The Feasibility of Less-Invasive Bentall Surgery: A Real-World Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Indications for Bentall Surgery
2.2. Statistical Analysis
2.3. Surgical Technique
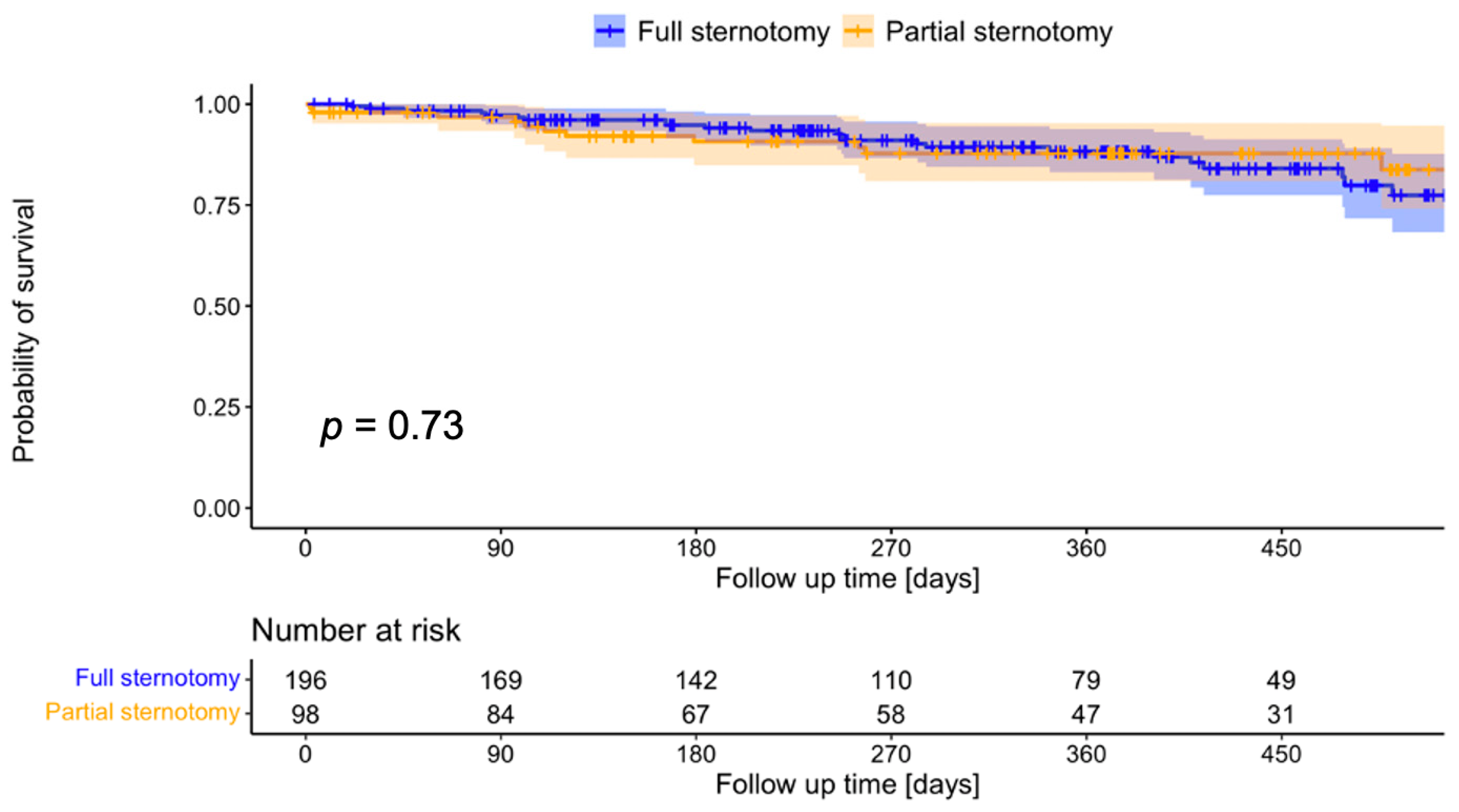
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bentall, H.; De Bono, A. A technique for complete replacement of the ascending aorta. Thorax 1968, 23, 338–339. [Google Scholar] [CrossRef]
- Etz, C.D.; Bischoff, M.S.; Bodian, C.; Roder, F.; Brenner, R.; Griepp, R.B.; Di Luozzo, G. The Bentall procedure: Is it the gold standard? A series of 597 consecutive cases. J. Thorac. Cardiovasc. Surg. 2010, 140, S64–S70. [Google Scholar] [CrossRef]
- Chang, C.; Raza, S.; Altarabsheh, S.E.; Delozier, S.; Sharma, U.M.; Zia, A.; Khan, M.S.; Neudecker, M.; Markowitz, A.H.; Sabik, J.F.; et al. Minimally Invasive Approaches to Surgical Aortic Valve Replacement: A Meta-Analysis. Ann. Thorac. Surg. 2018, 106, 1881–1889. [Google Scholar] [CrossRef]
- Haunschild, J.; van Kampen, A.; von Aspern, K.; Misfeld, M.; Davierwala, P.; Saeed, D.; Borger, M.A.; Etz, C.D. Supracommissural replacement of the ascending aorta and the aortic valve via partial versus full sternotomy-a propensity-matched comparison in a high-volume centre. Eur. J. Cardiothorac. Surg. 2021, 61, 479–487. [Google Scholar] [CrossRef]
- Levack, M.M.; Aftab, M.; Roselli, E.E.; Johnston, D.R.; Soltesz, E.G.; Gillinov, A.M.; Pettersson, G.B.; Griffin, B.; Grimm, R.; Hammer, D.F.; et al. Outcomes of a Less-Invasive Approach for Proximal Aortic Operations. Ann. Thorac. Surg. 2017, 103, 533–540. [Google Scholar] [CrossRef]
- Hillebrand, J.; Alshakaki, M.; Martens, S.; Scherer, M. Minimally Invasive Aortic Root Replacement with Valved Conduits through Partial Upper Sternotomy. Thorac. Cardiovasc. Surg. 2018, 66, 295–300. [Google Scholar] [CrossRef]
- Byrne, J.G.; Adams, D.H.; Couper, G.S.; Rizzo, R.J.; Cohn, L.H.; Aranki, S.F. Minimally-invasive aortic root replacement. Heart Surg. Forum 1999, 2, 326–329. [Google Scholar]
- Mikus, E.; Micari, A.; Calvi, S.; Salomone, M.; Panzavolta, M.; Paris, M.; Del Giglio, M. Mini-Bentall: An Interesting Approach for Selected Patients. Innovations 2017, 12, 41–45. [Google Scholar] [CrossRef]
- Bakir, I.; Casselman, F.; De Geest, R.; Wellens, F.; Foubert, L.; Degrieck, I.; Van Praet, F.; Vermeulen, Y.; Vanermen, H. Minimally invasive aortic root replacement: A bridge too far? J. Cardiovasc. Surg. 2007, 48, 85–91. [Google Scholar]
- R-Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Henry, L.; Hester, J. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Stuart, E.; King, G.; Imai, K.; Ho, D. MatchIt: Nonparametric preprocessing for parametric causal inference. J. Stat. Softw. 2011, 42, 1–28. [Google Scholar] [CrossRef]
- Nadarajah, S.; Bakar, S. A new R package for actuarial survival models. Comput. Stat. 2013, 28, 2139–5150. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2. Wiley Interdiscip. Rev. Comput. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]
- Di Eusanio, M.; Cefarelli, M.; Zingaro, C.; Capestro, F.; Matteucci, S.M.L.; D’alfonso, A.; Pierri, M.D.; Aiello, M.L.; Berretta, P. Mini Bentall operation: Technical considerations. Indian. J. Thorac. Cardiovasc. Surg. 2019, 35, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Harky, A.; Al-Adhami, A.; Chan, J.S.K.; Wong, C.H.M.; Bashir, M. Minimally Invasive Versus Conventional Aortic Root Replacement—A Systematic Review and Meta-Analysis. Heart Lung Circ. 2019, 28, 1841–1851. [Google Scholar] [CrossRef]
- Fröjd, V.; Jeppsson, A. Reexploration for Bleeding and Its Association With Mortality After Cardiac Surgery. Ann. Thorac. Surg. 2016, 102, 109–117. [Google Scholar] [CrossRef]
- Suen, W.S.; Mok, C.K.; Chiu, S.W.; Cheung, K.L.; Lee, W.T.; Cheung, D.; Das, S.R.; He, G.W. Risk factors for development of acute renal failure (ARF) requiring dialysis in patients undergoing cardiac surgery. Angiology 1998, 49, 789–800. [Google Scholar] [CrossRef]
- Oshita, T.; Hiraoka, A.; Nakajima, K.; Muraki, R.; Arimichi, M.; Chikazawa, G.; Yoshitaka, H.; Sakaguchi, T. A Better Predictor of Acute Kidney Injury After Cardiac Surgery: The Largest Area Under the Curve Below the Oxygen Delivery Threshold During Cardiopulmonary Bypass. J. Am. Heart Assoc. 2020, 9, e015566. [Google Scholar] [CrossRef]
- Modi, P.; Chitwood, W.R. Retrograde femoral arterial perfusion and stroke risk during minimally invasive mitral valve surgery: Is there cause for concern? Ann. Cardiothorac. Surg. 2013, 2, E1. [Google Scholar] [CrossRef]
- Meyer, A.; van Kampen, A.; Kiefer, P.; Sündermann, S.; Van Praet, K.M.; Borger, M.A.; Falk, V.; Kempfert, J. Minithoracotomy versus full sternotomy for isolated aortic valve replacement: Propensity matched data from two centers. J. Card. Surg. 2021, 36, 97–104. [Google Scholar] [CrossRef]
- Johnson, C.A.; Siordia, J.A.; Wood, K.L.; Robinson, D.A.; Knight, P.A. Right Mini-thoracotomy Bentall Procedure. Innovations 2018, 13, 328–331. [Google Scholar] [CrossRef]
| Unmatched | Matched | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total n = 768 | FS n = 670 | PS n = 98 | p-Value | SMD | Total n = 294 | FS n = 196 | PS n = 98 | p-Value | SMD | |
| Age | 59.4 ± 12.2 | 59.3 ± 12.2 | 60.1 ± 12.2 | 0.5 | 0.066 | 59.8 ± 12.2 | 59.7 ± 12.2 | 60.1 ± 12.2 | 0.8 | 0.038 |
| Male gender | 596 (77.6) | 522 (77.9) | 74 (75.5) | 0.7 | 0.057 | 222 (75.5) | 148 (75.5) | 74 (75.5) | 1 | 0 |
| BMI | 27.8 ± 4.6 | 27.9 ± 4.6 | 27.2 ± 4.7 | 0.1 | 0.161 | 27.1 ± 4.6 | 27.1 ± 4.6 | 27.2 ± 4.7 | 0.9 | 0.01 |
| Art. HTN | 584 (76) | 515 (76.9) | 69 (70.4) | 0.2 | 0.147 | 206 (70.1) | 137 (69.9) | 69 (70.4) | 1 | 0.011 |
| Pulm. HTN | 85 (11.1) | 71 (10.6) | 14 (14.3) | 0.4 | 0.112 | 39 (13.3) | 25 (12.8) | 14 (14.3) | 0.9 | 0.045 |
| Diabetes | 83 (10.8) | 75 (11.2) | 8 (8.2) | 0.5 | 0.103 | 21 (7.1) | 13 (6.6) | 8 (8.2) | 0.8 | 0.059 |
| COPD | 27 (3.5) | 25 (3.7) | 2 (2) | 0.6 | 0.101 | 6 (2) | 4 (2) | 2 (2) | 1 | 0 |
| PAD | 411 (53.5) | 367 (54.8) | 44 (44.9) | 0.09 | 0.199 | 132 (44.9) | 88 (44.9) | 44 (44.9) | 1 | 0 |
| eGFR | 97.4 (32.6) | 97.8 (32.7) | 94 (31.5) | 0.3 | 0.118 | 93.9 (30.6) | 94.7 (30.9) | 92.5 (30) | 0.6 | 0.072 |
| LVEF | 57.4 ± 12 | 57.1 ± 12.1 | 59.1 ± 11 | 0.1 | 0.038 | 59.4 ± 11.9 | 59.6 ± 12.5 | 59.2 ± 10.6 | 0.8 | 0.035 |
| BAV | 358 (46.6) | 314 (46.9) | 44 (44.9) | 0.8 | 0.039 | 138 (46.9) | 94 (48) | 44 (44.9) | 0.7 | 0.061 |
| severe AS | 358 (46.6) | 290 (43.3) | 68 (69.4) | <0.001 | 0.546 | 204 (69.4) | 136 (69.4) | 68 (69.4) | 1 | 0 |
| severe AR | 456 (59.4) | 418 (62.4) | 38 (38.8) | <0.001 | 0.486 | 119 (40.5) | 81 (41.3) | 38 (38.8) | 0.8 | 0.052 |
| Euro-SCORE II | 4.7 ± 2.1 | 4.7 ± 2.1 | 4.4 ± 2.1 | 0.3 | 0.121 | 4.4 ± 2 | 4.4 ± 2 | 4.4 ± 2 | 0.9 | 0.022 |
| Matched Cohorts | ||||
|---|---|---|---|---|
| Total n = 294 | FS n = 196 | PS n = 98 | p-Value | |
| Arterial cannulation | ||||
| Central | 280 (95.2) | 188 (95.9) | 92 (93.9) | 0.6 |
| Femoral | 8 (2.7) | 2 (1) | 6 (6.1) | 0.02 |
| Axillary | 6 (2) | 6 (3.1) | 0 (0) | 0.2 |
| Venous cannulation | ||||
| Central | 268 (91.2) | 192 (98) | 76 (77.6) | <0.001 |
| Femoral | 26 (8.8) | 4 (2) | 22 (22.4) | <0.001 |
| LV vent | ||||
| via pulmonary vein | 274 (93.2) | 195 (99.5) | 79 (80.6) | <0.001 |
| via pulmonary artery | 20 (6.8) | 1 (0.5) | 19 (19.4) | <0.001 |
| Concomitant procedures | ||||
| LAA occlusion | 2 (0.7) | 2 (1) | 0 (0) | 0.6 |
| morrow resection | 15 (5.1) | 8 (4.1) | 7 (7.1) | 0.3 |
| hemiarch replacement | 14 (4.8) | 8 (4.1) | 6 (6.1) | 0.7 |
| Biological valve prosthesis | 201 (68.4) | 123 (62.8) | 78 (79.6) | 0.003 |
| Matched Cohorts | |||||
|---|---|---|---|---|---|
| Total | FS | PS | OR (95% CI) | p-Value | |
| n = 294 | n = 196 | n = 98 | |||
| Procedure time [min] | 197 (171.3–230) | 192.5 (165–224) | 205 (180–243.8) | - | 0.002 |
| Bypass time [min] | 113.5 (98.3–136) | 114.5 (96.8–135.3) | 110.5 (101–137) | - | 0.9 |
| Aortic cross-clamp time [min] | 86 (74.3–99.8) | 87 (73.8–102) | 83.5 (76–97.75) | - | 0.6 |
| Median prosthesis size | 27 (25;27) | 25 (25;27) | 27 (25;27) | - | 0.4 |
| Conversion | - | - | 8 (8.2) | - | - |
| Emergency CABG | 12 (4.1) | 8 (4.1) | 4 (4.1) | 1 (0.2–3.8) | 1 |
| IABP | 8 (2.7) | 4 (2) | 4 (4) | 2 (0.4–11.2) | 0.4 |
| ECMO | 4 (1.4) | 2 (1) | 2 (2) | 1.3 (0.1–11.9) | 1 |
| Ventilation time [minutes] | 567 (372–942) | 670 (400–975) | 525 (342–921) | - | 0.2 |
| ICU length of stay [hours] | 19.9 (6.3–26.3) | 20.5 (6.7–26.5) | 17.8 (5.4–25.3) | - | 0.2 |
| IMCU length of stay [hours] | 36.3 (20–70.9) | 35.7 (19.5–70.8) | 37.2 (21.1–71) | - | 0.7 |
| Revision f. bleeding | 19 (6.5) | 8 (4.1) | 11 (11.2) | 3 (1.04–8.8) | 0.02 |
| Revision f. pericardial effusion | 22 (7.5) | 17 (8.7) | 5 (5.1) | 0.6 (0.2–1.7) | 0.4 |
| Revision f. coronary compl. | 5 (1.7) | 5 (2.6) | 0 (0) | 0 (0–2.2) | 0.2 |
| Revision f. sternal instability | 5 (1.7) | 4 (2) | 1 (1) | 0.5 (0.01–5.1) | 0.5 |
| GI complications | 14 (4.7) | 11 (5.6) | 3 (3.1) | 0.5 (0.09–2.1) | 0.4 |
| Stroke | 5 (1.7) | 2 (1) | 3 (3.1) | 3.05 (0.3–37.1) | 0.3 |
| Pacemaker | 10 (3.4) | 8 (4.1) | 2 (2) | 0.5 (0.05–2.5) | 0.5 |
| Respiratory failure | 36 (6.1) | 26 (13.3) | 10 (10.2) | 0.7 (0.3–1.7) | 0.6 |
| Dialysis | 18 (3.1) | 8 (4.1) | 10 (10.2) | 2.7 (0.9–8) | 0.07 |
| In-hospital death | 8 (2.7) | 4 (2) | 4 (4.1) | 2 (0.4–11.2) | 0.4 |
| Hospitalization [days] | 10 (8–14) | 10.5 (8.8–15) | 9.5 (8–12) | - | 0.02 |
| Minimally Invasive Operations Only | |||||
|---|---|---|---|---|---|
| Total | T-Incision | J/L-Incision | OR (95% CI) | p-Value | |
| n = 98 | n = 22 | n = 76 | |||
| Procedure time [minutes] | 205 (180–244) | 205 (190–229) | 206 (179–245) | - | 0.9 |
| Bypass time [minutes] | 111 (101–137) | 117 (107–145) | 110 (96–133) | - | 0.3 |
| Aortic cross-clamp time [minutes] | 84 (76–98) | 81 (76–94) | 85 (76–99) | - | 0.6 |
| Conversion to full sternotomy | 8 (8) | 2 (9.1) | 6 (7.9) | 1.2 (0.1–7.4) | 1 |
| Revision for bleeding | 11 (11.2) | 1 (4.5) | 10 (13.2) | 0.3 (0.01–2.5) | 0.5 |
| Revision for pericardial effusion | 5 (5) | 1 (4.5) | 4 (5.3) | 0.9 (0.02–9.3) | 1 |
| Revision for sternal instability | 1 () | 0 (0) | 1 (1.3) | 0 (0–19) | 1 |
| ECMO | 2 (2) | 0 (0) | 2 (2.6) | 0 (0–19) | 1 |
| IABP | 4 (4) | 0 (0) | 4 (5.3) | 0 (0–5.3) | 0.6 |
| Pacemaker | 2 (2) | 0 (0) | 2 (2.6) | 0 (9–19) | 1 |
| Stroke | 3 (3) | 0 (0) | 3 (3.9) | 0 (0–8.5) | 1 |
| In-hospital death | 4 (4) | 0 (0) | 4 (5.3) | 0 (0–5.3) | 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Kampen, A.; Etz, C.D.; Haunschild, J.; Misfeld, M.; Davierwala, P.; Leontyev, S.; Borger, M.A. The Feasibility of Less-Invasive Bentall Surgery: A Real-World Analysis. Life 2023, 13, 2204. https://doi.org/10.3390/life13112204
van Kampen A, Etz CD, Haunschild J, Misfeld M, Davierwala P, Leontyev S, Borger MA. The Feasibility of Less-Invasive Bentall Surgery: A Real-World Analysis. Life. 2023; 13(11):2204. https://doi.org/10.3390/life13112204
Chicago/Turabian Stylevan Kampen, Antonia, Christian D. Etz, Josephina Haunschild, Martin Misfeld, Piroze Davierwala, Sergey Leontyev, and Michael A. Borger. 2023. "The Feasibility of Less-Invasive Bentall Surgery: A Real-World Analysis" Life 13, no. 11: 2204. https://doi.org/10.3390/life13112204
APA Stylevan Kampen, A., Etz, C. D., Haunschild, J., Misfeld, M., Davierwala, P., Leontyev, S., & Borger, M. A. (2023). The Feasibility of Less-Invasive Bentall Surgery: A Real-World Analysis. Life, 13(11), 2204. https://doi.org/10.3390/life13112204