Oral Health and Behavior Patterns of Women with Eating Disorders—A Clinical Pilot Study
Abstract
:1. Introduction
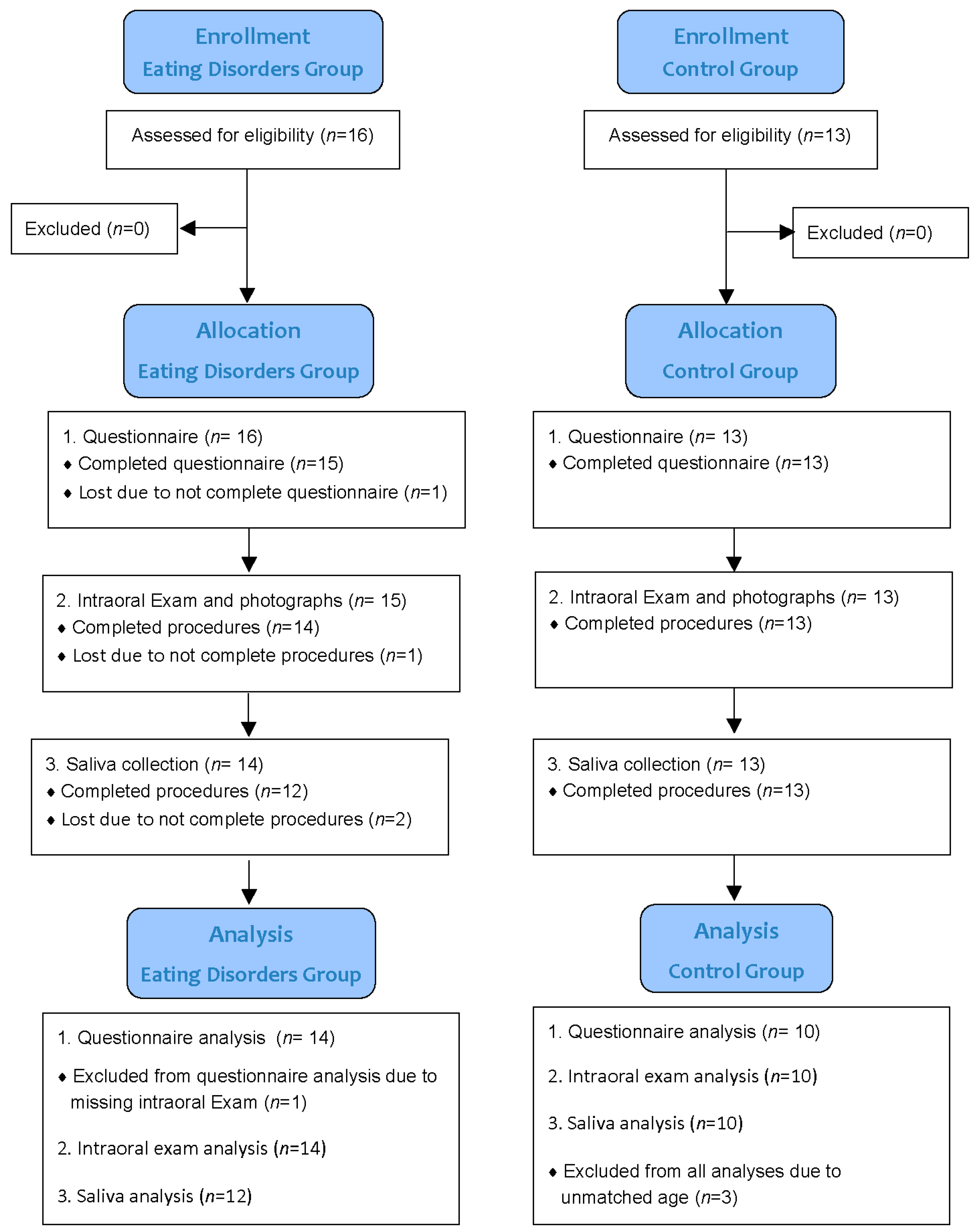
2. Materials and Methods
2.1. Study Design
2.2. Subject Recruitment and Background Information
2.3. Questionnaire: Eating Disorders and Oral Care Behaviors
2.4. Intraoral Examination
2.5. Saliva Collection and Analysis
2.6. Data and Statistical Analysis
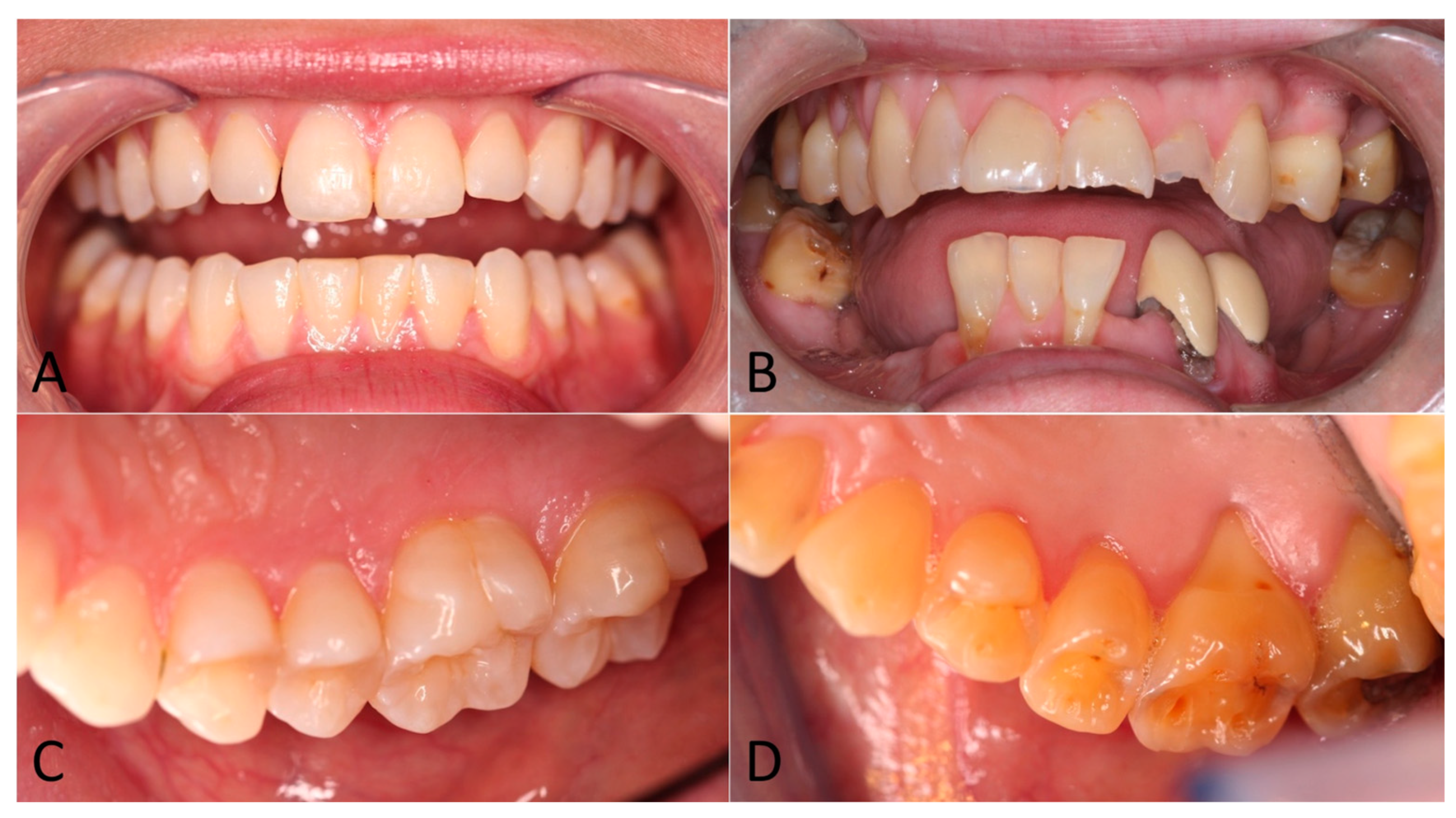
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lussi, A. Erosive tooth wear—A multifactorial condition of growing concern and increasing knowledge. Monogr. Oral. Sci. 2006, 20, 1–8. [Google Scholar] [PubMed]
- Ganss, C. Definition of erosion and links to tooth wear. Monogr. Oral. Sci. 2006, 20, 9–16. [Google Scholar] [PubMed]
- Schlueter, N.; Amaechi, B.T.; Bartlett, D.; Buzalaf, M.A.R.; Carvalho, T.S.; Ganss, C.; Hara, A.T.; Huysmans, M.-C.D.; Lussi, A.; Moazzez, R.; et al. Terminology of Erosive Tooth Wear: Consensus Report of a Workshop Organized by the ORCA and the Cariology Research Group of the IADR. Caries Res. 2020, 54, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Schlueter, N.; Luka, B. Erosive tooth wear—A review on global prevalence and on its prevalence in risk groups. Br. Dent. J. 2018, 224, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Okunseri, C.; Wong, M.C.M.; Yau, D.T.W.; McGrath, C.; Szabo, A. The relationship between consumption of beverages and tooth wear among adults in the United States. J. Public. Health Dent. 2015, 75, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Bretz, W.A. Oral profiles of bulimic women: Diagnosis and management. What is the evidence? J. Evid. Based Dent. Pract. 2002, 2, 267–272. [Google Scholar] [CrossRef] [PubMed]
- de Moor, R.J. Eating disorder-induced dental complications: A case report. J. Oral. Rehabil. 2004, 31, 725–732. [Google Scholar] [CrossRef]
- Johansson, A.K.; Norring, C.; Unell, L.; Johansson, A. Eating disorders and oral health: A matched case-control study. Eur. J. Oral. Sci. 2012, 120, 61–68. [Google Scholar] [CrossRef]
- Kavitha, P.R.; Vivek, P.; Hegde, A.M. Eating disorders and their implications on oral health–role of dentists. J. Clin. Pediatr. Dent. 2011, 36, 155–160. [Google Scholar] [CrossRef]
- Ohrn, R.; Enzell, K.; Angmar-Månsson, B. Oral status of 81 subjects with eating disorders. Eur. J. Oral. Sci. 1999, 107, 157–163. [Google Scholar] [CrossRef]
- Smink, F.R.; van Hoeken, D.; Hoek, H.W. Epidemiology, course, and outcome of eating disorders. Curr. Opin. Psychiatry 2013, 26, 543–548. [Google Scholar] [CrossRef]
- The National Eating Disorders Association. What Are Eating Disorders? Available online: https://www.nationaleatingdisorders.org/learn/general-information/what-are-eating-disorders (accessed on 20 October 2023).
- Conviser, J.H.; Fisher, S.D.; Mitchell, K.B. Oral care behavior after purging in a sample of women with bulimia nervosa. J. Am. Dent. Assoc. 2014, 145, 352–354. [Google Scholar] [CrossRef] [PubMed]
- Hara, A.T.; Lussi, A.; Zero, D.T. Biological factors. Monogr. Oral. Sci. 2006, 20, 88–99. [Google Scholar] [PubMed]
- Schlueter, N.; Ganss, C.; Pötschke, S.; Klimek, J.; Hannig, C. Enzyme activities in the oral fluids of patients suffering from bulimia: A controlled clinical trial. Caries Res. 2012, 46, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Amaechi, B.T.; Higham, S.M. Dental erosion: Possible approaches to prevention and control. J. Dent. 2005, 33, 243–252. [Google Scholar] [CrossRef] [PubMed]
- DeBate, R.D.; Plichta, S.B.; Tedesco, L.A.; Kerschbaum, W.E. Integration of oral health care and mental health services: Dental hygienists’ readiness and capacity for secondary prevention of eating disorders. J. Behav. Health Serv. Res. 2006, 33, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Willumsen, T.; Graugaard, P.K. Dental fear, regularity of dental attendance and subjective evaluation of dental erosion in women with eating disorders. Eur. J. Oral Sci. 2005, 113, 297–302. [Google Scholar] [CrossRef]
- Johnson, L.B.; Boyd, L.D.; Rainchuso, L.; Rothman, A.; Mayer, B. Eating disorder professionals’ perceptions of oral health knowledge. Int. J. Dent. Hyg. 2017, 15, 164–171. [Google Scholar] [CrossRef]
- Szupiany, T.; Pytko-Polończyk, J.; Rutkowski, K. Dental needs of psychiatric patient with eating disorders. Psychiatr. Pol. 2015, 49, 945–954. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®); American Psychiatric Publishing: Washington, DC, USA, 2013. [Google Scholar]
- World Health Organization. Oral Health Surveys: Basic Methods, 5th ed.; WHO Press: Geneva, Switzerland, 2013. [Google Scholar]
- Lussi, A.; Schaffner, M.; Hotz, P.; Suter, P. Dental erosion in a population of Swiss adults. Community Dent. Oral. Epidemiol. 1991, 19, 286–290. [Google Scholar] [CrossRef]
- Valena, V.; Young, W.G. Dental erosion patterns from intrinsic acid regurgitation and vomiting. Aust. Dent. J. 2002, 47, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Barron, R.P.; Carmichael, R.P.; A Marcon, M.; Sàndor, G.K.B. Dental erosion in gastroesophageal reflux disease. J. Can. Dent. Assoc. 2003, 69, 84–89. [Google Scholar] [PubMed]
- Otsu, M.; Hamura, A.; Ishikawa, Y.; Karibe, H.; Ichijyo, T.; Yoshinaga, Y. Factors affecting the dental erosion severity of patients with eating disorders. Biopsychosoc. Med. 2014, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Uhlen, M.-M.; Tveit, A.B.; Stenhagen, K.R.; Mulic, A. Self-induced vomiting and dental erosion—A clinical study. BMC Oral. Health 2014, 14, 92. [Google Scholar] [CrossRef]
- Kisely, S.; Baghaie, H.; Lalloo, R.; Johnson, N.W. Association between poor oral health and eating disorders: Systematic review and meta-analysis. Br. J. Psychiatry 2015, 207, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Nijakowski, K.; Jankowski, J.; Gruszczyński, D.; Surdacka, A. Eating Disorders and Dental Erosion: A Systematic Review. J. Clin. Med. 2023, 12, 6161. [Google Scholar] [CrossRef]
- Giraudeau, N.; Camman, P.; Pourreyron, L.; Inquimbert, C.; Lefebvre, P. The contribution of teledentistry in detecting tooth erosion in patients with eating disorders. Digit. Health 2021, 7, 20552076211019250. [Google Scholar] [CrossRef]
- Choi, J.; Price, J.; Ryder, S.; Siskind, D.; Solmi, M.; Kisely, S. Prevalence of dental disorders among people with mental illness: An umbrella review. Aust. New Zealand J. Psychiatry 2022, 56, 949–963. [Google Scholar] [CrossRef]
- Hermont, A.P.; Oliveira, P.A.D.; Martins, C.C.; Paiva, S.M.; Pordeus, I.A.; Auad, S.M. Tooth erosion and eating disorders: A systematic review and meta-analysis. PLoS ONE 2014, 9, e111123. [Google Scholar] [CrossRef]
- Zero, D.T.; Lussi, A. Erosion–chemical and biological factors of importance to the dental practitioner. Int. Dent. J. 2005, 55 (Suppl. S1), 285–290. [Google Scholar] [CrossRef]
- Young, W.G. The oral medicine of tooth wear. Aust. Dent. J. 2001, 46, 236–250. [Google Scholar] [CrossRef]
- Dynesen, A.W.; Bardow, A.; Petersson, B.; Nielsen, L.R.; Nauntofte, B. Salivary changes and dental erosion in bulimia nervosa. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2008, 106, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Lifante-Oliva, C.; López-Jornet, P.; Camacho-Alonso, F.; Esteve-Salinas, J. Study of oral changes in patients with eating disorders. Int. J. Dent. Hyg. 2008, 6, 119–122. [Google Scholar] [CrossRef]
- Guggenheimer, J.; Moore, P.A. Xerostomia: Etiology, recognition and treatment. J. Am. Dent. Assoc. 2003, 134, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Dawes, C. Factors Influencing Salivary Flow Rate and Composition, 4th ed.; Saliva and Oral Health; Edgar, M., Dawes, C., O’mullane, D., Eds.; Stephen Hancocks Limited.: London, UK, 2015; pp. 37–56. [Google Scholar]
- Paszynska, E.; Hernik, A.; Slopien, A.; Roszak, M.; Jowik, K.; Dmitrzak-Weglarz, M.; Tyszkiewicz-Nwafor, M. Risk of Dental Caries and Erosive Tooth Wear in 117 Children and Adolescents’ Anorexia Nervosa Population—A Case-Control Study. Front. Psychiatry 2022, 13, 874263. [Google Scholar] [CrossRef] [PubMed]
- Mehler, P.S. Medical complications of bulimia nervosa and their treatments. Int. J. Eat. Disord. 2011, 44, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Fukudo, S. Gastrointestinal symptoms and disorders in patients with eating disorders. Clin. J. Gastroenterol. 2015, 8, 255–263. [Google Scholar] [CrossRef]
- Johansson, A.-K.; Øvretvedt, T.M.; Reinholtsen, K.K.; Johansson, A. Eating Disorders: An Analysis of Self-Induced Vomiting, Binge Eating, and Oral Hygiene Behavior. Int. J. Clin. Pract. 2022, 2022, 6210372. [Google Scholar] [CrossRef]
- Tantbirojn, D.; Pintado, M.R.; Versluis, A.; Dunn, C.; Delong, R. Quantitative analysis of tooth surface loss associated with gastroesophageal reflux disease: A longitudinal clinical study. J. Am. Dent. Assoc. 2012, 143, 278–285. [Google Scholar] [CrossRef]
- Robb, N.D.; Smith, B.G.; Geidrys-Leeper, E. The distribution of erosion in the dentitions of patients with eating disorders. Br. Dent. J. 1995, 178, 171–175. [Google Scholar] [CrossRef]
- Oudkerk, J.; Grenade, C.; Davarpanah, A.; Vanheusden, A.; Vandenput, S.; Mainjot, A.K. Risk factors of tooth wear in permanent dentition: A scoping review. J. Oral. Rehabil. 2023, 50, 1110–1165. [Google Scholar] [CrossRef] [PubMed]
- Milosevic, A.; Brodie, D.A.; Slade, P.D. Dental erosion, oral hygiene, and nutrition in eating disorders. Int. J. Eat. Disord. 1997, 21, 195–199. [Google Scholar] [CrossRef]
- Harrison, J.L.; A George, L.; Cheatham, J.L.; Zinn, J. Dental effects and management of bulimia nervosa. Gen. Dent. 1985, 33, 65–68. [Google Scholar] [PubMed]
- Arfanakis, D.P.; Amaechi, B.T.; Bassiouny, M.A.; Dehghan, M.; Rechmann, P.; Zero, D. Clinical practice guidelines on the management of acid erosion. Gen. Dent. 2016, 64, e6–e17. [Google Scholar]
- Aranha, A.C.; Cde, P.E.; Cordás, T.A. Eating disorders. Part I: Psychiatric diagnosis and dental implications. J. Contemp. Dent. Pract. 2008, 9, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Eversole, S.L.; Saunders-Burkhardt, K.; Faller, R.V. Erosion Prevention Potential of an Over-the-Counter Stabilized SnF2 Dentifrice Compared to 5000 ppm F Prescription-Strength Products. J. Clin. Dent. 2015, 26, 44–49. [Google Scholar] [PubMed]
- Ganss, C.; Lussi, A.; Grunau, O.; Klimek, J.; Schlueter, N. Conventional and anti-erosion fluoride toothpastes: Effect on enamel erosion and erosion-abrasion. Caries Res. 2011, 45, 581–589. [Google Scholar] [CrossRef]
- Agrawal, N.; Shashikiran, N.D.; Singla, S.; Ravi, K.S.; Kulkarni, V.K. Effect of remineralizing agents on surface microhardness of primary and permanent teeth after erosion. J. Dent. Child. 2014, 81, 117–121. [Google Scholar]
- Dehghan, M.; Stanley, P.J.; Tantbirojn, D.; Versluis, A. Investigation of treatment options to minimize the effects of acid erosion on enamel. Gen. Dent. 2014, 62, e30–e33. [Google Scholar]
| ED | Control | p Value | ||
|---|---|---|---|---|
| Number of subjects (all female) | Sample statistic | 14 | 10 | |
| Age (years) | Mean ± SD | 33 ± 13 | 33 ± 10 | 0.90 (1) |
| Weight (lb) | Mean ± SD | 177 ± 71 | 166 ± 21 | 0.60 (1) |
| Ethnicity | Caucasian | 10 | 2 | |
| Black | 2 | 8 | ||
| Others | 2 | |||
| Duration of ED (years) | Mean ± SD | 17 ± 13 | 0 | |
| Active purging (vomiting) | percent | 64 | 0 | 0.002 (3) |
| Taking antidepressant | percent | 64 | 10 | 0.02 (3) |
| Taking antipsychotic | percent | 21 | 0 | 0.24 (3) |
| Have acid reflux | percent | 36 | 0 | 0.06 (3) |
| Take acidic drinks | percent | 93 | 100 | 0.99 (3) |
| Brushing after purging (vomiting) | percent | 31 | n/a | n/a |
| Feeling of dry mouth | percent | 15 | 20 | 0.99 (3) |
| DMFT | Mean ± SD | 11.57 ± 5.83 | 4.70 ± 4.72 | 0.006 (1) |
| Number of decayed teeth | Mean ± SD | 3.64 ± 3.89 | 2.30 ± 2.91 | 0.37 (1) |
| Number of filled teeth | Mean ± SD | 7.07 ± 4.18 | 2.40 ± 4.55 | 0.02 (1) |
| Number of missing teeth | Mean ± SD | 1.09 ± 2.47 | 0 | n/a |
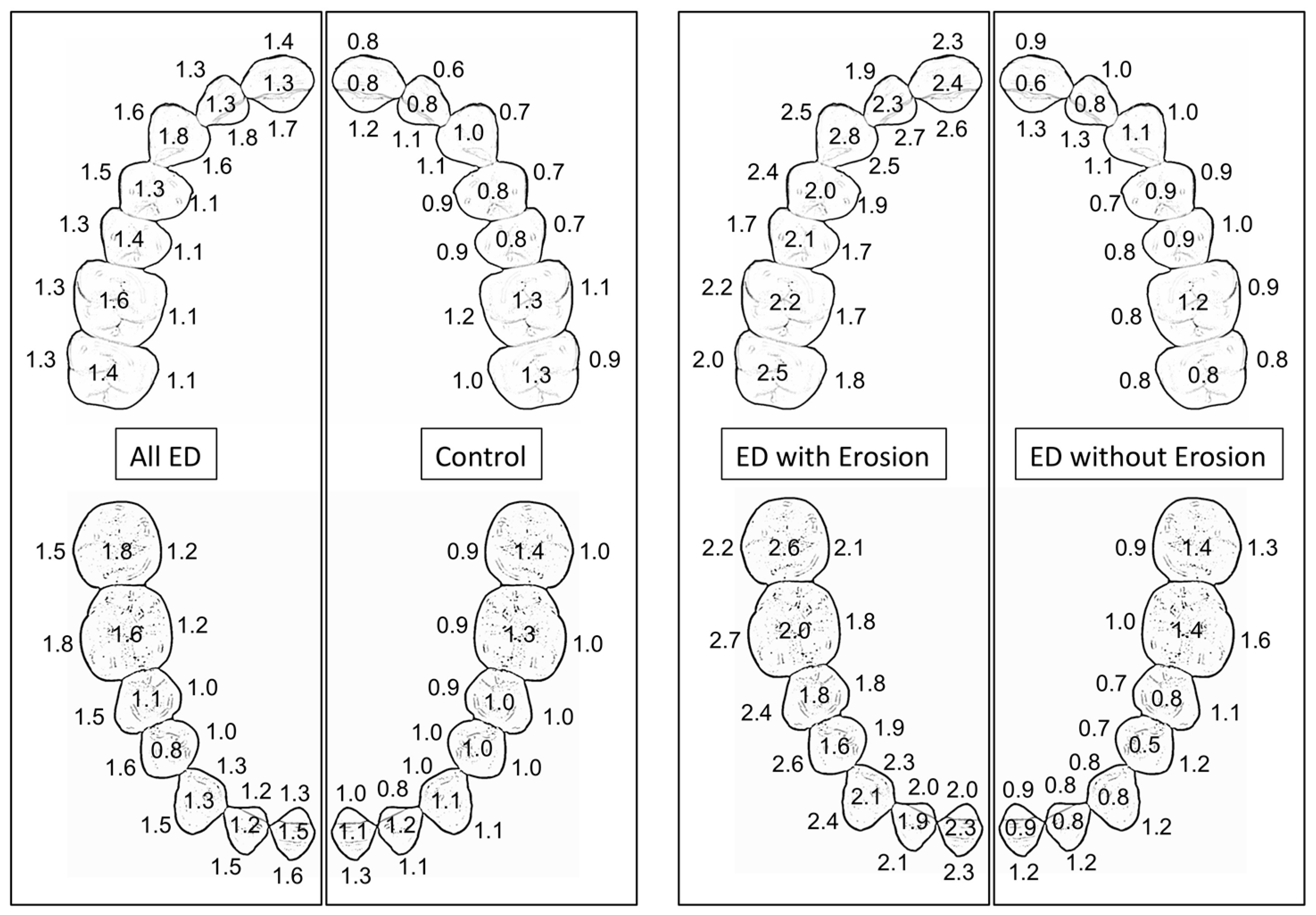
| Tooth erosion score (BEWE) | Mean ± SD | 1.38 ± 0.69 | 0.96 ± 0.38 | 0.08 (2) |
| ED | Control | p Value | |
|---|---|---|---|
| Number of subjects | 12 | 10 | |
| Resting saliva flow rate (mL/min) | 0.44 ± 0.26 | 0.61 ± 0.35 | 0.22 |
| Stimulated saliva flow rate (mL/min) | 1.80 ± 0.75 | 3.01 ± 1.30 | 0.02 |
| Resting saliva pH | 6.77 ± 0.44 | 6.92 ± 0.42 | 0.41 |
| Stimulated saliva pH | 7.25 ± 0.29 | 7.30 ± 0.30 | 0.66 |
| Buffering capacity (stimulated saliva) | 9.67 ± 2.81 | 10.80 ± 1.81 | 0.29 |
| ED with Extensive Tooth Erosion | ED without Extensive Tooth Erosion | |
|---|---|---|
| Tooth erosion score (BEWE) | 2.16 ± 0.20 | 0.95 ± 0.41 |
| Number of teeth with BEWE score ‘3’ per subject | 14.80 ± 4.02 | 0.22 ± 0.67 |
| Age (years) | 41 ± 6 | 32 ± 14 |
| Weight (lb) | 146 ± 42 | 202 ± 77 |
| Duration of ED (years) | 23 ± 4 | 14 ± 15 |
| Active purging (vomiting) (%) | 60 | 67 |
| Taking antidepressants (%) | 80 | 56 |
| Taking antipsychotic (%) | 20 | 22 |
| Have acid reflux (%) | 60 | 22 |
| Take acidic drinks (%) | 100 | 89 |
| Brushing after purging (vomiting) (%) | 80 | 0 |
| Feeling of dry mouth (%) | 20 | 13 |
| Resting saliva flow rate (mL/min) | 0.26 ± 0.10 | 0.53 ± 0.27 |
| Stimulated saliva flow rate (mL/min) | 1.70 ± 0.54 | 1.86 ± 0.87 |
| Resting saliva pH | 6.46 ± 0.60 | 6.92 ± 0.26 |
| Stimulated saliva pH | 7.20 ± 0.23 | 7.27 ± 0.33 |
| Buffering capacity | 11.75 ± 0.50 | 8.63 ± 2.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dehghan, M.; Tantbirojn, D.; Harrison, J.; Stewart, C.W.; Johnson, N.; Tolley, E.A.; Zhang, Y.H. Oral Health and Behavior Patterns of Women with Eating Disorders—A Clinical Pilot Study. Life 2023, 13, 2297. https://doi.org/10.3390/life13122297
Dehghan M, Tantbirojn D, Harrison J, Stewart CW, Johnson N, Tolley EA, Zhang YH. Oral Health and Behavior Patterns of Women with Eating Disorders—A Clinical Pilot Study. Life. 2023; 13(12):2297. https://doi.org/10.3390/life13122297
Chicago/Turabian StyleDehghan, Mojdeh, Daranee Tantbirojn, Janet Harrison, Colette W. Stewart, Nancy Johnson, Elizabeth A. Tolley, and Yanhui H. Zhang. 2023. "Oral Health and Behavior Patterns of Women with Eating Disorders—A Clinical Pilot Study" Life 13, no. 12: 2297. https://doi.org/10.3390/life13122297
APA StyleDehghan, M., Tantbirojn, D., Harrison, J., Stewart, C. W., Johnson, N., Tolley, E. A., & Zhang, Y. H. (2023). Oral Health and Behavior Patterns of Women with Eating Disorders—A Clinical Pilot Study. Life, 13(12), 2297. https://doi.org/10.3390/life13122297






