The Impact of Neck Cooling on Serum Oxidant/Antioxidant Status and HSP70 Levels during High-Intensity Cycling
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Baseline Assessment
2.3. Cycling and Neck Cooling Interventions
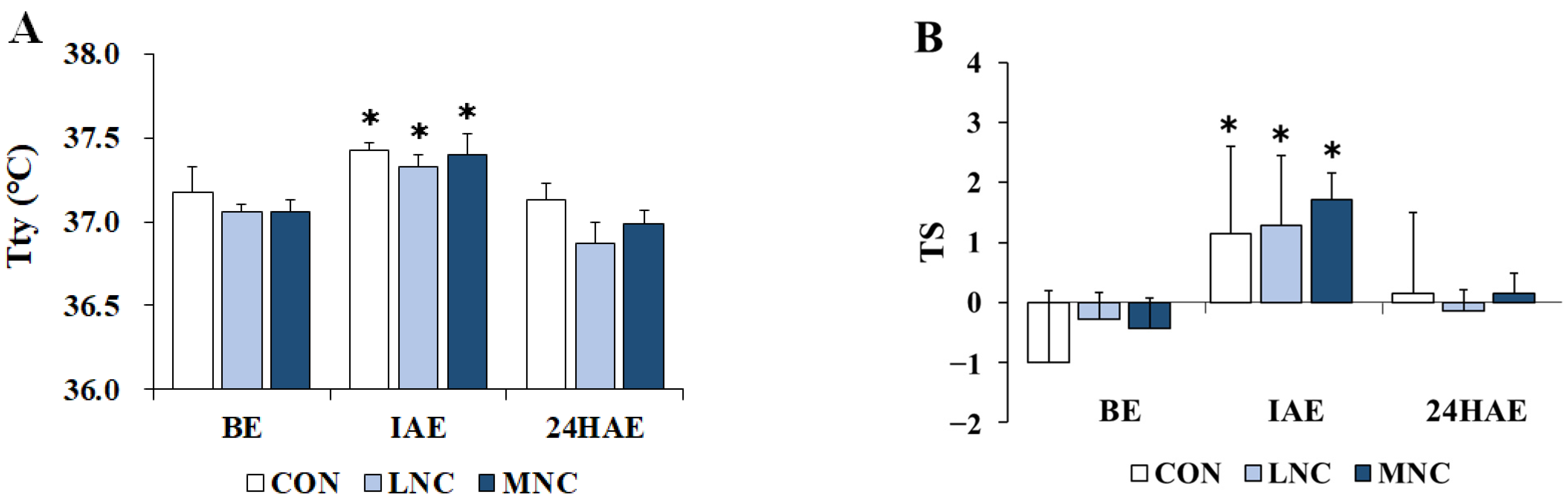
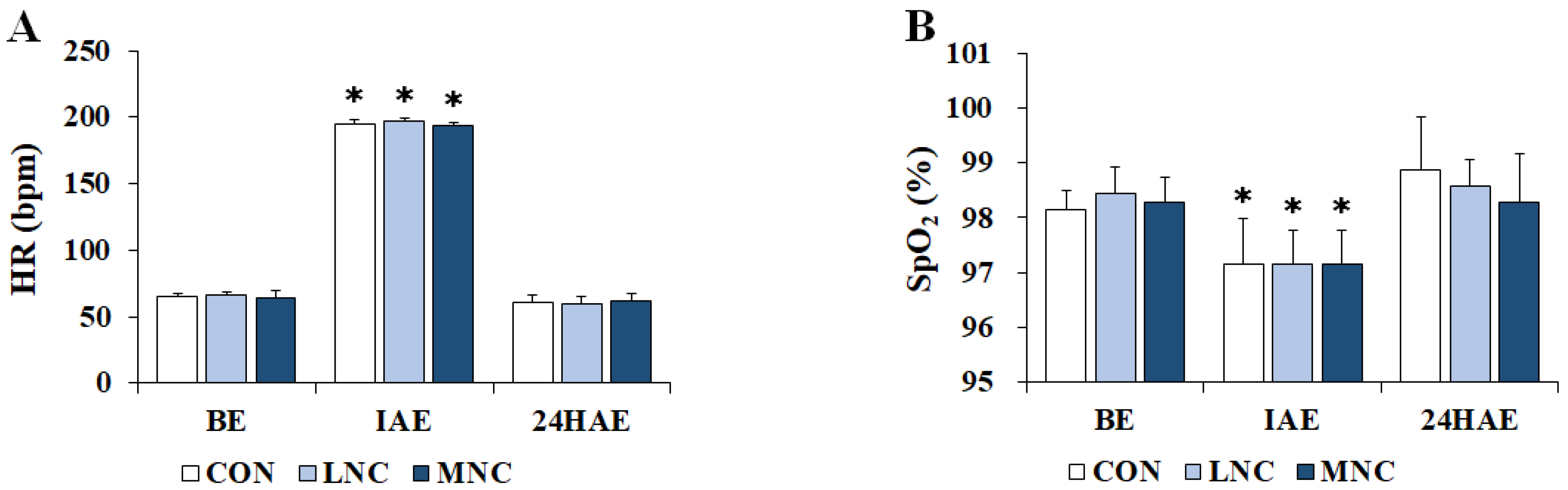
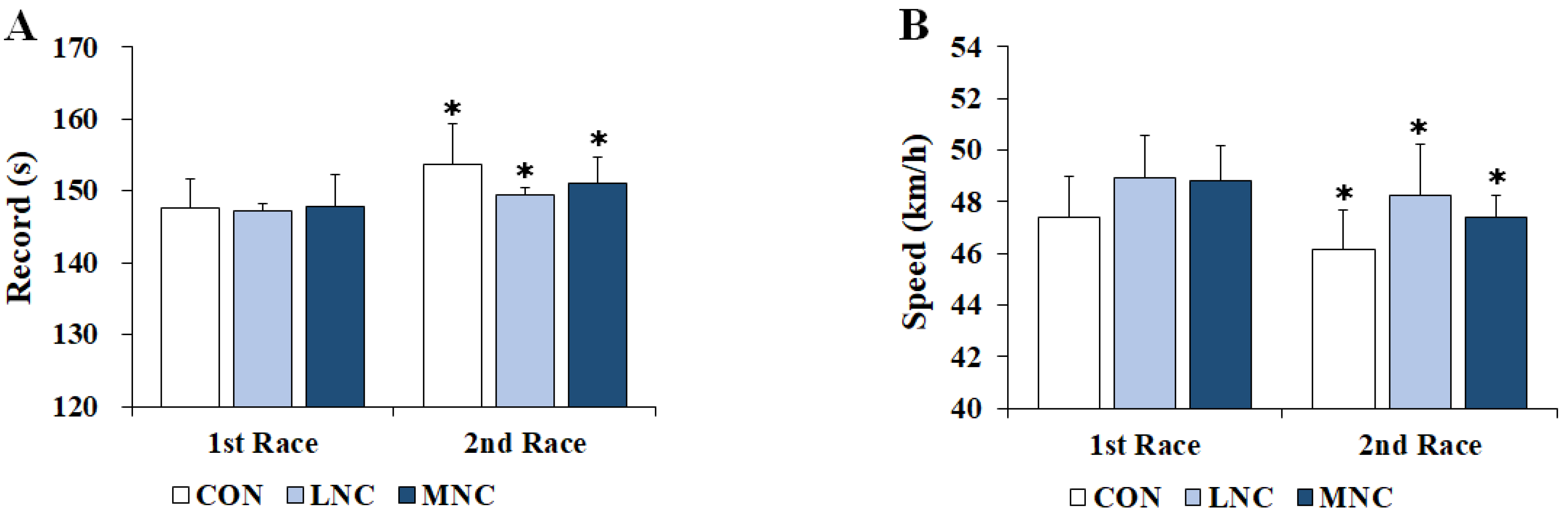
2.4. Thermoregulatory Responses, Physiological Variables, and Performance Measurement
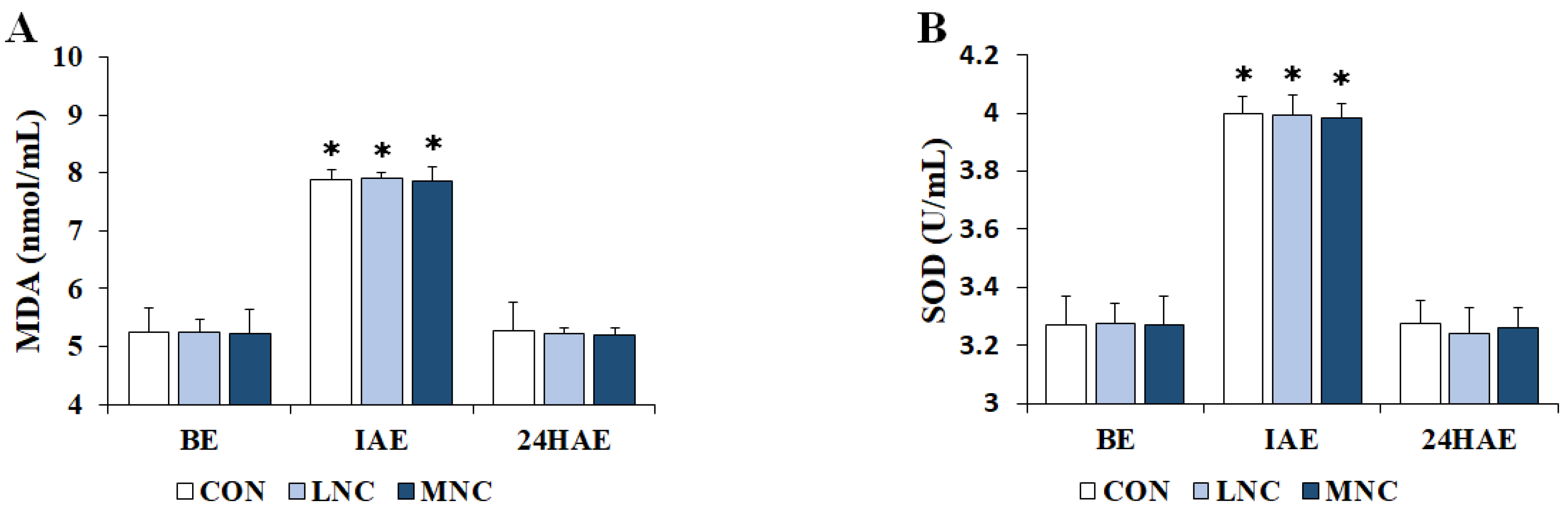
2.5. Blood Sampling and Analysis
2.6. Statistical Analyses
3. Results
3.1. Changes in Thermoregulatory Responses
3.2. Changes in Physiological Variables
3.3. Changes in Serum Oxidant/Antioxidant Status
3.4. Changes in Serum HSP70 Levels
3.5. Changes in Cycling Race Record and Speed
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sawka, M.N.; Leon, L.R.; Montain, S.J.; Sonna, L.A. Integrated physiological mechanisms of exercise performance, adaptation, and maladaptation to heat stress. Compr. Physiol. 2011, 1, 1883–1928. [Google Scholar] [PubMed]
- Nakamura, K. Central circuitries for body temperature regulation and fever. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, 1207–1228. [Google Scholar]
- Maunder, E.; Plews, D.J.; Merien, F.; Kilding, A.E. Exercise intensity regulates the effect of heat stress on substrate oxidation rates during exercise. Eur. J. Sport Sci. 2020, 20, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Nybo, L.; Rasmussen, P.; Sawka, M.N. Performance in the heat-physiological factors of importance for hyperthermia-induced fatigue. Compr. Physiol. 2014, 4, 657–689. [Google Scholar] [PubMed]
- Périard, J.D.; Eijsvogels, T.M.H.; Daanen, H.A.M. Exercise under heat stress: Thermoregulation, hydration, performance implications, and mitigation strategies. Physiol. Rev. 2021, 101, 1873–1979. [Google Scholar]
- Robertson, C.V.; Marino, F.E. Cerebral responses to exercise and the influence of heat stress in human fatigue. J. Therm. Biol. 2017, 63, 10–15. [Google Scholar]
- Cao, Y.; Lei, T.H.; Wang, F.; Yang, B.; Mündel, T. Head, Face and Neck Cooling as Per-cooling (Cooling During Exercise) Modalities to Improve Exercise Performance in the Heat: A Narrative Review and Practical Applications. Sports Med. Open. 2022, 8, 16. [Google Scholar] [CrossRef]
- Ross, M.L.; Garvican, L.A.; Jeacocke, N.A.; Laursen, P.B.; Abbiss, C.R.; Martin, D.T.; Burke, L.M. Novel precooling strategy enhances time trial cycling in the heat. Med. Sci. Sports Exerc. 2011, 43, 123–133. [Google Scholar] [CrossRef]
- Schulze, E.; Daanen, H.A.; Levels, K.; Casadio, J.R.; Plews, D.J.; Kilding, A.E.; Siegel, R.; Laursen, P.B. Effect of thermal state and thermal comfort on cycling performance in the heat. Int. J. Sports Physiol. Perform. 2015, 10, 655–663. [Google Scholar] [CrossRef]
- Pawłowska, M.; Mila-Kierzenkowska, C.; Boraczyński, T.; Boraczyński, M.; Szewczyk-Golec, K.; Sutkowy, P.; Wesołowski, R.; Budek, M.; Woźniak, A. The Influence of Ambient Temperature Changes on the Indicators of Inflammation and Oxidative Damage in Blood after Submaximal Exercise. Antioxidants 2022, 11, 2445. [Google Scholar] [CrossRef]
- Lindsay, A.; Carr, S.; Cross, S.; Petersen, C.; Lewis, J.G.; Gieseg, S.P. The physiological response to cold-water immersion following a mixed martial arts training session. Appl. Physiol. Nutr. Metab. 2017, 42, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Tyler, C.J.; Wild, P.; Sunderland, C. Practical neck cooling and time-trial running performance in a hot environment. Eur. J. Appl. Physiol. 2010, 110, 1063–1074. [Google Scholar] [CrossRef] [PubMed]
- Gordon, N.F.; Bogdanffy, G.M.; Wilkinson, J. Effect of a practical neck cooling device on core temperature during exercise. Med. Sci. Sports Exerc. 1990, 22, 245–249. [Google Scholar]
- Morton, J.P.; Kayani, A.C.; McArdle, A.; Drust, B. The exercise-induced stress response of skeletal muscle, with specific emphasis on humans. Sports Med. 2009, 39, 643–662. [Google Scholar] [CrossRef] [PubMed]
- Cooper, K.H. A means of assessing maximal oxygen intake. Correlation between field and treadmill testing. JAMA 1968, 203, 201–204. [Google Scholar] [CrossRef]
- Humphreys, M.A. “Why did the piggy bark”; some effects of language and context on the interpretation of words used in scales of warmth and thermal preference. In Proceedings of the Conference: Air Conditioning and the Low Carbon Cooling Challenge, Cumberland Lodge, Windsor, UK, 27–29 July 2008. [Google Scholar]
- Lim, C.L.; Byrne, C.; Lee, J.K. Human thermoregulation and measurement of body temperature in exercise and clinical settings. Ann. Acad. Med. Singap. 2008, 37, 347–353. [Google Scholar] [CrossRef]
- Hughson, R.L.; Green, H.J.; Houston, M.E.; Thomson, J.A.; MacLean, D.R.; Sutton, J.R. Heat injuries in Canadian mass participation runs. Can. Med. Assoc. J. 1980, 122, 1141–1144. [Google Scholar]
- Richards, R.; Richards, D. Fatal heat stroke in a “fun run”. Med. J. Aust. 1980, 2, 225–226. [Google Scholar] [CrossRef]
- Bright, F.M.; Chaseling, G.K.; Jay, O.; Morris, N.B. Self-paced exercise performance in the heat with neck cooling, menthol application, and abdominal cooling. J. Sci. Med. Sport 2019, 22, 371–377. [Google Scholar] [CrossRef]
- Hamada, S.; Torii, M.; Szygula, Z.; Adachi, K. Effect of partial body cooling on thermophysiological responses during cycling work in a hot environment. J. Therm. Biol. 2006, 31, 194–207. [Google Scholar] [CrossRef]
- Nybo, L. Hyperthermia and fatigue. J. Appl. Physiol. 2008, 104, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Crandall, C.G.; González-Alonso, J. Cardiovascular function in the heat-stressed human. Acta Physiol. 2010, 199, 407–423. [Google Scholar] [CrossRef] [PubMed]
- Torii, M.; Adachi, K.; Miyabayashi, T.; Arima, T.; Iwashita, M. Effect of bilateral carotid cooling with an ice pack on thermal responses during bicycle exercise. Environ. Ergon. 2005, 3, 113–119. [Google Scholar]
- Jackson, K.; Rubin, R.; Van Hoeck, N.; Hauert, T.; Lana, V.; Wang, H. The effect of selective head-neck cooling on physiological and cognitive functions in healthy volunteers. Transl. Neurosci. 2015, 6, 131–138. [Google Scholar] [CrossRef]
- Partridge, E.M.; Cooke, J.; McKune, A.J.; Pyne, D.B. Pre-Exercise Whole- or Partial-Body Cryotherapy Exposure to Improve Physical Performance: A Systematic Review. Sports 2021, 9, 135. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, D.; Jiang, H.; Song, Y.; Wang, Z.; Shao, L.; Liu, Y. Effects of physical exercise on biomarkers of oxidative stress in healthy subjects: A meta-analysis of randomized controlled trials. Open Life Sci. 2023, 18, 20220668. [Google Scholar] [CrossRef]
- Powers, S.K.; Deminice, R.; Ozdemir, M.; Yoshihara, T.; Bomkamp, M.P.; Hyatt, H. Exercise-induced oxidative stress: Friend or foe? J. Sport. Health Sci. 2020, 9, 415–425. [Google Scholar] [CrossRef]
- Cho, S.Y.; Chung, Y.S.; Yoon, H.K.; Roh, H.T. Impact of Exercise Intensity on Systemic Oxidative Stress, Inflammatory Responses, and Sirtuin Levels in Healthy Male Volunteers. Int. J. Environ. Res. Public. Health 2022, 19, 11292. [Google Scholar] [CrossRef]
- McAnulty, S.R.; McAnulty, L.; Pascoe, D.D.; Gropper, S.S.; Keith, R.E.; Morrow, J.D.; Gladden, L.B. Hyperthermia increases exercise-induced oxidative stress. Int. J. Sports Med. 2005, 26, 188–192. [Google Scholar] [CrossRef]
- Krüger, K.; Reichel, T.; Zeilinger, C. Role of heat shock proteins 70/90 in exercise physiology and exercise immunology and their diagnostic potential in sports. J. Appl. Physiol. 2019, 126, 916–927. [Google Scholar] [CrossRef]
- Dalgaard, L.B.; Ørtenblad, N.; Hvid, L.G.; Gejl, K.D. The expression of HSP70 in skeletal muscle is not associated with glycogen availability during recovery following prolonged exercise in elite endurance athletes. Eur. J. Appl. Physiol. 2022, 122, 1831–1842. [Google Scholar] [CrossRef] [PubMed]
- Roh, H.T.; Cho, S.Y.; So, W.Y.; Paik, I.Y.; Suh, S.H. Effects of different fluid replacements on serum HSP70 and lymphocyte DNA damage in college athletes during exercise at high ambient temperatures. J. Sport Health Sci. 2016, 5, 448–455. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pournot, H.; Bieuzen, F.; Duffield, R.; Lepretre, P.M.; Cozzolino, C.; Hausswirth, C. Short term effects of various water immersions on recovery from exhaustive intermittent exercise. Eur. J. Appl. Physiol. 2011, 111, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Bongers, C.C.; Hopman, M.T.; Eijsvogels, T.M. Cooling interventions for athletes: An overview of effectiveness, physiological mechanisms, and practical considerations. Temperature 2017, 4, 60–78. [Google Scholar] [CrossRef]
- Colvin, D.P.; Lokody, T. Development of a macroPCM neck cooling collar for athletes and runners. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition, Washington, DC, USA, 15–21 November 2003; Volume 37106, pp. 187–188. [Google Scholar]
- Moss, J.N.; Trangmar, S.J.; Mackenzie, R.W.A.; Tyler, C.J. The effects of pre- and per-cooling interventions used in isolation and combination on subsequent 15-min time-trial cycling performance in the heat. J. Sci. Med. Sport 2021, 24, 800–805. [Google Scholar] [CrossRef]
- Cuttell, S.A.; Kiri, V.; Tyler, C. A comparison of 2 practical cooling methods on cycling capacity in the heat. J. Athl. Train. 2016, 51, 525–532. [Google Scholar] [CrossRef]


| Characteristic | Mean ± SD | Range |
|---|---|---|
| Age (years) | 17.00 ± 0.76 | 16.0–18.0 |
| Height (cm) | 174.16 ± 3.05 | 169.1–177.5 |
| Weight (kg) | 65.13 ± 2.12 | 63.1–68.7 |
| BMI (kg/m2) | 21.50 ± 0.73 | 20.1–22.1 |
| SMM (kg) | 31.79 ± 0.96 | 30.7–33.5 |
| Fat Mass (kg) | 8.91 ± 1.63 | 6.2–11.0 |
| PBF (%) | 13.61 ± 2.26 | 9.8–16.3 |
| VO2max (mL/kg/min) | 53.38 ± 1.34 | 52.1–55.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, K.-S.; Roh, H.-T.; Cho, S.-Y. The Impact of Neck Cooling on Serum Oxidant/Antioxidant Status and HSP70 Levels during High-Intensity Cycling. Life 2023, 13, 2178. https://doi.org/10.3390/life13112178
Choi K-S, Roh H-T, Cho S-Y. The Impact of Neck Cooling on Serum Oxidant/Antioxidant Status and HSP70 Levels during High-Intensity Cycling. Life. 2023; 13(11):2178. https://doi.org/10.3390/life13112178
Chicago/Turabian StyleChoi, Kyung-Su, Hee-Tae Roh, and Su-Youn Cho. 2023. "The Impact of Neck Cooling on Serum Oxidant/Antioxidant Status and HSP70 Levels during High-Intensity Cycling" Life 13, no. 11: 2178. https://doi.org/10.3390/life13112178
APA StyleChoi, K.-S., Roh, H.-T., & Cho, S.-Y. (2023). The Impact of Neck Cooling on Serum Oxidant/Antioxidant Status and HSP70 Levels during High-Intensity Cycling. Life, 13(11), 2178. https://doi.org/10.3390/life13112178







