Imaging of Strictures in Crohn’s Disease
Abstract
:1. Introduction
2. What Is the Best Technique to Request for the Evaluation of a Patient with Small Bowel Stricture?
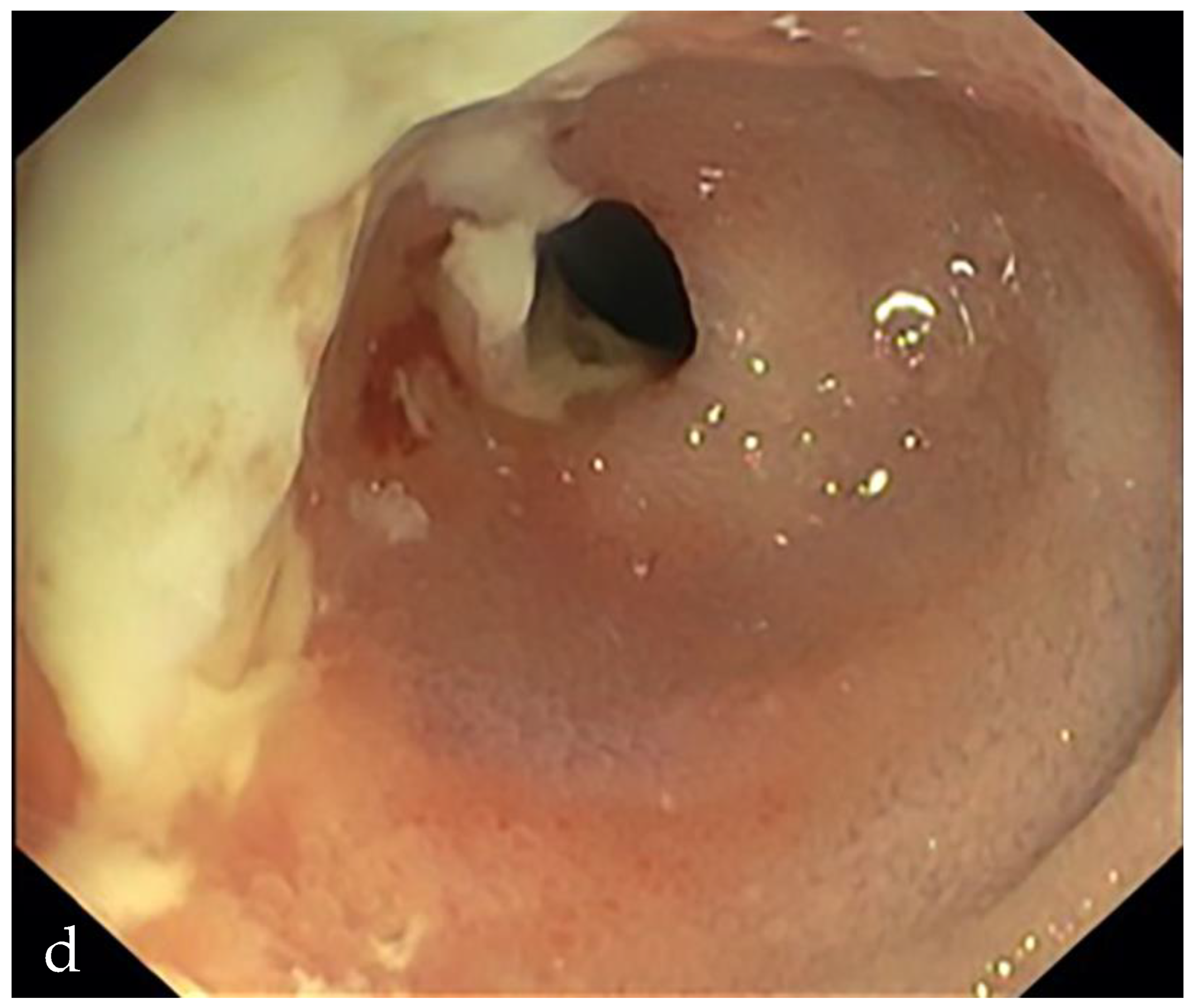
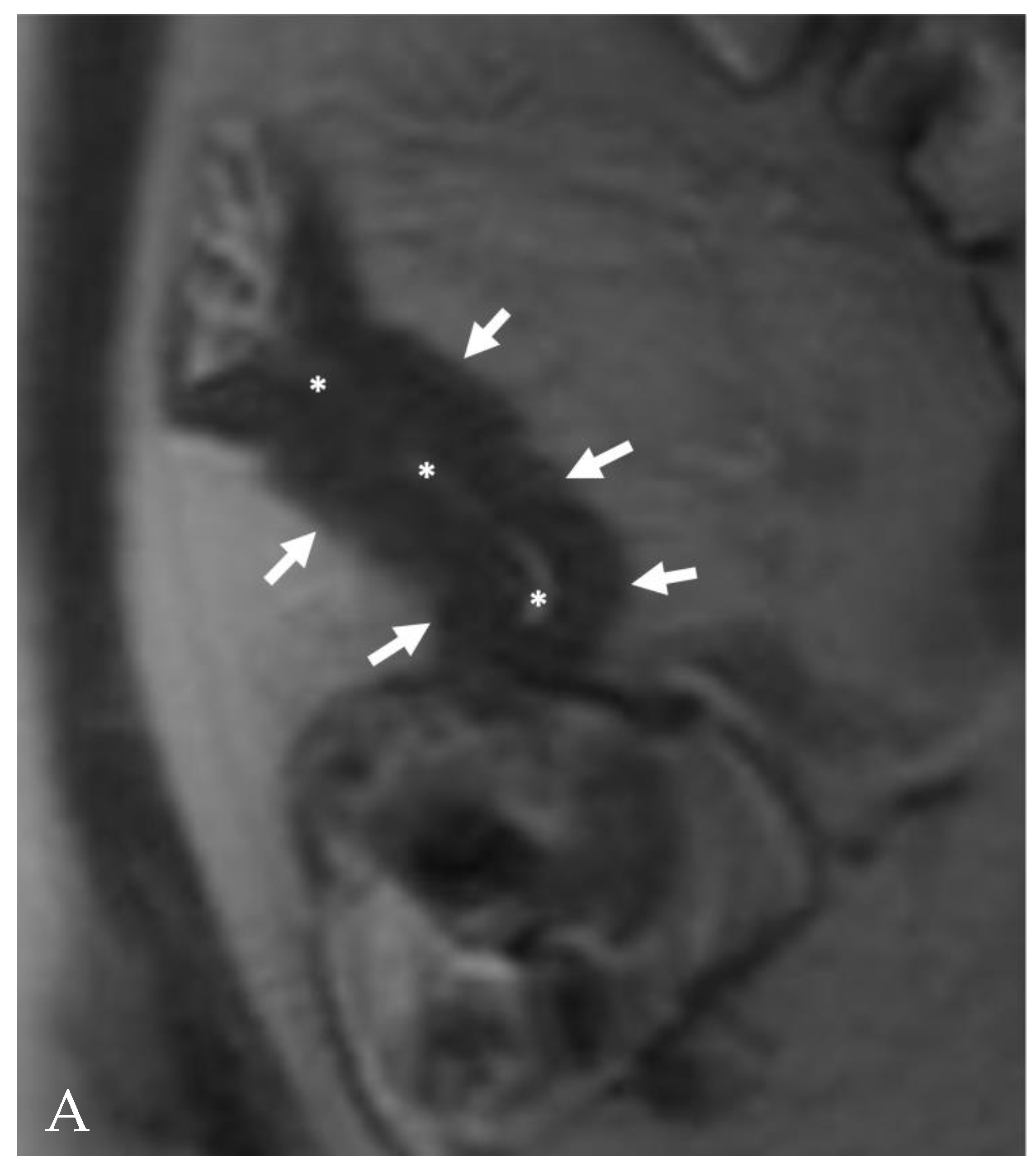
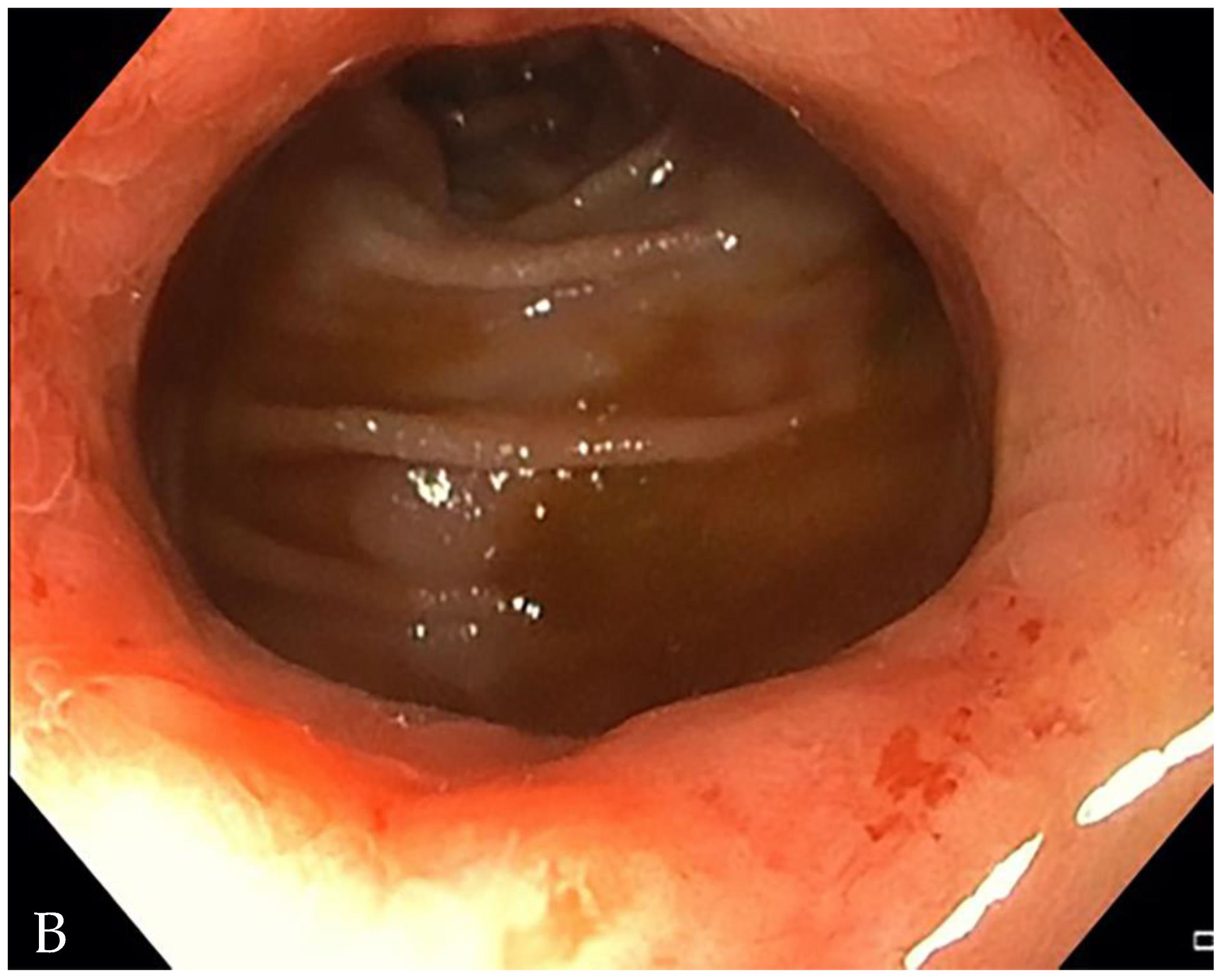
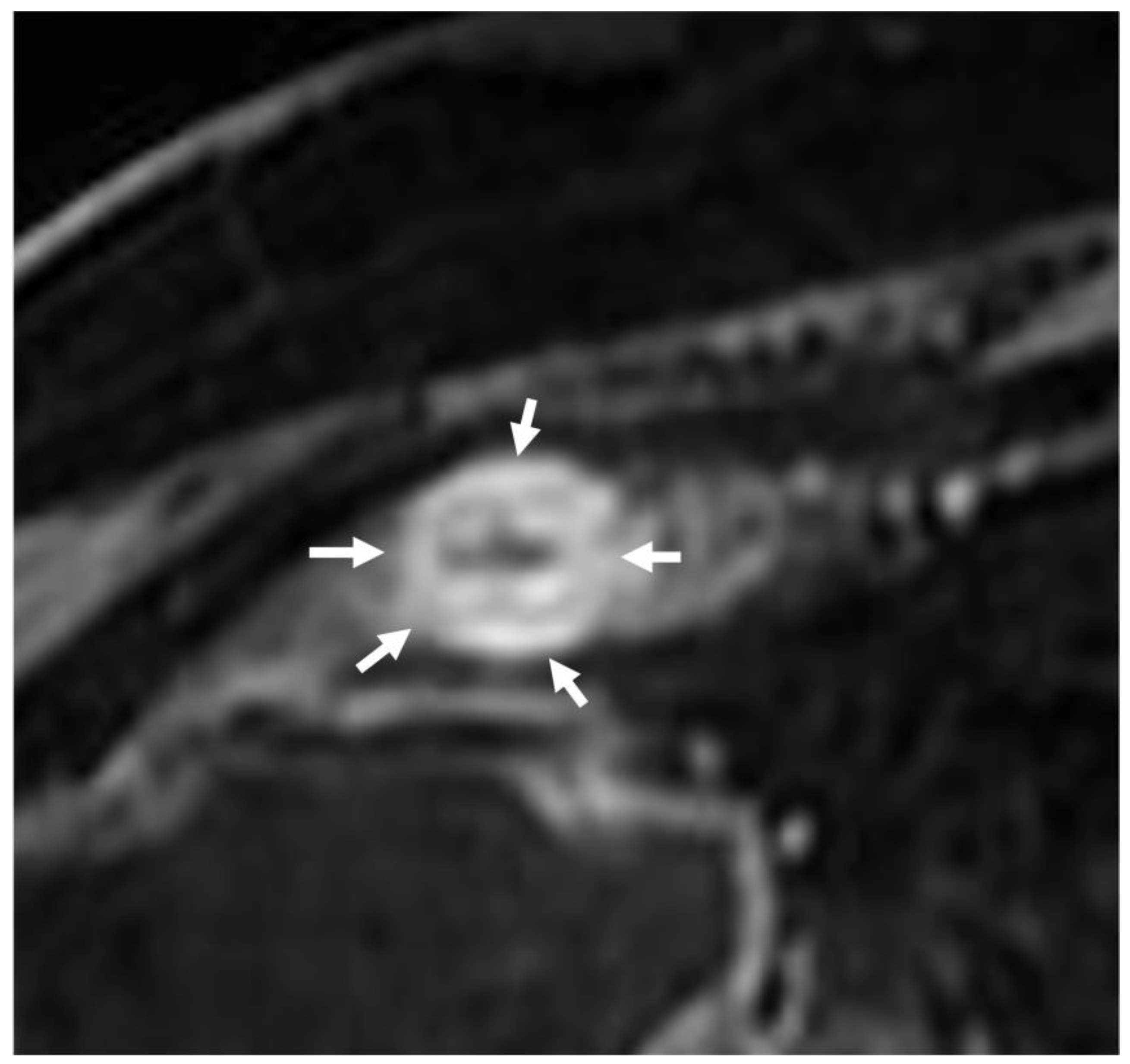
3. Is There a Small Bowel Stricture?
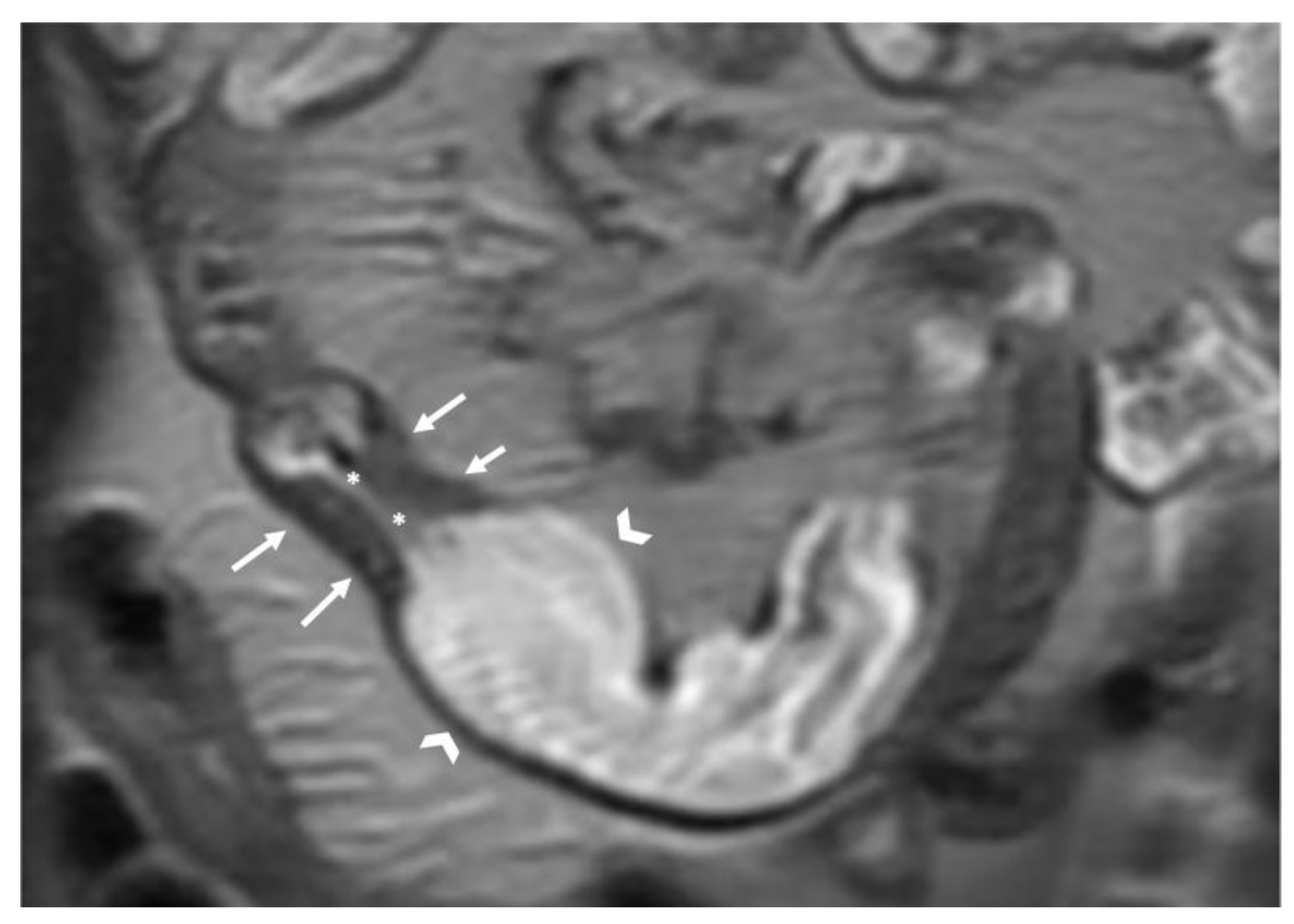
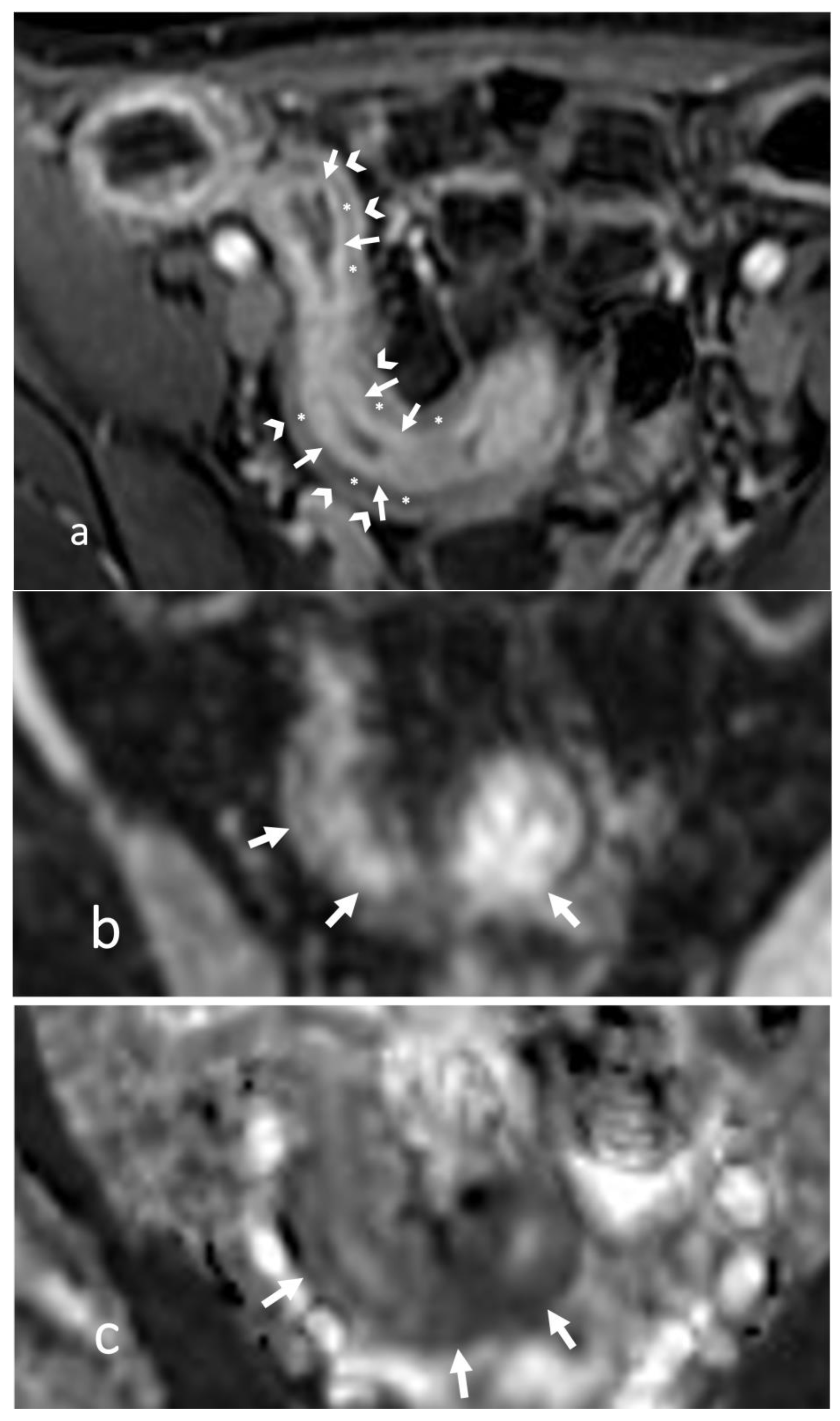
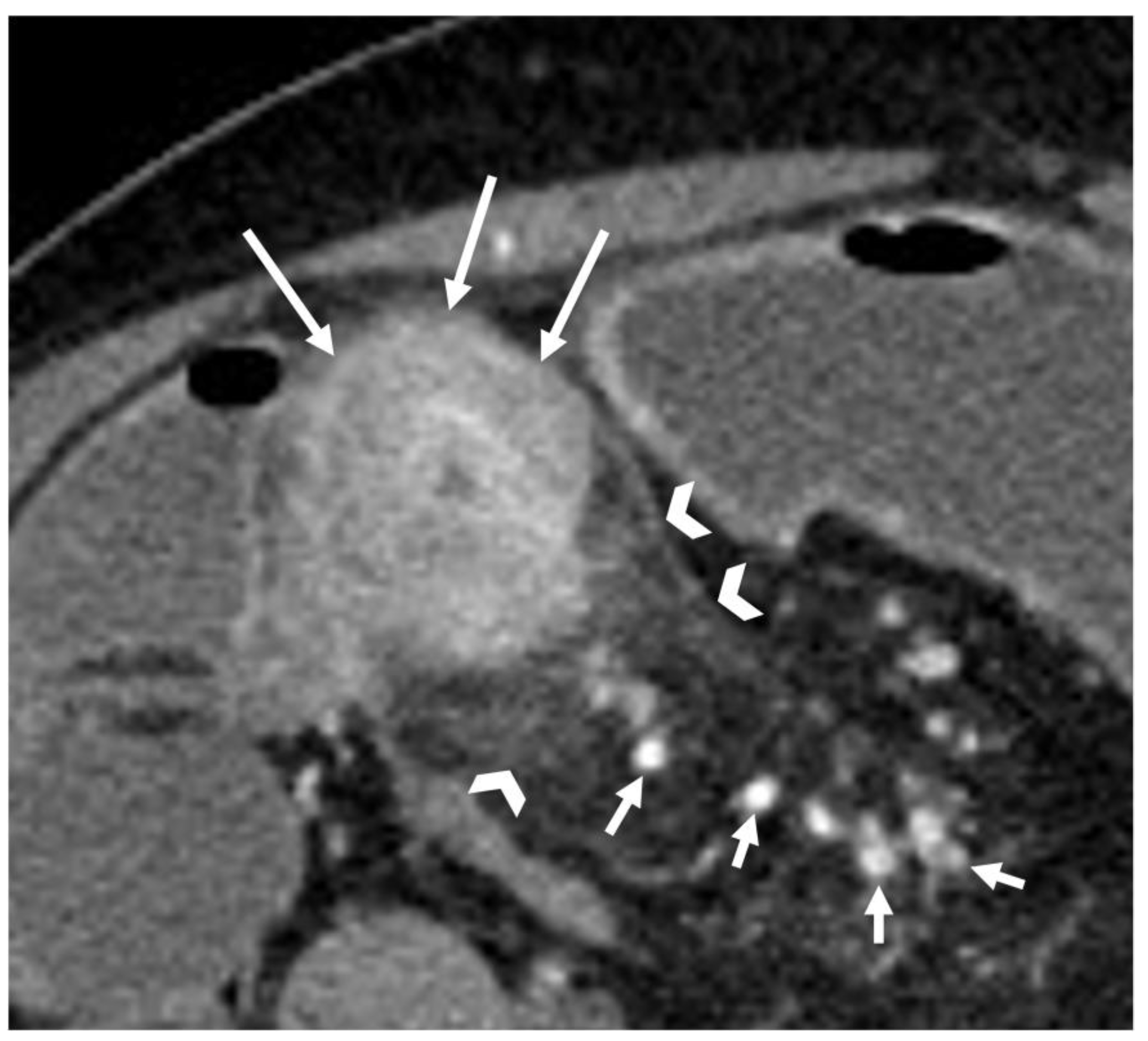
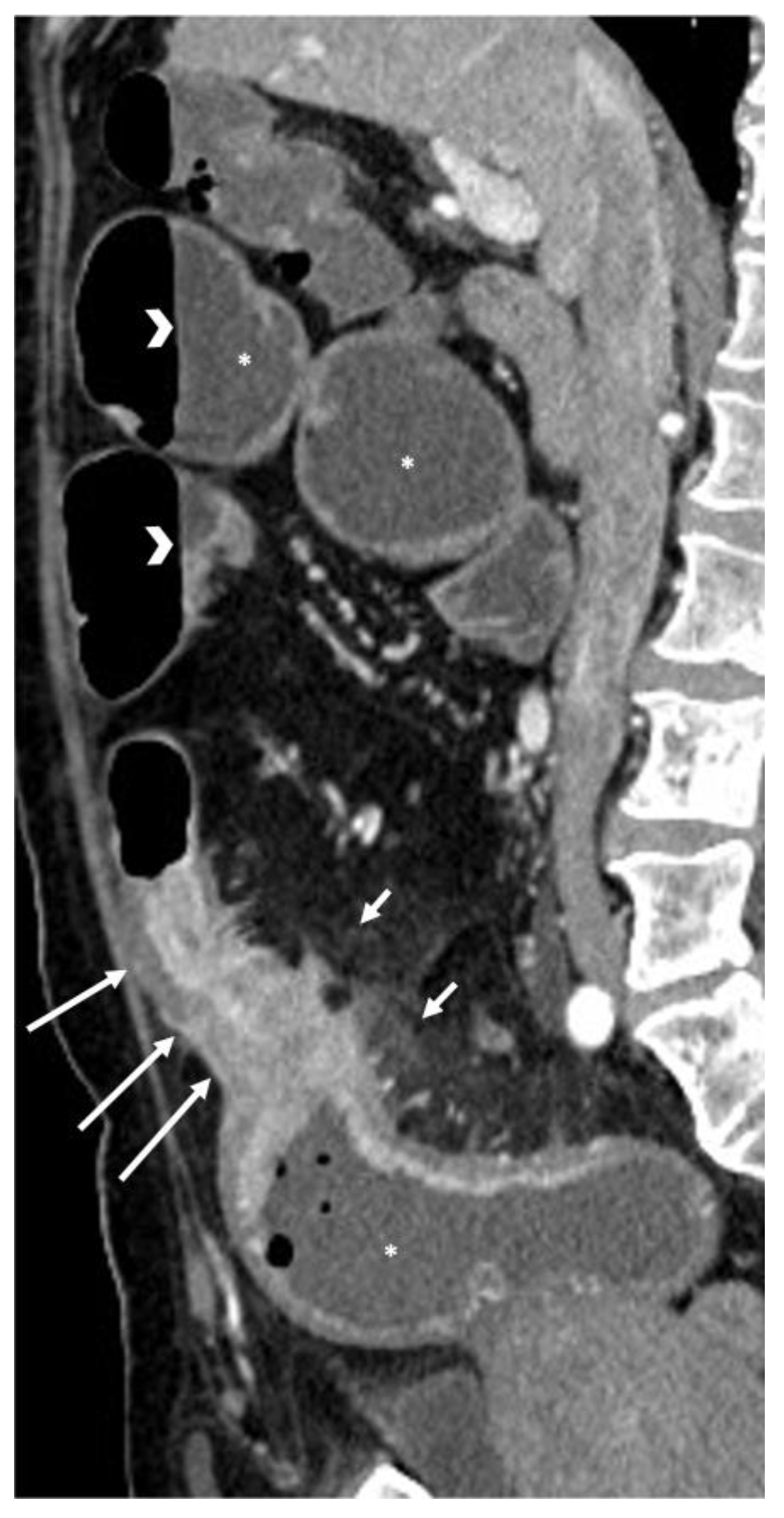
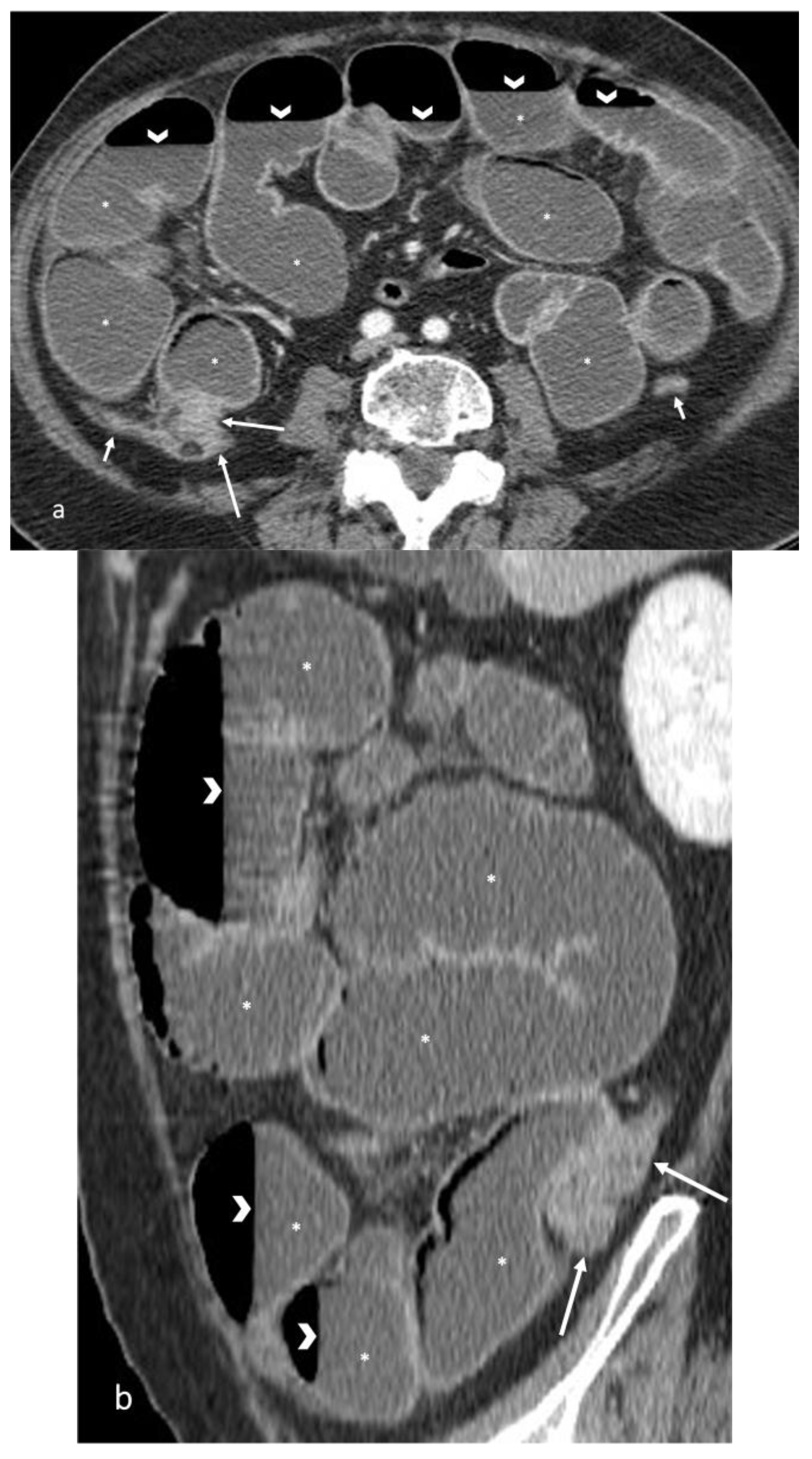
- The wall of a small bowel loop is defined thickened when its thickness (measured from mucosal to serosal layers) is more than 3 mm. According to the degree of thickness, small bowel thickening is defined as “mild” if the thickness is less than 1 cm, “moderate” if it is between 1 cm and 2 cm, and “marked” if it is more than 2 cm [20,21].
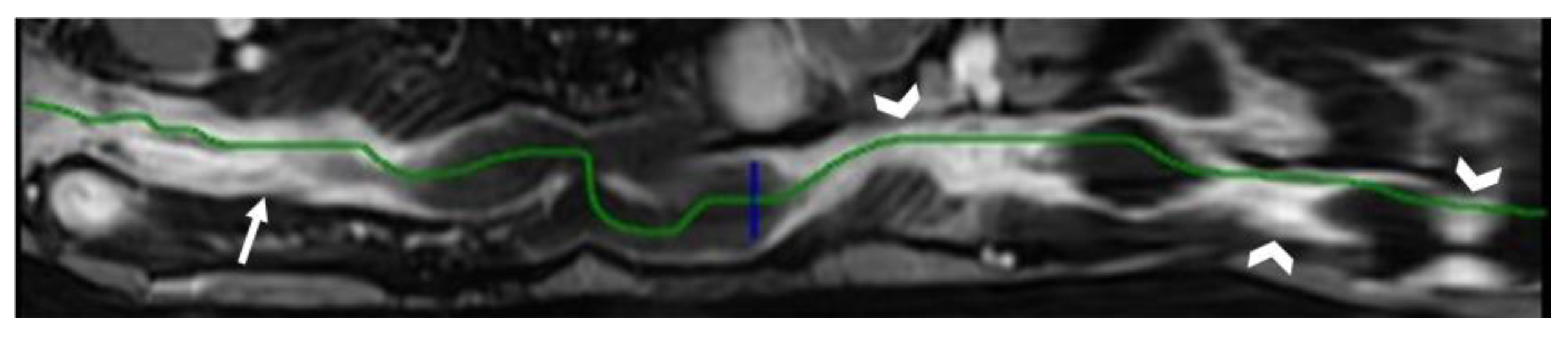
- Regarding the length of the bowel tract affected, a stricture is considered focal if it is less than 5 cm long, segmental if it is between 6 and 40 cm, and diffuse if it is longer than 40 cm [20,21,22]. In the case of multiple stenotic tracts, the healthy tracts interposed between the stenotic ones must not be considered.
- The minimum caliber of the lumen of the bowel loop affected by the stricture is typically considered pathological if it is less than 10 mm at the site of bowel wall thickening or if it is less than 50% compared to the adjacent bowel tracts [23]. Obviously, a correct valuation of this caliber presupposes good bowel preparation, in order to exclude false positives due to inadequate bowel loop distension.
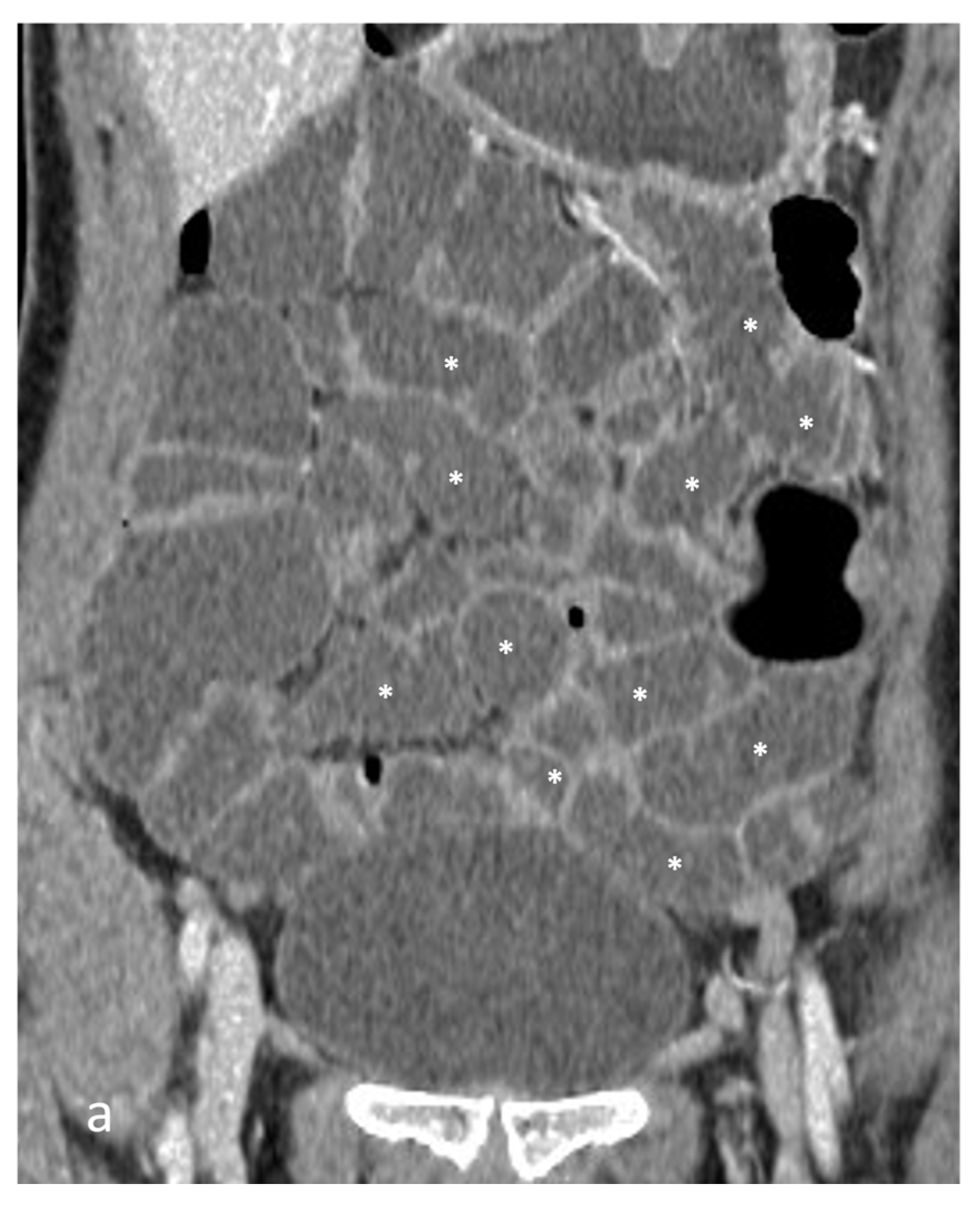
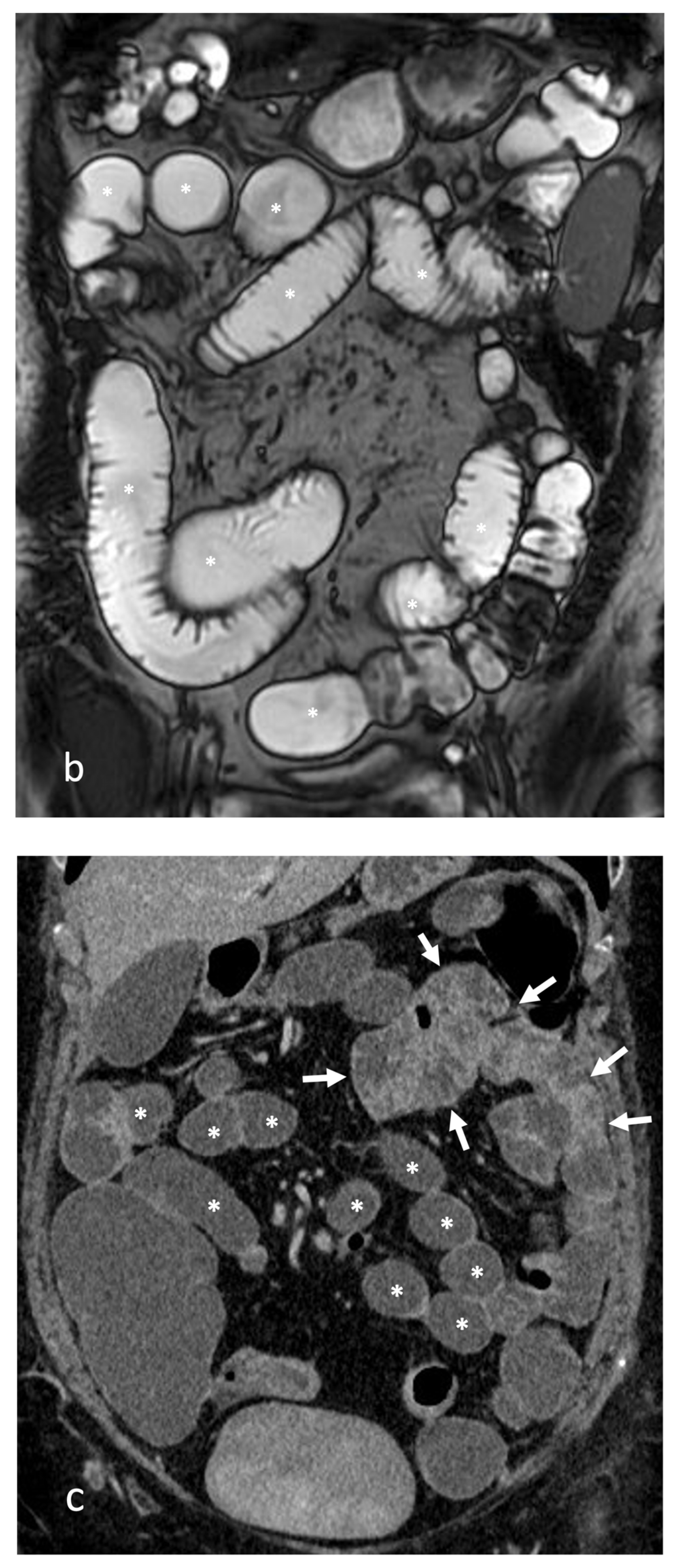
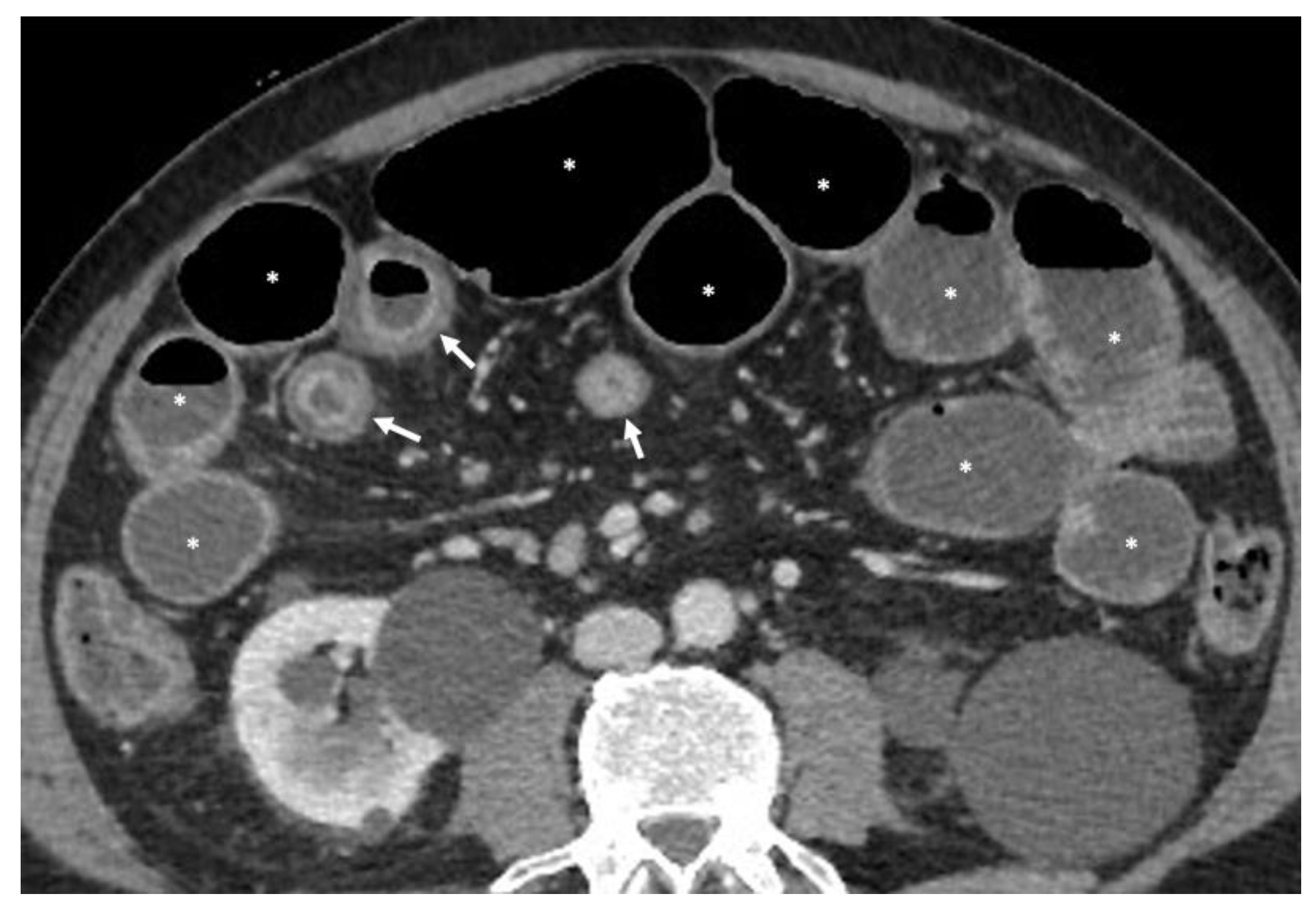
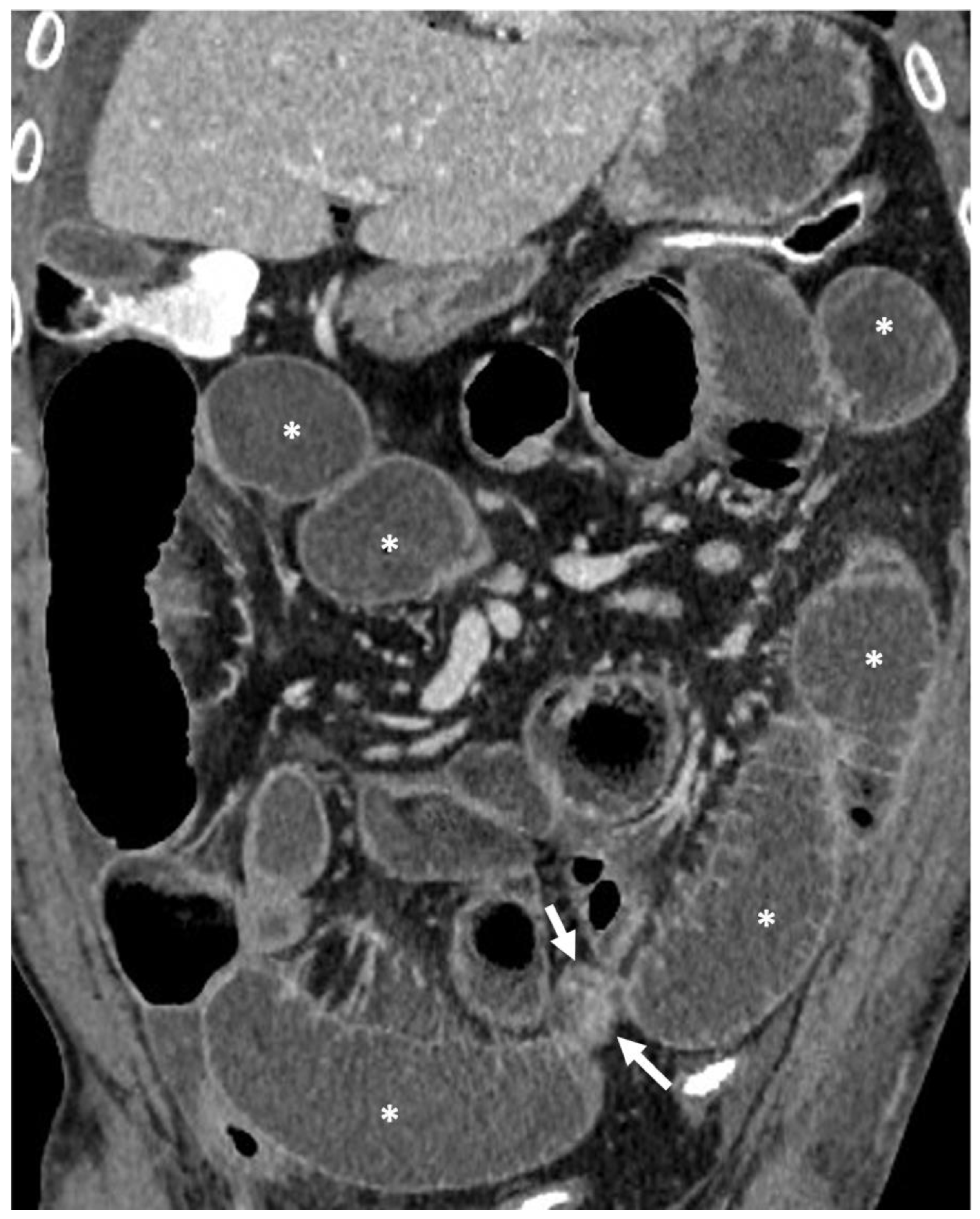
- A normal bowel loop caliber ranges between 2 and 2.5 cm. A bowel lumen is dilated when it has a maximum diameter greater than 2.5–3 cm. The dilation is mild when the upstream lumen is dilated up to 4 cm and severe when it is more than 4 cm (Figure 3) [24]. Pre-stenotic bowel dilatation should always be checked, as it is a sign related to bowel obstruction. Moderate to severe stenosis was determined via double-contrast imaging (conventional barium study) with a sufficient amount of injected air, and stenosis was defined as stenosis in which the lumen was less than one half that of neighboring healthy intestine [25].
4. Where Is the Stricture?
5. How Extensive Is the Disease?
6. Is the Stricture Fibrotic or Inflammatory?
7. Is There Another Cause of Stricture?
8. What Evaluation to Use after Treatment?
9. Discussion and Conclusions
- Previous surgery: no, yes. If yes, indicate type.
- Wall thickening: no, yes. If present, report:
- -
- Location: proximal jejunum, distal jejunum, proximal ileum, distal ileum, last ileal loop;
- -
- Type: symmetric-asymmetric;
- -
- Degree: mild (<1 cm), moderate (1–2 cm), marked (>2 cm);
- -
- Distribution: focal (<5 cm), segmental (6–40 cm), diffuse (<40 cm), indicate length;
- -
- Type of CE after contrast medium: stratified, homogeneous, non-homogeneous, fatty halo sign.
- Presence of stenosis: no, yes. If present, indicate lumen caliber.
- Upstream loop dilation: no, yes. If present, indicate caliber of the most dilated loop.
- Other findings:
- Desmoplastic reaction: no, yes.
- Lymph nodes: no, yes. If present, report location, morphological characteristics (short axis, CE, necrosis, etc.).
- Endo-abdominal fluid flaps: no, yes.
- Fistulae: no, yes. If present, indicate type (entero-enteric, entero-cutaneous, other).
- Sinus tract: no, yes.
- Abscesses/phlegmons: no, yes.If present, report location and diameters.
- Fibro-adipose proliferation: no, yes.
- Loco-regional hypervascularity: no, yes.
Author Contributions
Funding
Conflicts of Interest
References
- Rieder, F.; Latella, G.; Magro, F.; Yuksel, E.S.; Higgins, P.D.R.; Di Sabatino, A.; de Bruyn, J.R.; Rimola, J.; Brito, J.; Bettenworth, D.; et al. European Crohn’s and Colitis Organisation Topical Review on Prediction, Diagnosis and Management of Fibrostenosing Crohn’s Disease. J. Crohn’s Colitis 2016, 10, 873–885. [Google Scholar] [CrossRef]
- Maaser, C.; Sturm, A.; Vavricka, S.R.; Kucharzik, T.; Fiorino, G.; Annese, V.; Calabrese, E.; Baumgart, D.C.; Bettenworth, D.; Borralho Nunes, P.; et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J. Crohn’s Colitis 2019, 13, 144–164. [Google Scholar] [CrossRef]
- Simian, D. Inflammatory bowel disease: A descriptive study of 716 local Chilean patients. World J. Gastroenterol. 2016, 22, 5267. [Google Scholar] [CrossRef]
- Chan, W.P.W.; Mourad, F.; Leong, R.W.L. Crohn’s disease associated strictures. J. Gastroenterol. Hepatol. 2018, 33, 998–1008. [Google Scholar] [CrossRef]
- Minordi, L.M.; Vecchioli, A.; Mirk, P.; Bonomo, L. CT enterography with polyethylene glycol solution vs CT enteroclysis in small bowel disease. Br. J. Radiol. 2011, 84, 112–119. [Google Scholar] [CrossRef]
- Khatri, G.; Coleman, J.; Leyendecker, J.R. Magnetic Resonance Enterography for Inflammatory and Noninflammatory Conditions of the Small Bowel. Radiol. Clin. N. Am. 2018, 56, 671–689. [Google Scholar] [CrossRef]
- Chavoshi, M.; Mirshahvalad, S.A.; Kasaeian, A.; Djalalinia, S.; Kolahdoozan, S.; Radmard, A.R. Diagnostic Accuracy of Magnetic Resonance Enterography in the Evaluation of Colonic Abnormalities in Crohn’s Disease: A Systematic Review and Meta-Analysis. Acad. Radiol. 2021, 28, S192–S202. [Google Scholar] [CrossRef]
- Schmidt, S.A.; Baumann, J.A.; Stanescu-Siegmund, N.; Froehlich, E.; Brambs, H.J.; Juchems, M.S. Oral distension methods for small bowel MRI: Comparison of different agents to optimize bowel distension. Acta Radiol. 2016, 57, 1460–1467. [Google Scholar] [CrossRef]
- Sinha, R.; Rawat, S. MRI enterography with divided dose oral preparation: Effect on bowel distension and diagnostic quality. Indian J. Radiol. Imaging 2013, 23, 86–91. [Google Scholar] [CrossRef]
- Rieder, F.; Zimmermann, E.M.; Remzi, F.H.; Sandborn, W.J. Crohn’s disease complicated by strictures: A systematic review. Gut 2013, 62, 1072–1084. [Google Scholar] [CrossRef]
- Sleiman, J.; Chirra, P.; Gandhi, N.S.; Baker, M.E.; Lu, C.; Gordon, I.O.; Viswanath, S.E.; Rieder, F. Crohn’s disease related strictures in cross-sectional imaging: More than meets the eye? United Eur. Gastroenterol. J. 2022, 10, 1167–1178. [Google Scholar] [CrossRef]
- Colombel, J.F.; Solem, C.A.; Sandborn, W.J.; Booya, F.; Loftus, E.V.; Harmsen, W.S.; Zinsmeister, A.R.; Bodily, K.D.; Fletcher, J.G. Quantitative measurement and visual assessment of ileal Crohn’s disease activity by computed tomography enterography: Correlation with endoscopic severity and C reactive protein. Gut 2006, 55, 1561–1567. [Google Scholar] [CrossRef]
- Solem, C.A.; Loftus, E.V.; Fletcher, J.G.; Baron, T.H.; Gostout, C.J.; Petersen, B.T.; Tremaine, W.J.; Egan, L.J.; Faubion, W.A.; Schroeder, K.W.; et al. Small-bowel imaging in Crohn’s disease: A prospective, blinded, 4-way comparison trial. Gastrointest. Endosc. 2008, 68, 255–266. [Google Scholar] [CrossRef]
- Voderholzer, W.A. Small bowel involvement in Crohn’s disease: A prospective comparison of wireless capsule endoscopy and computed tomography enteroclysis. Gut 2005, 54, 369–373. [Google Scholar] [CrossRef]
- Fiorino, G.; Bonifacio, C.; Peyrin-Biroulet, L.; Minuti, F.; Repici, A.; Spinelli, A.; Fries, W.; Balzarini, L.; Montorsi, M.; Malesci, A.; et al. Prospective comparison of computed tomography enterography and magnetic resonance enterography for assessment of disease activity and complications in ileocolonic Crohn’s disease. Inflamm. Bowel Dis. 2011, 17, 1073–1080. [Google Scholar] [CrossRef]
- Turetschek, K.; Schober, E.; Wunderbaldinger, P.; Bernhard, C.; Schima, W.; Puespoek, A.; Vogelsang, H.; Moeschl, P.; Mostbeck, G. Findings at Helical CT–Enteroclysis in Symptomatic Patients with Crohn Disease: Correlation With Endoscopic and Surgical Findings. J. Comput. Assist. Tomogr. 2002, 26, 488–492. [Google Scholar] [CrossRef]
- Panés, J.; Bouzas, R.; Chaparro, M.; García-Sánchez, V.; Gisbert, J.P.; Martínez De Guereñu, B.; Mendoza, J.L.; Paredes, J.M.; Quiroga, S.; Ripollés, T.; et al. Systematic review: The use of ultrasonography, computed tomography and magnetic resonance imaging for the diagnosis, assessment of activity and abdominal complications of Crohn’s disease. Aliment. Pharmacol. Ther. 2011, 34, 125–145. [Google Scholar] [CrossRef]
- Negaard, A.; Paulsen, V.; Sandvik, L.; Berstad, A.E.; Borthne, A.; Try, K.; Lygren, I.; Storaas, T.; Klow, N.-E. A prospective randomized comparison between two MRI studies of the small bowel in Crohn’s disease, the oral contrast method and MR enteroclysis. Eur. Radiol. 2007, 17, 2294–2301. [Google Scholar] [CrossRef]
- Rieder, F.; Bettenworth, D.; Ma, C.; Parker, C.E.; Williamson, L.A.; Nelson, S.A.; van Assche, G.; Di Sabatino, A.; Bouhnik, Y.; Stidham, R.W.; et al. An expert consensus to standardise definitions, diagnosis and treatment targets for anti-fibrotic stricture therapies in Crohn’s disease. Aliment. Pharmacol. Ther. 2018, 48, 347–357. [Google Scholar] [CrossRef]
- Minordi, L.M.; Larosa, L.; Barbaro, B.; Angelino, A.; Broglia, D.; Cipri, C.; Scaldaferri, F.; Manfredi, R.; Natale, L. How the Radiologist Must Reason for a Correct Diagnosis in Patients with Small Bowel Mural Thickening Studied by CT or MRI: A Pictorial Review. Curr. Probl. Diagn. Radiol. 2023, 52, 393–411. [Google Scholar] [CrossRef]
- Akcalar, S.; Turkbey, B.; Karcaaltincaba, M.; Akpinar, E.; Akhan, O. Small bowel wall thickening: MDCT evaluation in the emergency room. Emerg. Radiol. 2011, 18, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.; Oliveira, M.I.; Castro, R.; Araújo, B.; Viamonte, B.; Cunha, R. Bowel wall thickening at CT: Simplifying the diagnosis. Insights Imaging 2014, 5, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Bettenworth, D.; Nowacki, T.M.; Cordes, F.; Buerke, B.; Lenze, F. Assessment of stricturing Crohn’s disease: Current clinical practice and future avenues 2016 Inflammatory Bowel Disease: Global view. World J. Gastroenterol. 2016, 22, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Bruining, D.H.; Zimmermann, E.M.; Loftus, E.V.; Sandborn, W.J.; Sauer, C.G.; Strong, S.A.; Fletcher, J.G. Consensus recommendations for evaluation, interpretation, and utilization of computed tomography and magnetic resonance enterography in patients with small bowel Crohn’s disease. Radiology 2018, 286, 776–799. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Matsui, T.; Yano, Y.; Tsurumi, K.; Okado, Y.; Matsushima, Y.; Koga, A.; Takahashi, H.; Ninomiya, K.; Ono, Y.; et al. Long-term course of Crohn’s disease in Japan: Incidence of complications, cumulative rate of initial surgery, and risk factors at diagnosis for initial surgery. J. Gastroenterol. Hepatol. 2015, 30, 1713–1719. [Google Scholar] [CrossRef] [PubMed]
- Morar, P.S.; Faiz, O.; Warusavitarne, J.; Brown, S.; Cohen, R.; Hind, D.; Abercrombie, J.; Ragunath, K.; Sanders, D.S.; Arnott, I.; et al. Systematic review with meta-analysis: Endoscopic balloon dilatation for Crohn’s disease strictures. Aliment. Pharmacol. Ther. 2015, 42, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Minordi, L.M.; Larosa, L.; Brizi, M.G.; Armuzi, A.; Manfredi, R. Length of the healthy and pathological small intestine in patients with Crohn’s disease: Calculations using computed tomography and magnetic resonance enterography. Diagn. Interv. Radiol. 2023, 29, 24–28. [Google Scholar] [CrossRef]
- Venkatasamy, A.; Minault, Q.; Veillon, F. Fat halo sign. Abdom. Radiol. 2018, 43, 2533–2534. [Google Scholar] [CrossRef]
- Punwani, S.; Rodriguez-Justo, M.; Bainbridge, A.; Greenhalgh, R.; De Vita, E.; Bloom, S.; Cohen, R.; Windsor, A.; Obichere, A.; Hansmann, A.; et al. Mural Inflammation in Crohn Disease: Location-Matched Histologic Validation of MR Imaging Features. Radiology 2009, 252, 712–720. [Google Scholar] [CrossRef]
- Foti, P.V.; Travali, M.; Farina, R.; Palmucci, S.; Coronella, M.; Spatola, C.; Puzzo, L.; Garro, R.; Inserra, G.; Riguccio, G.; et al. Can Conventional and Diffusion-Weighted MR Enterography Biomarkers Differentiate Inflammatory from Fibrotic Strictures in Crohn’s Disease? Medicina 2021, 57, 265. [Google Scholar] [CrossRef]
- Minordi, L.M.; Bevere, A.; Papa, A.; Larosa, L.; Manfredi, R. CT and MRI Evaluations in Crohn’s Complications: A Guide for the Radiologist. Acad. Radiol. 2022, 29, 1206–1227. [Google Scholar] [CrossRef] [PubMed]
- Du, J.F.; Lu, B.L.; Huang, S.Y.; Mao, R.; Zhang, Z.W.; Cao, Q.H.; Chen, Z.H.; Li, S.Y.; Qin, Q.L.; Sun, C.H.; et al. A novel identification system combining diffusion kurtosis imaging with conventional magnetic resonance imaging to assess intestinal strictures in patients with Crohn’s disease. Abdom. Radiol. 2021, 46, 936–947. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.N.; Li, X.H.; Lin, J.J.; Huang, S.Y.; Cao, Q.H.; Chen, Z.H.; Sun, C.H.; Zhang, Z.W.; Rieder, F.; Rimola, J.; et al. Magnetisation transfer imaging adds information to conventional MRIs to differentiate inflammatory from fibrotic components of small intestinal strictures in Crohn’s disease. Eur. Radiol. 2020, 30, 1938–1947. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.K.; Lu, B.L.; Huang, S.Y.; Chen, Y.J.; Li, Z.P.; Rimola, J. Cross-sectional imaging for assessing intestinal fibrosis in Crohn’s disease. J. Dig. Dis. 2020, 21, 342–350. [Google Scholar] [CrossRef]
- Coimbra, A.; Rimola, J.; Cuatrecasas, M.; De Hertogh, G.; Van Assche, G.; Vanslembrouck, R.; Glerup, H.; Nielsen, A.H.; Hagemann-Madsen, R.; Bouhnik, Y.; et al. Magnetic Resonance Enterography and Histology in Patients with Fibrostenotic Crohn’s Disease: A Multicenter Study. Clin. Transl. Gastroenterol. 2022, 13, e00505. [Google Scholar] [CrossRef]
- Fornasa, F.; Benassuti, C.; Benazzato, L. Role of magnetic resonance enterography in differentiating between fibrotic and active inflammatory small bowel stenosis in patients with crohn’s disease. J. Clin. Imaging Sci. 2011, 1, 35. [Google Scholar] [CrossRef]
- Rami Reddy, S.R.; Cappell, M.S. A Systematic Review of the Clinical Presentation, Diagnosis, and Treatment of Small Bowel Obstruction. Curr. Gastroenterol. Rep. 2017, 19, 28. [Google Scholar] [CrossRef]
- Tay, G.S.; Binion, D.G.; Eastwood, D.; Otterson, M.F. Multivariate analysis suggests improved perioperative outcome in Crohn’s disease patients receiving immunomodulator therapy after segmental resection and/or strictureplasty. Surgery 2003, 134, 565–572. [Google Scholar] [CrossRef]
- Cosnes, J.; Nion-Larmurier, I.; Beaugerie, L.; Afchain, P.; Tiret, E.; Gendre, J.P. Impact of the increasing use of immunosuppressants in Crohn’s disease on the need for intestinal surgery. Gut 2005, 54, 237–241. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Oussalah, A.; Williet, N.; Pillot, C.; Bresler, L.; Bigard, M.A. Impact of azathioprine and tumour necrosis factor antagonists on the need for surgery in newly diagnosed Crohn’s disease. Gut 2011, 60, 930–936. [Google Scholar] [CrossRef]
- Louis, E.; Boverie, J.; Dewit, O.; Baert, F.; De Vos, M.; D’Haens, G. Treatment of small bowel subocclusive Crohn’s disease with infliximab: An open pilot study. Acta Gastro-Enterol. Belg. 2007, 70, 15–19. [Google Scholar]
- Limketkai, B.N.; Bayless, T.M. Editorial: Can stenosis in ileal Crohn’s disease be prevented by current therapy? Am. J. Gastroenterol. 2013, 108, 1755–1756. [Google Scholar] [CrossRef] [PubMed]
- Reese, G.E.; Nanidis, T.; Borysiewicz, C.; Yamamoto, T.; Orchard, T.; Tekkis, P.P. The effect of smoking after surgery for Crohn’s disease: A meta-analysis of observational studies. Int. J. Colorectal. Dis. 2008, 23, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Scimeca, D.; Mocciaro, F.; Cottone, M.; Montalbano, L.M.; D’Amico, G.; Olivo, M.; Orlando, R.; Orlando, A. Efficacy and safety of endoscopic balloon dilation of symptomatic intestinal Crohn’s disease strictures. Dig Liver Dis. 2011, 43, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Thomas-Gibson, S.; Brooker, J.C.; Hayward, C.M.; Shah, S.G.; Williams, C.B.; Saunders, B.P. Colonoscopic balloon dilation of Crohn’s strictures: A review of long-term outcomes. Eur. J. Gastroenterol. Hepatol. 2003, 15, 485–488. [Google Scholar] [CrossRef]
- Hagel, A.F.; Hahn, A.; Dauth, W.; Matzel, K.; Konturek, P.C.; Neurath, M.F.; Raithel, M. Outcome and complications of endoscopic balloon dilatations in various types of ileocaecal and colonic stenosis in patients with Crohn’s disease. Surg. Endosc. 2014, 10, 2966–2972. [Google Scholar] [CrossRef]
- Hassan, C.; Zullo, A.; De Francesco, V.; Ierardi, E.N.; Giustini, M.; Pitidis, A.; Taggi, F.; Winn, S.; Morini, S. Systematic review: Endoscopic dilatation in Crohn’s disease. Aliment. Pharmacol. Ther. 2007, 26, 1457–1464. [Google Scholar] [CrossRef]
- Ono, Y.; Hirai, F.; Matsui, T.; Beppu, T.; Yano, Y.; Takatsu, N.; Takaki, Y.; Nagahama, T.; Hisabe, T.; Yao, K.; et al. Value of concomitant endoscopic balloon dilation for intestinal stricture during long-term infliximab therapy in patients with Crohn’s disease. Dig. Endosc. 2012, 24, 432–438. [Google Scholar] [CrossRef]
- Froehlich, F.; Juillerat, P.; Pittet, V.; Felley, C.; Mottet, C.; Vader, J.P.; Michetti, P.; Gonvers, J.J. Maintenance of surgically induced remission of Crohn’s disease. Digestion 2007, 76, 130–135. [Google Scholar] [CrossRef]
- Bouguen, G.; Siproudhis, L.; Bretagne, J.-F.; Bigard, M.A.; Peyrin-Biroulet, L. Nonfistulizing perianal Crohn’s disease: Clinical features, epidemiology, and treatment. Inflamm. Bowel Dis. 2010, 16, 1431–1442. [Google Scholar] [CrossRef]
- Geiger, T.M.; Miedema, B.W.; Tsereteli, Z.; Sporn, E.; Thaler, K. Stent placement for benign colonic stenosis: Case report, review of the literature, and animal pilot data. Int. J. Color. Dis. 2008, 23, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
| Sequence | Trade Name | Imaging Plane |
|---|---|---|
| Balanced steady-state free procession (bSSFP) | Balanced FFE/TurboFISP/TrueFISP/FIESTA | Axial and coronal |
| T2-weighted fat-suppressed | FSE/TSE | Axial |
| 3D cinematic bSSFP | Coronal | |
| 3D T1-weighted fat-suppressed post-contrast images at 45 and 75 s | VIBE/LAVA | Coronal |
| Delayed 3D T1-weighted fat-suppressed post-contrast images at 120 s | VIBE/LAVA | Axial |
| Diffusion-weighted imaging (DWI) | Axial | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minordi, L.M.; Larosa, L.; Bevere, A.; D’Angelo, F.B.; Pierro, A.; Cilla, S.; Del Ciello, A.; Scaldaferri, F.; Barbaro, B. Imaging of Strictures in Crohn’s Disease. Life 2023, 13, 2283. https://doi.org/10.3390/life13122283
Minordi LM, Larosa L, Bevere A, D’Angelo FB, Pierro A, Cilla S, Del Ciello A, Scaldaferri F, Barbaro B. Imaging of Strictures in Crohn’s Disease. Life. 2023; 13(12):2283. https://doi.org/10.3390/life13122283
Chicago/Turabian StyleMinordi, Laura Maria, Luigi Larosa, Antonio Bevere, Francesca Bice D’Angelo, Antonio Pierro, Savino Cilla, Annemilia Del Ciello, Franco Scaldaferri, and Brunella Barbaro. 2023. "Imaging of Strictures in Crohn’s Disease" Life 13, no. 12: 2283. https://doi.org/10.3390/life13122283
APA StyleMinordi, L. M., Larosa, L., Bevere, A., D’Angelo, F. B., Pierro, A., Cilla, S., Del Ciello, A., Scaldaferri, F., & Barbaro, B. (2023). Imaging of Strictures in Crohn’s Disease. Life, 13(12), 2283. https://doi.org/10.3390/life13122283





