Infrared Spectral Signatures of Nucleobases in Interstellar Ices I: Purines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Apparatus
2.2. Preparation and Quantification of Purines Embedded in Interstellar Ice Analogues
3. Results
3.1. Mid-Infrared Absorption Spectra of Purines at 300 and 20 K
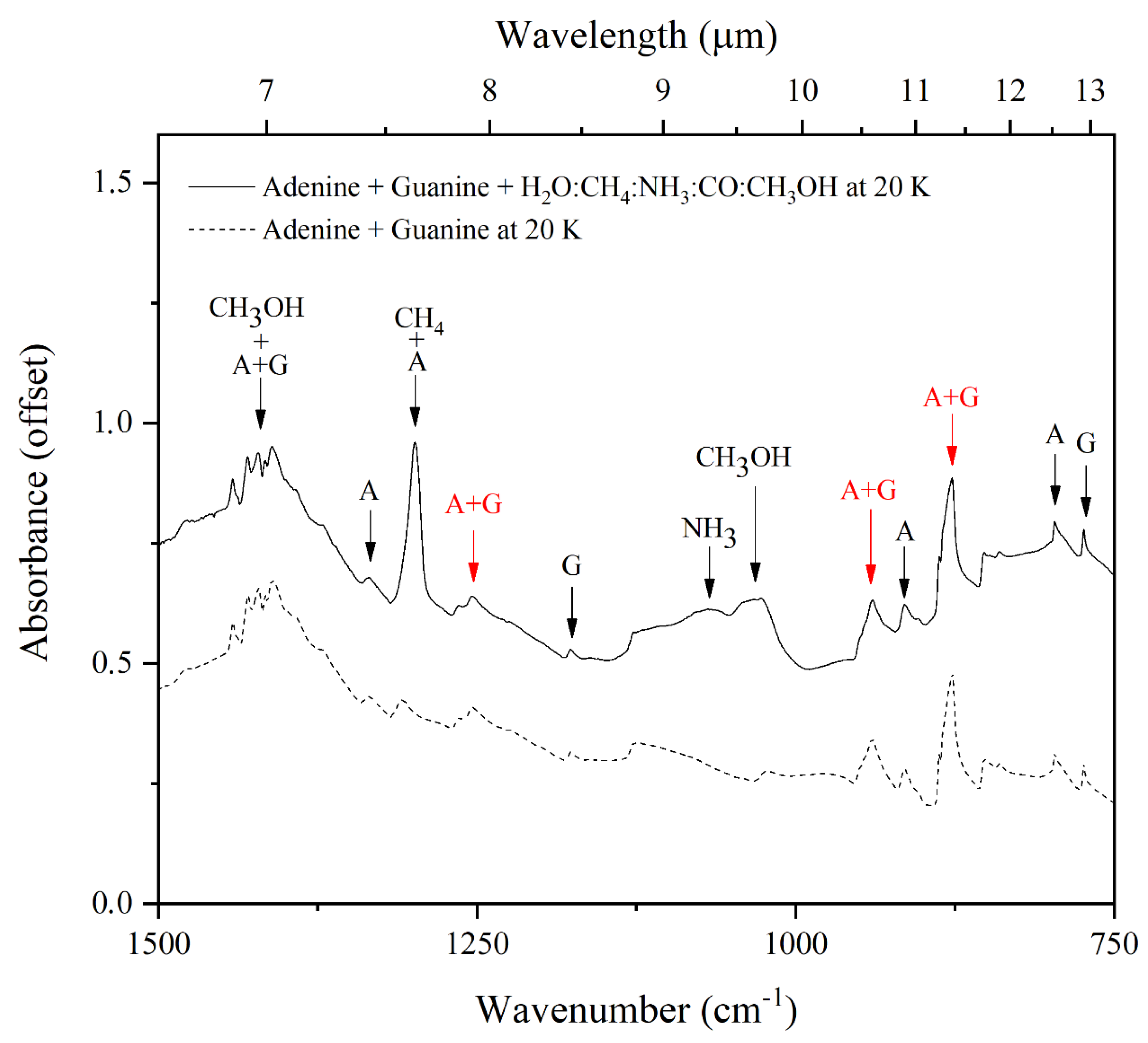
3.2. Mid-Infrared Absorption Spectra of Purines Embedded within Interstellar Ice Analogues at 20 K
4. Astrochemical Implications
| ν (cm−1) | λ (μm) | Assignment † | ψ (km mol−1) |
|---|---|---|---|
| 1255 | 7.97 | βCH (A), βNH (A), and νCN (G) | 23 (A); 16 (G) |
| 940 | 10.64 | γCH (A), β-ring (G), and γC=O (G) | 21 (A); 10 (G) |
| 878 | 11.39 | ring deformation (A) and γNH (G) | 4.1 (A); 19 (G) |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Herbst, E.; van Dishoeck, E.F. Complex organic interstellar molecules. Annu. Rev. Astron. Astrophys. 2009, 47, 427–480. [Google Scholar] [CrossRef]
- Rosemeyer, H. The chemodiversity of purine as a constituent of natural products. Chem. Biodivers. 2004, 1, 361–401. [Google Scholar] [CrossRef]
- McGuire, B.A. 2021 census of interstellar, circumstellar, extragalactic, protoplanetary disk, and exoplanetary molecules. Astrophys. J. Suppl. Ser. 2022, 259, 30. [Google Scholar] [CrossRef]
- Oba, Y.; Takano, Y.; Naraoka, H.; Watanabe, N.; Kouchi, A. Nucleobase synthesis in interstellar ices. Nat. Commun. 2019, 10, 4413. [Google Scholar] [CrossRef] [PubMed]
- Nuevo, M.; Milam, S.N.; Sandford, S.A. Nucleobases and prebiotic molecules in organic residues produced from the ultraviolet photo-irradiation of pyrimidine in NH3 and H2O + NH3 ices. Astrobiology 2012, 12, 295–314. [Google Scholar] [CrossRef]
- Materese, C.K.; Nuevo, M.; Sandford, S.A. The formation of nucleobases from the ultraviolet photoirradiation of purine in simple astrophysical ice analogues. Astrobiology 2017, 17, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Stoks, P.G.; Schwartz, A.W. Uracil in carbonaceous meteorites. Nature 1979, 282, 709–710. [Google Scholar] [CrossRef]
- Callahan, M.P.; Smith, K.E.; Cleaves, H.J.; Ruzicka, J.; Stern, J.C.; Glavin, D.P.; House, C.H.; Dworkin, J.P. Carbonaceous meteorites contain a wide range of extraterrestrial nucleobases. Proc. Nat. Acad. Sci. USA 2011, 108, 13995–13998. [Google Scholar] [CrossRef]
- Burton, A.S.; Stern, J.C.; Elsila, J.E.; Glavin, D.P.; Dworkin, J.P. Understanding prebiotic chemistry through the analysis of extraterrestrial amino acids and nucleobases in meteorites. Chem. Soc. Rev. 2012, 41, 5459–5472. [Google Scholar] [CrossRef]
- Oba, Y.; Takano, Y.; Furukawa, T.; Koga, T.; Glavin, D.P.; Dworkin, J.P.; Naraoka, H. Identifying the wide diversity of extraterrestrial purine and pyrimidine nucleobases in carbonaceous meteorites. Nat. Commun. 2022, 13, 2008. [Google Scholar] [CrossRef]
- Saladino, R.; Crestini, C.; Cossetti, C.; Di Mauro, E.; Deamer, D. Catalytic effects of Murchison material: Prebiotic synthesis and degradation of RNA precursors. Orig. Life Evol. Biosph. 2011, 41, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, J.D. The origin of life—Out of the blue. Angew. Chem. Int. Ed. 2016, 55, 104–121. [Google Scholar] [CrossRef] [PubMed]
- Saladino, R.; Crestini, C.; Neri, V.; Brucato, J.R.; Colangeli, L.; Ciciriello, F.; Di Mauro, E.; Costanzo, G. Synthesis and degradation of nucleic acid components by formamide and cosmic dust analogues. ChemBioChem 2005, 6, 1368–1374. [Google Scholar] [CrossRef] [PubMed]
- Saladino, R.; Crestini, C.; Pino, S.; Costanzo, G.; Di Mauro, E. Formamide and the origin of life. Phys. Life Rev. 2012, 9, 84–104. [Google Scholar] [CrossRef] [PubMed]
- Saladino, R.; Carota, E.; Botta, G.; Kapralov, M.; Timoshenko, G.N.; Rozanov, A.Y.; Krasavin, E.; Di Mauro, E. Meteorite-catalysed syntheses of nucleosides and of other prebiotic compounds from formamide under proton irradiation. Proc. Nat. Acad. Sci. USA 2015, 112, E2746–E2755. [Google Scholar] [CrossRef]
- Saladino, R.; Carota, E.; Botta, G.; Kapralov, M.; Timoshenko, G.N.; Rozanov, A.Y.; Krasavin, E.; Di Mauro, E. First evidence on the role of heavy ion irradiation of meteorites and formamide in the origin of biomolecules. Orig. Life Evol. Biosph. 2016, 46, 515–521. [Google Scholar] [CrossRef]
- Ferus, M.; Civiš, S.; Mládek, A.; Šponer, J.; Juha, L.; Šponer, J.E. On the road from formamide ices to nucleobases: IR spectroscopic observation of a direct reaction between cyano radicals and formamide in a high-energy impact event. J. Am. Chem. Soc. 2012, 134, 20788–20796. [Google Scholar] [CrossRef]
- Ferus, M.; Pietrucci, F.; Saitta, A.M.; Knížek, A.; Kubelík, O.; Shestivska, V.; Civiš, V. Formation of nucleobases in a Miller-Urey reducing atmosphere. Proc. Nat. Acad. Sci. USA 2017, 114, 4306–4311. [Google Scholar] [CrossRef]
- Schutte, W.A.; Boogert, A.C.A.; Tielens, A.G.G.M.; Whittet, D.C.B.; Gerakines, P.A.; Chiar, J.E.; Ehrenfreund, P.; Greenberg, J.M.; van Dishoeck, E.F.; de Graauw, T. Weak ice absorption features at 7.24 and 7.41 μm in the spectrum of the obscured young stellar object W33A. Astron. Astrophys. 1999, 343, 966–976. [Google Scholar]
- Fortenberry, R.C. Quantum astrochemical spectroscopy. Int. J. Quantum Chem. 2017, 117, 81–91. [Google Scholar] [CrossRef]
- Rosa, C.A.; Emilio, M.; Andrade, L.; de Souza, A.D.; Bendjoya, P.; Pacheco, E.J.; Bergantini, A.; Lage, C. Proposed infrared spectral signatures for the search of purines and pyrimidines towards interstellar medium objects—A laboratory and observational study. Astrobiology 2023. submitted. [Google Scholar]
- Rosa, C.A.; Bergantini, A.; da Silveira, E.F.; Emilio, M.; Andrade, L.; Pacheco, E.J.; Mason, N.J.; Lage, C. Characterising infrared spectral signatures of nucleobases embedded in a mixture of common interstellar volatiles. Mon. Not. R. Astron. Soc. 2023. submitted. [Google Scholar]
- Öberg, K.I.; Boogert, A.C.A.; Pontoppidan, K.M.; van den Broek, S.; van Dishoeck, E.F.; Bottinelli, S.; Blake, G.A.; Evans, N.J. The Spitzer Ice Legacy: Ice evolution from cores to protostars. Astrophys. J. 2011, 740, 109. [Google Scholar] [CrossRef]
- Öberg, K.I. Photochemistry and astrochemistry: Photochemical pathways to interstellar complex organic molecules. Chem. Rev. 2016, 116, 9631–9663. [Google Scholar] [CrossRef] [PubMed]
- Hudgins, D.M.; Allamandola, L.J.; Sandford, S.A. Complex organic molecules in space: The carriers of the interstellar infrared emission features. Adv. Space Res. 1997, 19, 999–1008. [Google Scholar] [CrossRef]
- Abplanalp, M.J.; Kaiser, R.I. On the formation of complex organic molecules in the interstellar medium: Untangling the chemical complexity of carbon monoxide-hydrocarbon containing ice analogues exposed to ionising radiation via a combined infrared and reflectron time-of-flight analysis. Phys. Chem. Chem. Phys. 2019, 21, 16949–16980. [Google Scholar]
- Bergantini, A.; Maksyutenko, P.; Kaiser, R.I. On the formation of the C2H6O isomers ethanol (C2H5OH) and dimethyl ether (CH3OCH3) in star-forming regions. Astrophys. J. 2017, 841, 96. [Google Scholar] [CrossRef]
- Sandford, S.A.; Nuevo, M.; Bera, P.P.; Lee, T.J. Prebiotic astrochemistry and the formation of molecules of astrobiological interest in interstellar clouds and protostellar disks. Chem. Rev. 2020, 120, 4616–4659. [Google Scholar] [CrossRef]
- de Barros, A.L.F.; Bergantini, A.; Domaracka, A.; Rothard, H.; Boduch, P.; da Silveira, E.F. Radiolysis of NH3:CO ice mixtures—Implications for Solar System and interstellar ices. Mon. Not. R. Astron. Soc. 2020, 499, 2162–2172. [Google Scholar] [CrossRef]
- Mifsud, D.V.; Herczku, P.; Sulik, B.; Juhász, Z.; Vajda, I.; Rajta, I.; Ioppolo, S.; Mason, N.J.; Strazzulla, G.; Kaňuchová, Z. Proton and electron irradiations of CH4:H2O mixed ices. Atoms 2023, 11, 19. [Google Scholar] [CrossRef]
- McClure, M.K.; Rocha, W.R.M.; Pontoppidan, K.M.; Crouzet, N.; Chu, L.E.U.; Dartois, E.; Lamberts, T.; Noble, J.A.; Pendleton, Y.J.; Perotti, G.; et al. An Ice Age JWST inventory of dense molecular cloud ices. Nat. Astron. 2023, 7, 431–443. [Google Scholar] [CrossRef]
- Herczku, P.; Mifsud, D.V.; Ioppolo, S.; Juhász, Z.; Kaňuchová, Z.; Kovács, S.T.S.; Traspas Muiña, A.; Hailey, P.A.; Rajta, I.; Vajda, I.; et al. The Ice Chamber for Astrophysics-Astrochemistry (ICA): A new experimental facility for ion impact studies of astrophysical ice analogues. Rev. Sci. Instrum. 2021, 92, 084501. [Google Scholar] [CrossRef] [PubMed]
- Mifsud, D.V.; Juhász, Z.; Herczku, P.; Kovács, S.T.S.; Ioppolo, S.; Kaňuchová, Z.; Czentye, M.; Hailey, P.A.; Traspas Muiña, A.; Mason, N.J.; et al. Electron irradiation and thermal chemistry studies of interstellar and planetary ice analogues at the ICA astrochemistry facility. Eur. J. Phys. D 2021, 75, 182. [Google Scholar] [CrossRef]
- Gibb, E.L.; Whittet, D.C.B.; Boogert, A.C.A.; Tielens, A.G.G.M. Interstellar ice: The Infrared Space Observatory legacy. Astrophys. J. Suppl. Ser. 2004, 151, 35. [Google Scholar] [CrossRef]
- Boogert, A.D.A.; Gerakines, P.A.; Whittet, D.C.B. Observations of the icy universe. Annu. Rev. Astron. Astrophys. 2015, 53, 541–581. [Google Scholar] [CrossRef]
- van Dishoeck, E.F. Astrochemistry of dust, ice, and gas: Introduction and overview. Faraday Discuss. 2014, 168, 9–47. [Google Scholar] [CrossRef]
- Croft, S.K.; Lunine, J.I.; Kargel, J. Equation of state of ammonia-water liquid: Derivation and planetological applications. Icarus 1988, 73, 279–293. [Google Scholar] [CrossRef]
- Bouilloud, M.; Fray, N.; Bénilan, Y.; Cottin, H.; Gazeau, M.-C.; Jolly, A. Bibliographic review and new measurements of the infrared band strengths of pure molecules at 25 K: H2O, CO2, CO, CH4, NH3, CH3OH, HCOOH, and H2CO. Mon. Not. R. Astron. Soc. 2015, 451, 2145–2160. [Google Scholar] [CrossRef]
- Saïagh, K.; Cloix, M.; Frany, N.; Cottin, H. VUV and mid-UV photoabsorption cross section of thin films of adenine: Applications on its photochemistry in the Solar System. Planet. Space Sci. 2014, 90, 90–99. [Google Scholar] [CrossRef]
- Saïagh, K.; Cottin, H.; Aleian, A.; Fray, N. VUV and mid-UV photoabsorption cross section of thin films of guanine and uracil: Applications on their photochemistry in the Solar System. Astrobiology 2015, 15, 268–282. [Google Scholar] [CrossRef]
- Mathlouthi, M.; Seuvre, A.M.; Koenig, J.L. FT-IR and laser-Raman spectra of guanine and guanosine. Carbohydr. Res. 1986, 146, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Szczepaniak, K.; Szczesniak, M. Matrix isolation infrared studies of nucleic acid constituents: Part 4. Guanine and 9-methylguanine monomers and their keto-enol tautomerism. J. Mol. Struct. 1987, 156, 29–42. [Google Scholar] [CrossRef]
- Nowak, M.J.; Lapinski, L.; Kwiatkowski, J.S.; Leszczyński, J. Molecular structure and infrared spectra of adenine. Experimental matrix isolation and density functional theory study of adenine 15N isotopomers. J. Phys. Chem. 1996, 100, 3527–3534. [Google Scholar] [CrossRef]
- Colarusso, P.; Zhang, K.; Guo, B.; Bernath, P.F. The infrared spectra of uracil, thymine, and adenine in the gas phase. Chem. Phys. Lett. 1997, 269, 39–48. [Google Scholar] [CrossRef]
- Mohamed, T.A.; Shabaan, I.A.; Zoghaib, W.M.; Husband, J.; Farag, R.S.; Alajhaz, A.E.-N.M.A. Tautomerism, normal coordinate analysis, vibrational assignments, calculated IR, Raman, and NMR spectra of adenine. J. Mol. Struct. 2009, 938, 263–276. [Google Scholar] [CrossRef]
- Lopes, R.P.; Marques, P.M.; Valero, R.; Tomkinson, J.; Batista de Carvalho, L.A.E. Guanine: A combined study using vibrational spectroscopy and theoretical methods. J. Spectrosc. 2012, 27, 2691–2699. [Google Scholar] [CrossRef]
- Lopes, R.P.; Valero, R.; Tomkinson, J.; Marques, P.M.; Batista de Carvalho, L.A.E. Applying vibrational spectroscopy to the study of nucleobases—Adenine as a case study. New J. Chem. 2013, 37, 2691–2699. [Google Scholar] [CrossRef]
- Fornaro, T.; Biczysko, M.; Montiab, S.; Barone, V. Dispersion corrected DFT approaches for anharmonic vibrational frequency calculations: Nucleobases and their dimers. Phys. Chem. Chem. Phys. 2014, 16, 10112–10128. [Google Scholar] [CrossRef]
- Chen, F.; Wu, B.; Elad, N.; Gal, A.; Liu, Y.; Ma, Y.; Qi, L. Controlled crystallisation of anhydrous guanine β nano-platelets via an amorphous precursor. CrystEngComm 2019, 21, 3586–3591. [Google Scholar] [CrossRef]
- Rocha, W.R.M.; Rachid, M.G.; Olsthoorn, B.; van Dishoeck, E.F.; McClure, M.K.; Linnartz, H. LIDA: The Leiden Ice Database for Astrochemistry. Astron. Astrophys. 2022, 668, A63. [Google Scholar] [CrossRef]
- Mifsud, D.V.; Hailey, P.A.; Herczku, P.; Juhász, Z.; Kovács, S.T.S.; Sulik, B.; Ioppolo, S.; Kaňuchová, Z.; McCullough, R.W.; Paripás, B.; et al. Laboratory experiments on the radiation astrochemistry of water ice phases. Eur. Phys. J. D 2022, 76, 87. [Google Scholar] [CrossRef]
- Gálvez, Ó.; Maté, B.; Martín-Llorente, B.; Herrero, V.J.; Escribano, R. Phases of solid methanol. J. Phys. Chem. A 2009, 113, 3321–3329. [Google Scholar] [CrossRef]
- Müller, B.; Giuliano, B.M.; Goto, M.; Caselli, P. Spectroscopic measurements of CH3OH in layered and mixed interstellar ice analogues. Astron. Astrophys. 2021, 652, A126. [Google Scholar] [CrossRef]
- Sanchez, R.A.; Ferris, J.P.; Orgel, L.E. Studies in prebiotic synthesis: IV. Conversion of 4-aminoimidazole-5-carbonitrile derivatives to purines. J. Mol. Biol. 1968, 38, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Hayatsu, R.; Studier, M.H.; Oda, A.; Fuse, K.; Anders, E. Origin of organic matter in early Solar System—II. Nitrogen compounds. Geochim. Cosmochim. Acta 1968, 32, 175–190. [Google Scholar] [CrossRef]
- Hayatsu, R.; Studier, M.H.; Matsuoka, S.; Anders, E. Origin of organic matter in early Solar System—VI. Catalytic synthesis of nitriles, nitrogen bases and porphyrin-like pigments. Geochim. Cosmochim. Acta 1972, 36, 555–571. [Google Scholar] [CrossRef]
- Voet, A.B.; Schwartz, A.W. Prebiotic adenine synthesis from HCN—Evidence for a newly discovered major pathway. Bioorg. Chem. 1983, 12, 8–17. [Google Scholar] [CrossRef]
- Yuasa, S.; Flory, D.; Basile, B.; Oró, J. Abiotic synthesis of purines and other heterocyclic compounds by the action of electrical discharges. J. Mol. Evol. 1984, 21, 76–80. [Google Scholar] [CrossRef]
- Levy, M.; Miller, S.L.; Oró, J. Production of guanine from NH4CN polymerisations. J. Mol. Evol. 1999, 49, 165–168. [Google Scholar] [CrossRef]
- Levy, M.; Miller, S.L.; Brinton, K.; Bada, J.L. Prebiotic synthesis of adenine and amino acids under Europa-like conditions. Icarus 2000, 145, 609–613. [Google Scholar] [CrossRef]
- Miyakawa, S.; Cleaves, H.J.; Miller, S.L. The cold origin of life: Implications based on pyrimidines and purines produced from frozen ammonium cyanide solutions. Orig. Life Evol. Biosph. 2002, 32, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Barks, H.L.; Buckley, R.; Grieves, G.A.; Di Mauro, E.; Hud, N.V.; Orlando, T.M. Guanine, adenine, and hypoxanthine production in UV-irradiated formamide solutions: Relaxation of the requirements for prebiotic purine nucleobase formation. ChemBioChem 2010, 11, 1240–1243. [Google Scholar] [CrossRef] [PubMed]
- Tielens, A.G.G.M. The molecular universe. Rev. Mod. Phys. 2013, 85, 1021. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, X.; Zhang, D.; Zhang, W.; Liu, G.; Zhen, J. Laboratory formation and photochemistry of covalently bonded polycyclic aromatic nitrogen heterocycle (PANH) clusters in the gas phase. Mon. Not. R. Astron. Soc. 2020, 498, 1–11. [Google Scholar] [CrossRef]
- Mattioda, A.L.; Cruz-Diaz, G.A.; Ging, A.; Barnhardt, M.; Boersma, C.; Allamandola, L.J.; Schneider, T.; Vaughn, J.; Phillips, B.; Ricca, A. Formation of complex organic molecules (COMs) from polycyclic aromatic hydrocarbons (PAHs): Implications for ISM IR emission plateaus and Solar System organics. ACS Earth Space Chem. 2020, 4, 2227–2245. [Google Scholar] [CrossRef]
- Hudson, R.L. An IR investigation of solid amorphous ethanol—Spectra, properties, and phase changes. Spectrochim. Acta A 2017, 187, 82–86. [Google Scholar] [CrossRef]
- Hudson, R.L.; Gerakines, P.A.; Ferrante, R.F. IR spectra and properties of solid acetone, an interstellar and cometary molecule. Spectrochim. Acta A 2018, 193, 33–39. [Google Scholar] [CrossRef]
- Iglesias-Groth, S.; Cataldo, F. Mid- and far-infrared spectroscopy of nucleobases: Molar extinction coefficients, integrated molar absorptivity, and temperature dependence of the main bands. Mon. Not. R. Astron. Soc. 2023, 523, 1756–1771. [Google Scholar] [CrossRef]
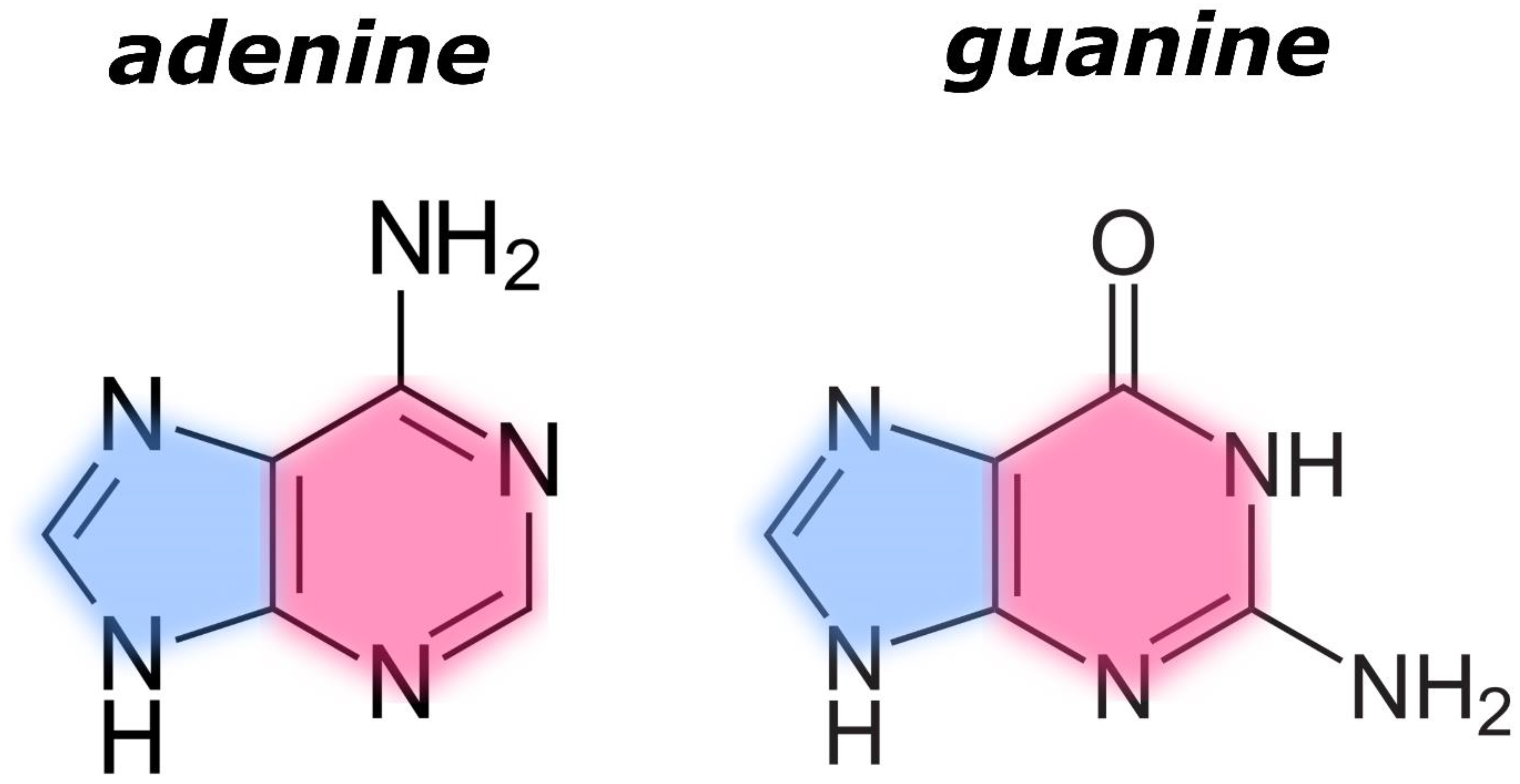
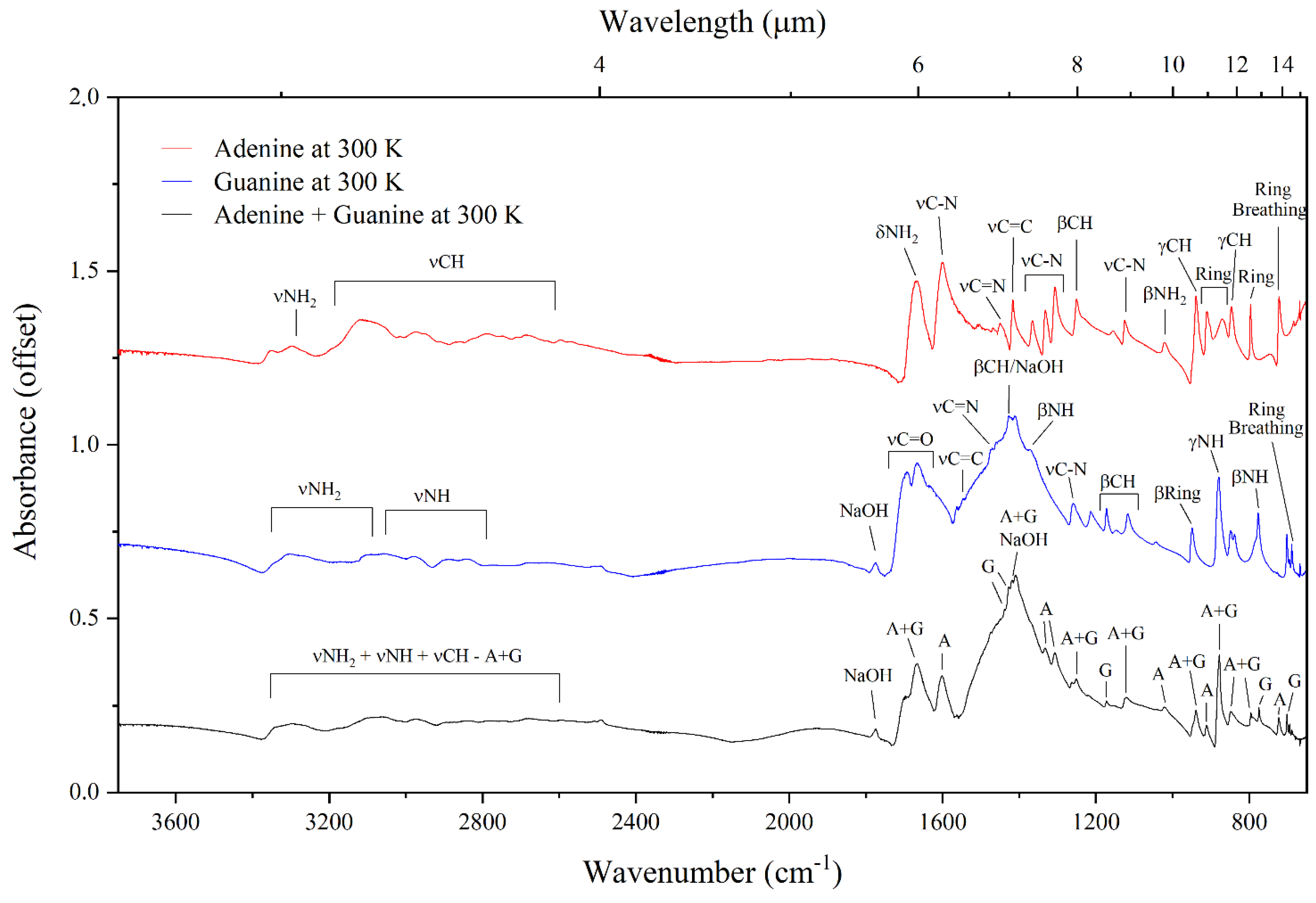
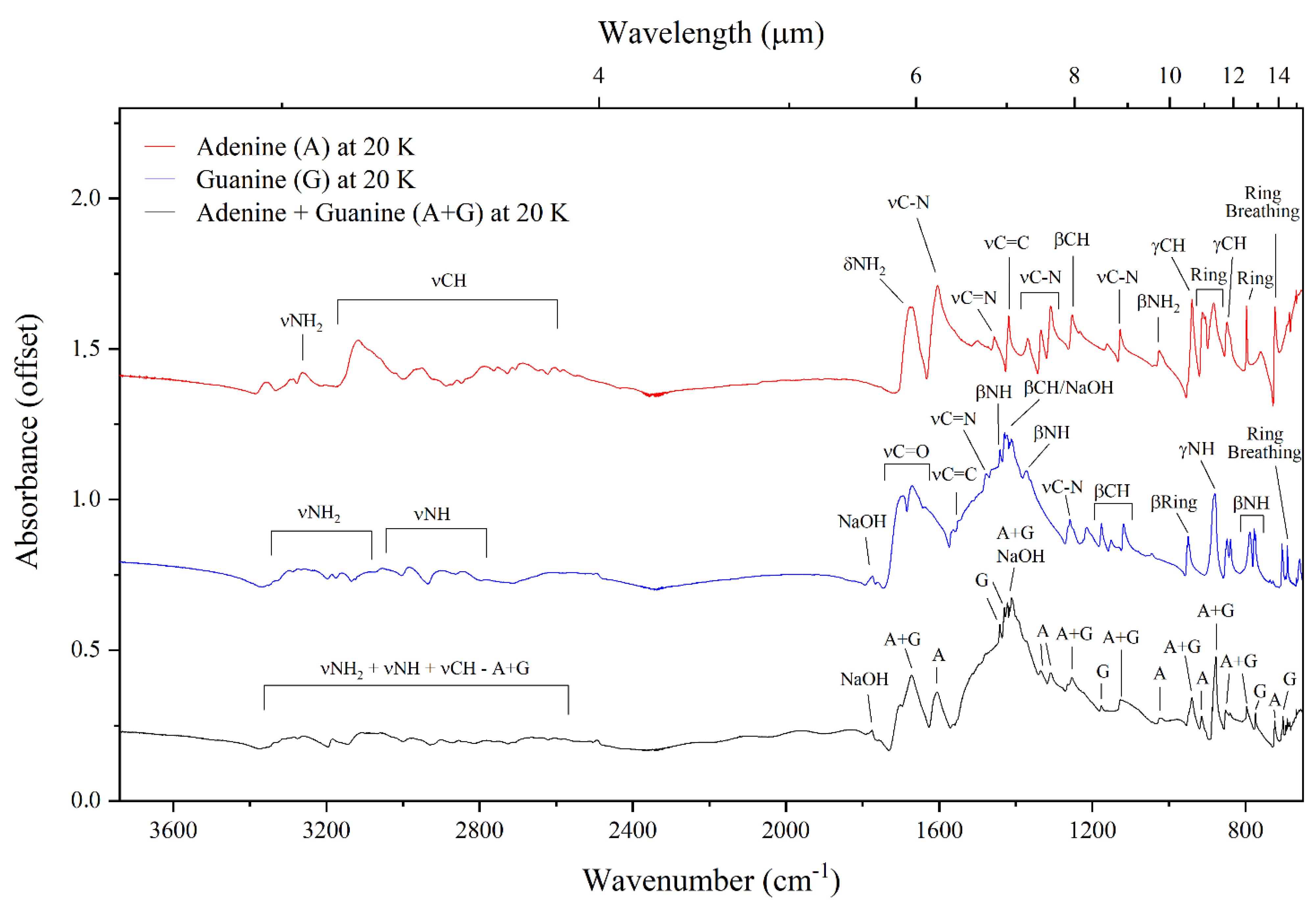

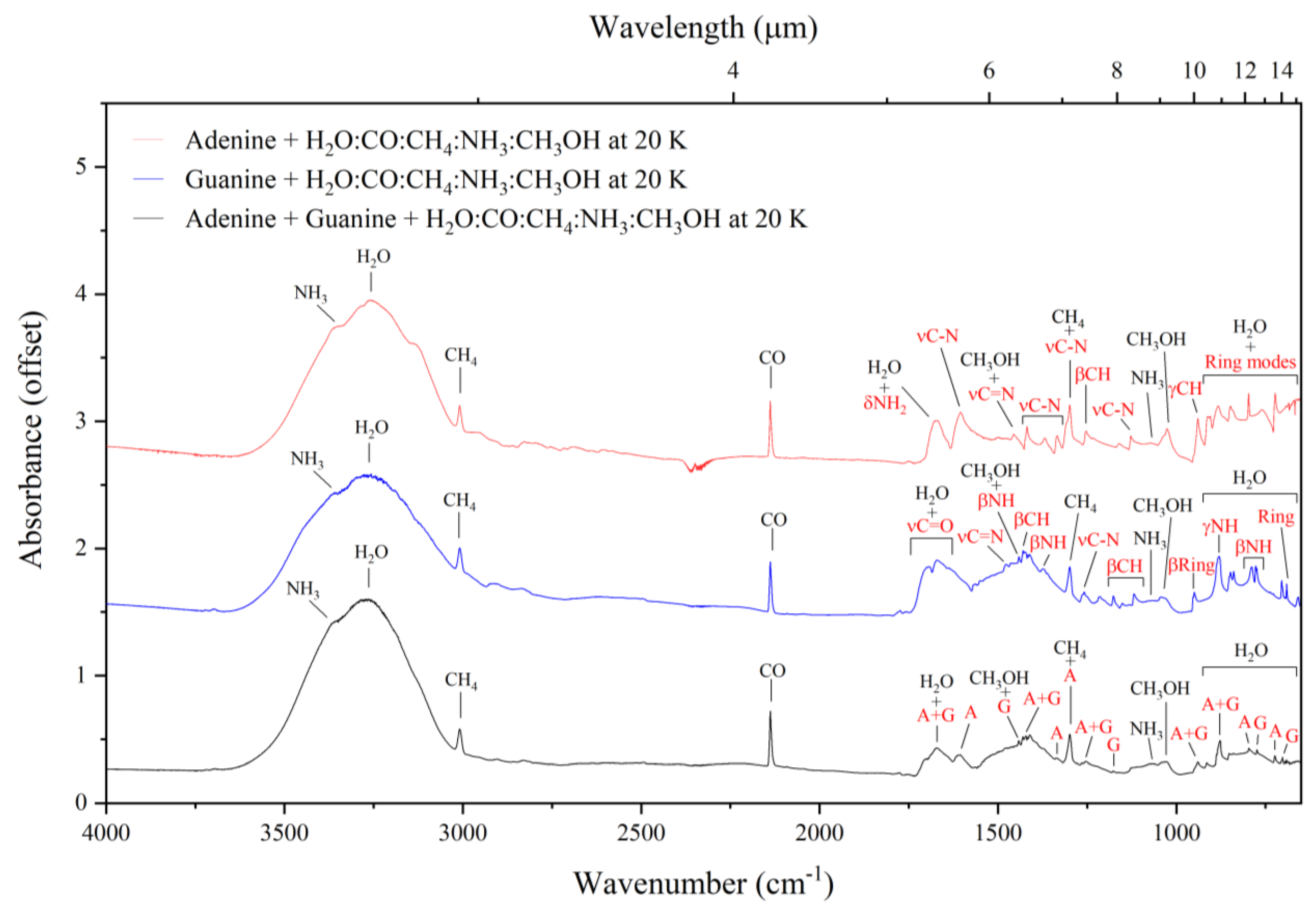
| Adenine | Guanine | Adenine–Guanine Mixture † | ||||||
|---|---|---|---|---|---|---|---|---|
| ν (cm−1) | Assignment | Reference | ν (cm−1) | Assignment | Reference | ν (cm−1) | Assignment | Reference |
| 3354 | νNH | [45] | 3159 | νsymNH2 | [46] | 3284 | νNH2 (A) | [45] |
| 3286 | νNH2 | [45] | 2896 | νNH | [46] | 3257 | νNH2 (A) | [47] |
| 3263 | νNH2 | [47] | 2841 | νNH | [41,42,46] | 3184 | νNH2 (G) | [46] |
| 3117 | νCH | [45,47] | 1694 | νC=O and βNH2 | [41,46] | 2978 | νCH (G) | [45,47] |
| 2950 | νCH | [45,47] | 1670 | νC=O and βNH2 | [40,41,46] | 2901 | νNH (G) | [46] |
| 2788 | νCH | [39,47] | 1551 | νC=C | [41,46] | 2854 | νNH (G) | [41,42,46] |
| 2686 | νCH | [39,47] | 1480 | νCN and νC=N | [41] | 2782 | νCH (A) | [39,47] |
| 1675 | δscisNH2 | [39,45,47] | 1441 | βNH | [40,41,42] | 1672 | νC=O (G), βNH2 (G), and δscisNH2 (A) | [39,40,41,45,46,47] |
| 1604 | νCN and νCC | [39,43,44,45,47,48] | 1429 | βCH and NaOH | [41] | 1605 | νCN (A) and νCC (A) | [39,43,44,45,47,48] |
| 1499 | ν-ring | [45] | 1372 | βNH, βCH, and νCN | [40,41,42] | 1442 | βNH (G) | [40,41,42] |
| 1456 | νC=N and βCH | [43,44,47,48] | 1258 | νCN | [40,41] | 1430 | βCH (G) and NaOH | [41] |
| 1419 | νC=C, νCN, and βCH | [39,43,44,45,47,48] | 1214 | νCNH2 | [41] | 1422 | νC=C (A), νCN (A), and βCH (A) | [39,43,44,45,47,48] |
| 1369 | νCN and βCH | [39,43,44,45,48] | 1176 | βCH | [41,42] | 1336 | νCN (A) and βCH (A) | [39,43,44,45,47,48] |
| 1334 | νCN and βCH | [39,43,44,45,47,48] | 1151 | βCH | [41] | 1309 | νCN (A) | [39,43,44,45,48] |
| 1309 | νCN | [39,43,44,45,48] | 1119 | βCH | [40,42] | 1253 | βCH (A), βNH (A), and νCN (G) | [39,40,41,43,44,45,47] |
| 1253 | βCH and βNH | [39,43,44,45,47,48] | 950 | β-ring and γC=O | [42] | 1178 | βCH (G) | [41,42] |
| 1128 | νCN | [39,43,44,45,47,48] | 880 | γNH | [41] | 1127 | νCN (A) and βCH (G) | [39,40,43,44,45,47,48] |
| 1025 | βrockNH2 and νC=N | [39,43,44,45,47,48] | 848 | νCC and β-ring | [41,42] | 1023 | βrockNH2 (A) and νC=N (A) | [39,43,44,45,47,48] |
| 940 | γCH | [43,44,45,47,48] | 789 | βNH | [41] | 940 | γCH (A), β-ring (G), and γC=O (G) | [42,43,44,45,48] |
| 913 | Β-ring and νC=C | [43,44,45,47,48] | 778 | βNH | [41,42] | 915 | β-ring (A) and νC=C (A) | [43,44,45,47,48] |
| 883 | ring deformation | [45] | 705 | β-ring | [41] | 877 | ring deformation (A) and γNH (G) | [41,45] |
| 848 | γCH | [45] | 691 | ring breathing | [41] | 852 | γCH (A), νCC (G), β-ring (G) | [41,42,45] |
| 797 | ring torsion | [45] | 797 | ring torsion (A) and βNH (G) | [41,45] | |||
| 723 | ring breathing | [43,45,47,48] | 774 | βNH (G) | [41,42] | |||
| 724 | ring breathing (A) | [43,45,47,48] | ||||||
| 702 | β-ring (G) | [41] | ||||||
| 691 | ring breathing (G) | [41] | ||||||
| Adenine + H2O:NH3:CH4:CO:CH3OH | Guanine + H2O:NH3:CH4:CO:CH3OH | Adenine–Guanine Mixture + H2O:NH3:CH4:CO:CH3OH | |||||
|---|---|---|---|---|---|---|---|
| ν (cm−1) | Assignment | ν (cm−1) | Assignment | ν (cm−1) | Assignment | ||
| 3362 | ν3 (NH3) | 3362 | ν3 (NH3) | 3362 | ν3 (NH3) | ||
| 3257 | ν3 (H2O) | 3257 | ν3 (H2O) | 3270 | ν3 (H2O) | ||
| 3009 | ν3 (CH4) | 3009 | ν3 (CH4) | 3009 | ν3 (CH4) | ||
| 2138 | ν (CO) | 2913 | combination modes (CH3OH) | 2913 | combination modes (CH3OH) | ||
| 1671 | δscisNH2 (A) and ν2 (H2O) | 2830 | ν3 (CH3OH) | 2830 | ν3 (CH3OH) | ||
| 1605 | νCN (A) and νCC (A) | 2138 | ν (CO) | 2138 | ν (CO) | ||
| 1456 | νC=N (A), βCH (A), and ν10 (CH3OH) | 1692 | νC=O (G), βNH2 (G), and ν2 (H2O) | 1670 | νC=O (G), βNH2 (G), δscisNH2 (A), and ν2 (H2O) | ||
| 1419 | νC=C (A), νCN (A), and βCH (A) | 1670 | νC=O (G) and ν2 (H2O) | 1606 | νCN (A), νCC (A), and ν2 (H2O) | ||
| 1369 | νCN (A) and βCH (A) | 1478 | νC=N (G) and νCN (G) | 1442 | βNH (G) and ν10 (CH3OH) | ||
| 1334 | νCN (A) and βCH (A) | 1441 | βNH (G) and ν10 (CH3OH) | 1422 | βCH (G), νC=C (A), νCN (A), and βCH (A) | ||
| 1299 | νCN (A) and ν4 (CH4) | 1430 | βCH (G) | 1336 | νCN (A) and βCH (A) | ||
| 1253 | βCH (A) and βNH (A) | 1375 | βNH (G), βCH (G), and νCN (G) | 1299 | νCN (A) and ν4 (CH4) | ||
| 1128 | νCN (A) | 1299 | ν4 (CH4) | 1255 | βCH (A), βNH (A), and νCN (G) | ||
| 1069 | ν2 (NH3) | 1258 | νCN (G) | 1178 | βCH (G) | ||
| 1026 | ν8 (CH3OH) | 1214 | νCNH2 (G) | 1070 | ν2 (NH3) | ||
| 940 | γCH (A) | 1176 | βCH (G) | 1035 | ν8 (CH3OH) | ||
| 910 | β-ring (A) and νC=C (A) | 1153 | βCH (G) | 940 | γCH (A), β-ring (G), and γC=O (G) | ||
| 883 | ring deformation (A) | 1118 | βCH (G) | 915 | β-ring (A) and νC=C (A) | ||
| 848 | γCH (A) | 1070 | ν2 (NH3) | 878 | ring deformation (A) and νL (H2O) | ||
| 797 | ring torsion (A) and νL (H2O) | 1038 | ν8 (CH3OH) | 797 | ring torsion (A) and νL (H2O) | ||
| 723 | ring breathing (A) and νL (H2O) | 950 | β-ring (G) and γC=O (G) | 774 | βNH (G) and νL (H2O) | ||
| 880 | γNH (G) | 724 | ring breathing (A) and νL (H2O) | ||||
| 848 | νCC (G) and β-ring (G) | 702 | β-ring (G) and νL (H2O) | ||||
| 799 | βNH (G) and νL (H2O) | 691 | ring breathing (G) and νL (H2O) | ||||
| 778 | βNH (G) and νL (H2O) | ||||||
| 705 | β-ring (G) and νL (H2O) | ||||||
| 691 | ring breathing (G) and νL (H2O) | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosa, C.A.; Bergantini, A.; Herczku, P.; Mifsud, D.V.; Lakatos, G.; Kovács, S.T.S.; Sulik, B.; Juhász, Z.; Ioppolo, S.; Quitián-Lara, H.M.; et al. Infrared Spectral Signatures of Nucleobases in Interstellar Ices I: Purines. Life 2023, 13, 2208. https://doi.org/10.3390/life13112208
Rosa CA, Bergantini A, Herczku P, Mifsud DV, Lakatos G, Kovács STS, Sulik B, Juhász Z, Ioppolo S, Quitián-Lara HM, et al. Infrared Spectral Signatures of Nucleobases in Interstellar Ices I: Purines. Life. 2023; 13(11):2208. https://doi.org/10.3390/life13112208
Chicago/Turabian StyleRosa, Caroline Antunes, Alexandre Bergantini, Péter Herczku, Duncan V. Mifsud, Gergő Lakatos, Sándor T. S. Kovács, Béla Sulik, Zoltán Juhász, Sergio Ioppolo, Heidy M. Quitián-Lara, and et al. 2023. "Infrared Spectral Signatures of Nucleobases in Interstellar Ices I: Purines" Life 13, no. 11: 2208. https://doi.org/10.3390/life13112208
APA StyleRosa, C. A., Bergantini, A., Herczku, P., Mifsud, D. V., Lakatos, G., Kovács, S. T. S., Sulik, B., Juhász, Z., Ioppolo, S., Quitián-Lara, H. M., Mason, N. J., & Lage, C. (2023). Infrared Spectral Signatures of Nucleobases in Interstellar Ices I: Purines. Life, 13(11), 2208. https://doi.org/10.3390/life13112208








