muscle sodium channels. J. Biol. Chem. 1985, 260, 9280–9288. [Google Scholar] [CrossRef]
- Buczek, O.; Bulaj, G.; Olivera, B.M. Conotoxins and the posttranslational modification of secreted gene products. Cell. Mol. Life Sci. 2005, 62, 3067–3079. [Google Scholar] [CrossRef]
- Zhang, B.-B.; Zhao, C.; Wang, X.-S.; He, L.; Du, W.-H. Effects of 4-hydroxyproline stereochemistry on α-conotoxin solution conformation. Acta Phys. Chim. Sin. 2013, 29, 1080–1087. [Google Scholar]
- Kim, G.; Weiss, S.J.; Levine, R.L. Methionine oxidation and reduction in proteins. Biochim. Biophys. Acta 2014, 1840, 901–905. [Google Scholar] [CrossRef]
- Pietzsch, J. Measurement of 5-hydroxy-2-2aminovaleric acid as a specific marker of iron-mediated oxidation of proline and arginine side-chain residues of low-density lipoprotein apolipoprotein B-100. Biochem. Biophys. Res. Commun. 2000, 270, 852–857. [Google Scholar] [CrossRef]
- Dean, R.T.; Wolff, S.P.; McElligott, M.A. Histidine and proline are important sites of free radical damage to proteins. Free Rad. Res. Comms. 1989, 7, 97–103. [Google Scholar] [CrossRef]
- Katz, M.J.; Acevedo, J.M.; Loenarz, C.; Galagovsky, D.; Liu-Yi, P.; Pérez-Pepe, M.; Thalhammer, A.; Sekirnik, R.; Ge, W.; Melani, M.; et al. Sudestada1, a Drosophila ribosomal prolyl-hydroxylase required for mRNA translation, cell homeostasis, and organ growth. Proc. Natl. Acad. Sci. USA 2014, 111, 4025–4030. [Google Scholar] [CrossRef]
- Gäde, G. Peptides of the adipokinetic hormone/red pigment-concentrating hormone family—A new take on biodiversity. In Trends in Comparative Endocrinology and Neurobiology; Annals of the New York Academy of Science; Vaudry, H., Roubos, E.W., Coast, G.M., Vallarino, M., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2009; Volume 1163, pp. 125–136. [Google Scholar]
- Simat, T.J.; Steinhart, H. Oxidation of free tryptophan and tryptophane residues in peptides and proteins. J. Agric. Food Chem. 1998, 46, 490–498. [Google Scholar] [CrossRef]
- Bellmaine, S.; Schnellbaecher, A.; Zimmer, A. Reactivity and degradation products of tryptophan in solution and proteins. Free Radic. Biol. Med. 2020, 20, 696–718. [Google Scholar] [CrossRef]
- Scheidegger, D.; Larsen, G.; Kivatinitz, S.C. Oxidative consequences of UV irradiation on isolated milk proteins: Effects of hydrogen peroxide and bivalent metal ions. Int. Dairy J. 2016, 55, 64–71. [Google Scholar] [CrossRef]
- Koivumäki, T.P.; Gürbüz, G.; Heinonen, I.M. Tryptophan and cysteine oxidation products dominate in α-lactalbumin-derived peptides analyzed with LC-MSn. J. Food Sci. 2017, 82, 2062–2069. [Google Scholar] [CrossRef] [PubMed]
- Dyer, J.M.; Bringans, S.D.; Bryson, W.G. Characterisation of photo-oxidation products within photoyellowed wool proteins: Tryptophan and tyrosine derived chromophores. Photochem. Photobiol. Sci. 2006, 5, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Barnett, G.V.; Balakrishnan, G.; Chennamsetty, N.; Hoffman, L.; Bongers, J.; Tao, L.; Huang, Y.; Slaney, T.; Das, T.K.; Leone, A.; et al. Probing the tryptophan environment in therapeutic proteins: Implications for higher order structure on tryptophan oxidation. J. Pharm. Sci. 2019, 108, 1944–1952. [Google Scholar] [CrossRef] [PubMed]
- Grosvenor, A.J.; Morton, J.D.; Dyer, J.M. Profiling of residue-level photo-oxidative damage in peptides. Amino Acids 2010, 39, 285–296. [Google Scholar] [CrossRef]
- Bayer, M.; Tsiskarishvili, N.; Stegemann, A.; Böhm, M.; König, S. Fast oxidation of α-melanocyte stimulating hormone and derived peptides under laboratory conditions causes irreproducible results—Insights from studies of prolylcarboxypeptidase in human cell types. Pigment. Cell Melanoma Res. 2019, 33, 378–382. [Google Scholar] [CrossRef]
- Pattison, D.I.; Rahmanto, A.S.; Davies, M.J. Photo-oxidation of proteins. Photochem. Photobiol. Sci. 2012, 11, 38–53. [Google Scholar] [CrossRef]
- Castaño, C.; Vignoni, M.; Vicendo, P.; Oliveros, E.; Thomas, A.H. Degradation of tyrosine and tryptophan residues of peptides by type I photosensitized oxidation. J. Photochem. Photobiol. B Biol. 2016, 164, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.L. Ozone in ambient air as a source of adventitious oxidation. A mass spectrometric study. Anal. Chem. 2006, 78, 4352–4362. [Google Scholar] [CrossRef]
- Kotiaho, T.; Eberlin, M.N.; Vainiotalo, P.; Kostiainen, R. Electrospray mass and tandem mass spectrometry identification of ozone oxidation products of amino acids and small peptides. J. Am. Soc. Mass Spectrom. 2000, 11, 526–535. [Google Scholar] [CrossRef]
- Perdivara, I.; Deterding, L.J.; Przybylski, M.; Tomer, K.B. Mass spectrometric identification of oxidative modifications of tryptophan residues in proteins: Chemical artifact or posttranslational modification? J. Am. Soc. Mass Spectrom. 2010, 21, 1114–1117. [Google Scholar] [CrossRef] [PubMed]
- Galetskiy, D.; Lohscheider, J.N.; Kononikhin, A.S.; Popov, I.A.; Nikolaev, E.N.; Adamska, I. Mass spectrometric characterization of photooxidative protein modifications in Arabidopsis thaliana thylakoid membranes. Rapid Commun. Mass Spectrom. 2011, 25, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, M.; Todorovsky, T.; Kuleva, N.; Hoffmann, R. Quantitative evaluation of tryptophan oxidation in actin and troponin I from skeletal muscles using a rat model of acute oxidative stress. Proteomics 2010, 10, 2692–2700. [Google Scholar] [CrossRef] [PubMed]
- Puddick, J.; Prinsep, M.R.; Wood, S.A.; Miles, C.O.; Rise, F.; Cary, S.C.; Hamilton, D.P.; Wilkins, A.L. Structural characterization of new microcystins containing tryptophan and oxidized tryptophan residues. Mar. Drugs. 2013, 11, 3025–3045. [Google Scholar] [CrossRef] [PubMed]
- Thackray, S.J.; Mowat, C.G.; Chapman, S.K. Exploring the mechanism of tryptophan 2,3-dioxygenase. Biochem. Soc. Trans. 2008, 36, 1120–1123. [Google Scholar] [CrossRef]
- Frydman, R.B.; Tomaro, M.L.; Frydman, B. Pyrrolooxygenase: Its action on tryptophan-containing enzymes and peptides. Biochim. Biophys. Acta 1972, 284, 80–89. [Google Scholar] [CrossRef]
- Frydman, B.; Frydman, R.B.; Tomaro, M.L. Pyrrolooxygenases: A new type of oxidases. Mol. Cell Biochem. 1973, 2, 121–136. [Google Scholar] [CrossRef]
- König, S.; Marco, H.G.; Gäde, G. The proline effect and the tryptophan immonium ion assist in de novo sequencing of adipokinetic hormones. Sci. Rep. 2023, 13, 10894. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Marco, H.G.; Scheich, N.; He, S.; Wang, Z.; Gäde, G.; McMahon, D.P. Comparative analysis of adipokinetic hormones and their receptors in Blattodea reveals novel patterns of gene evolution. Insect Mol. Biol. 2023, 32, 615–633. [Google Scholar] [CrossRef]
- Gäde, G.; Šimek, P.; Marco, H.G. A novel adipokinetic peptide from the corpus cardiacum of the primitive caeliferan pygmy grasshopper Tetrix subulata (Caelifera, Tetrigidae). Peptides 2015, 68, 43–49. [Google Scholar] [CrossRef]
- Gäde, G. On the release and action of the hypertrehalosaemic hormone from the cockroach Nauphoeta cinerea. Z. Naturforsch. 1988, 43, 108–116. [Google Scholar] [CrossRef]
- Gäde, G.; Šimek, P.; Marco, H.G. Two novel tyrosine-containing peptides (Tyr4) of the adipokinetic hormone family in beetles of the families Coccinellidae and Silphidae. Amino Acids 2015, 47, 2323–2333. [Google Scholar] [CrossRef] [PubMed]
- Gäde, G.; Goldsworthy, G.J.; Kegel, G.; Keller, R. Single step purification of locust adipokinetic hormones I and II by reversed-phase high-performance liquid-chromatography, and amino acid composition of the hormone II. Hoppe-Seyler Z. Physiol. Chem. 1984, 365, 393–398. [Google Scholar] [CrossRef]




| Species | Sequence | MW | Name |
|---|---|---|---|
| Nezara viridula | pQLNF S Hyp G W amide | 945.434 | [Hyp6] Panbo-RPCH [11] |
| Kalotermes flavicollis | pQVNF S Hyp G W amide | 931.419 | [Hyp6]-Manto-CC |
| Blaberus atropos | pQVNF S Hyp G WGT amide | 1089.4880 | [Hyp6]-Bladi-HrTH |
| Blaberus atropos | pQLNF S Hyp G WGF amide | 1149.5244 | [Hyp6]-BlaatHRTH |
| Eublaberus posticus | pQVNF S Hyp G WGT amide | 1089.4880 | [Hyp6]-Bladi-HrTH |
| Episymploce sundaica | pQVNF S Hyp G WGT amide | 1089.4880 | [Hyp6]-Bladi-HrTH |
| Episymploce sundaica | pQLNF S Hyp G WGP amide | 1099.5087 | [Hyp6]-Lobde-HrTH |
| Asiablatta kyotensis | pQVNF S Hyp G WGT amide | 1089.4880 | [Hyp6]-Bladi-HrTH |
| Asiablatta kyotensis | pQLNF S Hyp G WGV amide | 1101.5244 | [Hyp6]-Asiky-HrTH |
| Panchlora nivea | pQVNF S Hyp G WGT amide | 1089.4880 | [Hyp6]-Bladi-HrTH |
| Panchlora nivea | pQLNF S Hyp G WGT amide | 1103.5037 | [Hyp6]-Panni-HrTH |
| Supella longipalpa | pQVNF S Hyp G WGT amide | 1089.4880 | [Hyp6]-Bladi-HrTH |
| Loboptera decipiens | pQVNF S Hyp G WGT amide | 1089.4880 | [Hyp6]-Bladi-HrTH |
| Loboptera decipiens | pQLNF S Hyp G WGP amide | 1099.5087 | [Hyp6]-Lobde-HrTH |
| Xestoblatta cavicola | pQVNF S Hyp G WGT amide | 1089.4880 | [Hyp6]-Bladi-HrTH |
| Nauphotea cinera | pQVNF S Hyp G WGT amide | 1089.4880 | [Hyp6]-Bladi-HrTH |
| Aptera fusca | pQVNF S Hyp G WGT amide | 1089.4880 | [Hyp6]-Bladi-HrTH |
| Haematopota pluvialis | pQLTF T Hyp G W amide | 946.455 | [Hyp6]-Haepl-AKH [12] |
| Ergaula capucina | pQLNF S Hyp N W amide | 1002.4560 | [Hyp6]-Tenmo-HrTH [13] |
| References | ||||
|---|---|---|---|---|
| X | Hyp | G | [20,22] | |
| P | Hyp | G | [20,22,23,25] | |
| S | Hyp | G | this study, [25] | |
| T | Hyp | G | this study | |
| A | Hyp | G | [23] | |
| R | Hyp | G | [24] | |
| S | Hyp | A | [25] | |
| D | Hyp | V | [25] | |
| T | Hyp | P | [26,27] | |
| T | Hyp | N | [25] | |
| T | Hyp | N | this study | |
| S | Hyp | N | this study | |
| S | Hyp | E | [25] | |
| K | Hyp | Q | [26,27] | |
| A | Hyp | S | [25] | |
| R | Hyp | T | [28] | |
| T | Hyp | Hyp | K | [26,27] |
| P | Hyp | K | [26,27] | |
| T | Hyp | Hyp | R | [26,27] |
| P | Hyp | R | [26,27] | |
| D | Hyp | R | [28] | |
| Treatment | n | [Carbohydrates] T0min (µg/µL) | [Carbohydrates] T90min (µg/µL) | Difference (µg/µL) | p * |
|---|---|---|---|---|---|
| Distilled water | 20 | 17.87 ± 2.94 | 18.44 ± 3.46 | 0.57 ± 2.13 | NS |
| Bladi-HrTH | 5 | 20.38 ± 3.89 | 36.46 ± 9.41 | 16.08 ± 6.29 | 0.002 |
| [Hyp]-Bladi-HrTH | 19 | 15.96 ± 2.89 | 31.06 ± 4.86 | 15.10 ± 5.64 | 0.0006 |
| Blaat-HrTH | 9 | 15.44 ± 3.62 | 24.10 ± 6.51 | 8.66 ± 6.67 | 0.002 |
| [Hyp]-Blaat-HrTH | 18 | 17.61 ± 2.75 | 24.07 ± 1.32 | 6.46 ± 1.44 | 0.000001 |
| [Hyp]-Manto-CC | 5 | 17.78 ± 1.44 | 30.16 ± 4.32 | 12.38 ± 5.17 | 0.009 |
| [Hyp]-Tenmo-HrTH | 5 | 18.69 ± 0.95 | 30.37 ± 1.95 | 11.67 ± 1.80 | 0.0001 |
| Peram-CAH-I | 9 | 18.95 ± 2.94 | 35.92 ± 4.39 | 16.97 ± 3.61 | 0.0000003 |
| P. americana (0.1 gland pair equivalent) | 12 | 15.00 ± 2.02 | 45.86 ± 7.06 | 30.86 ± 6.61 | 0.000001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
König, S.; Marco, H.G.; Gäde, G. Oxidation Products of Tryptophan and Proline in Adipokinetic Hormones—Artifacts or Post-Translational Modifications? Life 2023, 13, 2315. https://doi.org/10.3390/life13122315
König S, Marco HG, Gäde G. Oxidation Products of Tryptophan and Proline in Adipokinetic Hormones—Artifacts or Post-Translational Modifications? Life. 2023; 13(12):2315. https://doi.org/10.3390/life13122315
Chicago/Turabian StyleKönig, Simone, Heather G. Marco, and Gerd Gäde. 2023. "Oxidation Products of Tryptophan and Proline in Adipokinetic Hormones—Artifacts or Post-Translational Modifications?" Life 13, no. 12: 2315. https://doi.org/10.3390/life13122315
APA StyleKönig, S., Marco, H. G., & Gäde, G. (2023). Oxidation Products of Tryptophan and Proline in Adipokinetic Hormones—Artifacts or Post-Translational Modifications? Life, 13(12), 2315. https://doi.org/10.3390/life13122315







