The Essential Strategies to Mitigate Cardiotoxicity Caused by Doxorubicin
Abstract
:1. Introduction
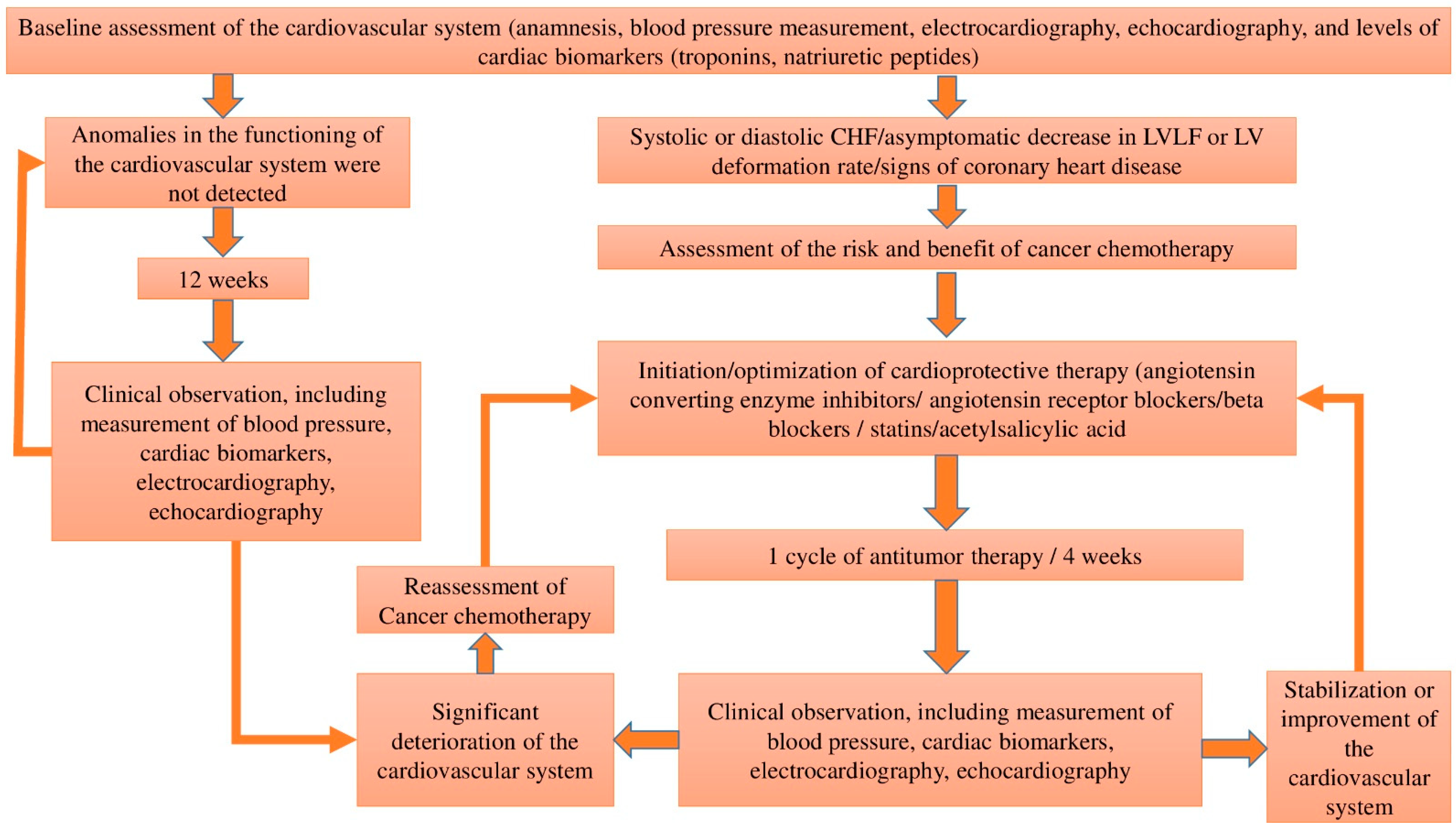
2. Early Diagnosis of Anthracycline Drugs Cardiotoxicity
3. Eliminating the Risk Factors Common for CVDs and Cancer
4. Reduction of Cumulative Dose and Prolonged Intravenous Infusion of Anthracyclines
5. Liposomal Doxorubicin
6. Exosomal Delivery of Doxorubicin
7. Dexrazoxane
8. Therapeutic Options for Anthracycline-Induced Cardiotoxicity
Statins
9. Beta-Adrenoblockers
10. RAAS Inhibitors
11. The Use of Human-Induced Pluripotent Stem Cells (hPSCs)-Cardiomyocytes
12. The Role of Genome-Wide Association Studies (GWAS Studies) in Identifying the Risk of Doxorubicin-Induced Cardiotoxicity
13. New Potential Targets and Drugs to Prevent Cardiotoxicity
13.1. mTORC1, Mammalian Target of Rapamycin Complex 1; ROS, Reactive Oxygen Species; and AMPK, AMP-Mediated Protein Kinase
13.2. Natural Phytocompounds: Resveratrol, Flavonoids, Vitamin E, and Lotusine
14. Mitochondrial-Targeted Cardioprotective Strategies
15. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blaes, A.; Prizment, A.; Koene, R.J.; Konety, S. Cardio-oncology Related to Heart Failure: Common Risk Factors Between Cancer and Cardiovascular Disease. Heart Fail. Clin. 2017, 13, 367–380. [Google Scholar] [CrossRef]
- Meijers, W.C.; de Boer, R.A. Common risk factors for heart failure and cancer. Cardiovasc. Res. 2019, 115, 844–853. [Google Scholar] [CrossRef]
- Chaulin, A.M.; Duplyakov, D.V. Environmental factors and cardiovascular diseases. Hyg. Sanit. 2021, 100, 223–228. (In Russian) [Google Scholar] [CrossRef]
- Ferlay, J.; Steliarova-Foucher, E.; Lortet-Tieulent, J.; Rosso, S.; Coebergh, J.W.W.; Comber, H.; Forman, D.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef]
- Beasley, G.S.; Towbin, J.A. Acquired and modifiable cardiovascular risk factors in patients treated for cancer. J. Thromb. Thrombolysis. 2021, 51, 846–853. [Google Scholar] [CrossRef]
- Tran, P.; Banerjee, P. Iatrogenic Decompensated Heart Failure. Curr. Heart Fail. Rep. 2020, 17, 21–27. [Google Scholar] [CrossRef]
- Chaulin, A.M.; Duplyakov, D.V. Comorbidity in chronic obstructive pulmonary disease and cardiovascular disease. Cardiovasc. Ther. Prev. 2021, 20, 2539. (In Russian) [Google Scholar] [CrossRef]
- Bluethmann, S.M.; Mariotto, A.B.; Rowland, J.H. Anticipating the “silver tsunami”: Prevalence trajectories and comorbidity burden among older Cancer survivors in the United States. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1029–1036. [Google Scholar] [CrossRef]
- Armenian, S.H.; Xu, L.; Ky, B.; Sun, C.; Farol, L.T.; Pal, S.K.; Douglas, P.S.; Bhatia, S.; Chao, C. Cardiovascular disease among survivors of adult-onset cancer: A community-based retrospective cohort study. J. Clin. Oncol. 2016, 34, 1122–1130. [Google Scholar] [CrossRef]
- Chaulin, A.M.; Abashina, O.E.; Duplyakov, D.V. Pathophysiological mechanisms of cardiotoxicity in chemotherapeutic agents. Russ. Open Med. J. 2020, 9, e0305. [Google Scholar] [CrossRef]
- Lee, C.H.; Zhang, J.F.; Yuan, K.S.; Wu, A.T.H.; Wu, S.Y. Risk of cardiotoxicity induced by adjuvant anthracycline-based chemotherapy and radiotherapy in young and old Asian women with breast cancer. Strahlenther. Onkol. 2019, 195, 629–639. (In English) [Google Scholar] [CrossRef]
- Cardinale, D.; Colombo, A.; Lamantia, G.; Colombo, N.; Civelli, M.; De Giacomi, G.; Rubino, M.; Veglia, F.; Fiorentini, C.; Cipolla, C.M. Anthracycline-induced cardiomyopathy: Clinical relevance and response to pharmacologic therapy. J. Am. Coll. Cardiol. 2010, 55, 213–220. [Google Scholar] [CrossRef]
- Lyon, A.R.; Dent, S.; Stanway, S.; Earl, H.; Brezden-Masley, C.; Cohen-Solal, A.; Tocchetti, C.G.; Moslehi, J.J.; Groarke, J.D.; Bergler-Klein, J.; et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: A position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur. J. Heart Fail. 2020, 22, 1945–1960. [Google Scholar] [CrossRef]
- Chaulin, A.M. Elevation Mechanisms and Diagnostic Consideration of Cardiac Troponins under Conditions Not Associated with Myocardial Infarction. Life 2021, 11, 1175. [Google Scholar] [CrossRef]
- Khan, M.A.; Singh, M.; Khan, M.S.; Ahmad, W.; Najmi, A.K.; Ahmad, S. Alternative approach for mitigation of doxorubicin-induced cardiotoxicity using herbal agents. Curr. Clin. Pharmacol. 2014, 9, 288–297. [Google Scholar] [CrossRef]
- Chaulin, A.M.; Duplyakov, D.V. Arrhythmogenic effects of doxorubicin. Complex Issues Cardiovasc. Dis. 2020, 9, 69–80. [Google Scholar] [CrossRef]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Muñoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The task force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef]
- Armenian, S.H.; Lacchetti, C.; Barac, A.; Carver, J.; Constine, L.S.; Denduluri, N.; Dent, S.; Douglas, P.S.; Durand, J.-B.; Ewer, M.; et al. Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2017, 35, 893–911. [Google Scholar] [CrossRef]
- Lancellotti, P.; Suter, T.M.; López-Fernández, T.; Galderisi, M.; Lyon, A.R.; Van der Meer, P.; Solal, A.C.; Zamorano, J.-L.; Jerusalem, G.; Moonen, M.; et al. Cardio-Oncology Services: Rationale, organization, and implementation. Eur. Heart J. 2019, 40, 1756–1763. [Google Scholar] [CrossRef]
- Tilemann, L.M.; Heckmann, M.B.; Katus, H.A.; Lehmann, L.H.; Müller, O.J. Cardio-oncology: Conflicting priorities of anticancer treatment and cardiovascular outcome. Clin. Res. Cardiol. 2018, 107, 271–280. [Google Scholar] [CrossRef]
- Henning, R.J.; Harbison, R.D. Cardio-oncology: Cardiovascular complications of cancer therapy. Future Cardiol. 2017, 13, 379–396. [Google Scholar] [CrossRef]
- Gripp, E.A.; Oliveira, G.E.; Feijó, L.A.; Garcia, M.I.; Xavier, S.S.; Sousa, A.S. Global Longitudinal Strain Accuracy for Cardiotoxicity Prediction in a Cohort of Breast Cancer Patients During Anthracycline and/or Trastuzumab Treatment. Arq. Bras. Cardiol. 2018, 110, 140–150. [Google Scholar] [CrossRef]
- Runowicz, C.D.; Leach, C.R.; Henry, N.L.; Henry, K.S.; Mackey, H.T.; Cowens-Alvarado, R.L.; Cannady, R.S.; Pratt-Chapman, M.; Edge, S.B.; Jacobs, L.A.; et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. J. Clin. Oncol. 2016, 34, 611–635. [Google Scholar] [CrossRef] [PubMed]
- Chaulin, A.M.; Duplyakov, D.V. Increased natriuretic peptides, not associated with heart failure. Russ. J. Cardiol. 2020, 25, 4140. [Google Scholar] [CrossRef]
- Čelutkienė, J.; Pudil, R.; López-Fernández, T.; Grapsa, J.; Nihoyannopoulos, P.; Bergler-Klein, J.; Cohen-Solal, A.; Farmakis, D.; Tocchetti, C.G.; von Haehling, S.; et al. Role of cardiovascular imaging in cancer patients receiving cardiotoxic therapies: A position statement on behalf of the Heart Failure Association (HFA), the European Association of Cardiovascular Imaging (EACVI) and the Cardio-Oncology Council of the European Society of Cardiology (ESC). Eur. J. Heart Fail. 2020, 22, 1504–1524, Erratum in Eur. J. Heart Fail. 2021, 23, 345. [Google Scholar] [CrossRef]
- de Souza, T.F.; Silva, T.Q.A.; Costa, F.O.; Shah, R.; Neilan, T.G.; Velloso, L.; Nadruz, W.; Brenelli, F.; Sposito, A.C.; Matos-Souza, J.R.; et al. Anthracycline Therapy Is Associated With Cardiomyocyte Atrophy and Preclinical Manifestations of Heart Disease. JACC Cardiovasc. Imaging 2018, 11, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.; Colombo, A.; Bacchiani, G.; Tedeschi, I.; Meroni, C.A.; Veglia, F.; Civelli, M.; Lamantia, G.; Colombo, N.; Curigliano, G.; et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 2015, 131, 1981–1988. [Google Scholar] [CrossRef]
- Armenian, S.H.; Hudson, M.M.; Mulder, R.L.; Chen, M.H.; Constine, L.S.; Dwyer, M.; Nathan, P.C.; E Tissing, W.J.; Shankar, S.; Sieswerda, E.; et al. Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: A report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2015, 16, e123–e136. [Google Scholar] [CrossRef]
- Pudil, R.; Mueller, C.; Čelutkienė, J.; Henriksen, P.A.; Lenihan, D.; Dent, S.; Barac, A.; Stanway, S.; Moslehi, J.; Suter, T.M.; et al. Role of serum biomarkers in cancer patients receiving cardiotoxic cancer therapies: A position statement from the Cardio-Oncology Study Group of the Heart Failure Association and the Cardio-Oncology Council of the European Society of Cardiology. Eur. J. Heart Fail. 2020, 22, 1966–1983. [Google Scholar] [CrossRef]
- Ananthan, K.; Lyon, A.R. The Role of Biomarkers in Cardio-Oncology. J. Cardiovasc. Transl. Res. 2020, 13, 431–450. [Google Scholar] [CrossRef]
- Chaulin, A.M.; Abashina, O.E.; Duplyakov, D.V. High-sensitivity cardiac troponins: Detection and central analytical characteristics. Cardiovasc. Ther. Prev. 2021, 20, 2590. [Google Scholar] [CrossRef]
- Jones, M.; O’gorman, P.; Kelly, C.M.; Mahon, N.; FitzGibbon, M.C. High-sensitive cardiac troponin-I facilitates timely detection of subclinical anthracycline-mediated cardiac injury. Ann. Clin. Biochem. 2017, 54, 149–157. [Google Scholar] [CrossRef]
- Christenson, E.S.; James, T.; Agrawal, V.; Park, B.H. Use of biomarkers for the assessment of chemotherapy-induced cardiac toxicity. Clin. Biochem. 2015, 48, 223–235. [Google Scholar] [CrossRef]
- Sawaya, H.; Sebag, I.A.; Plana, J.C.; Januzzi, J.L.; Ky, B.; Tan, T.C.; Cohen, V.; Banchs, J.; Carver, J.R.; Wiegers, S.E.; et al. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ. Cardiovasc. Imaging 2012, 5, 596–603. [Google Scholar] [CrossRef]
- Ky, B.; Putt, M.; Sawaya, H.; French, B.; Januzzi, J.L.; Sebag, I.A.; Plana, J.C.; Cohen, V.; Banchs, J.; Carver, J.R.; et al. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J. Am. Coll. Cardiol. 2014, 63, 809–816. [Google Scholar] [CrossRef]
- Demissei, B.G.; Freedman, G.; Feigenberg, S.J.; Plastaras, J.P.; Maity, A.; Smith, A.M.; McDonald, C.; Sheline, K.; Simone, C.B., 2nd; Lin, L.L.; et al. Early changes in cardiovascular biomarkers with contemporary thoracic radiation therapy for breast cancer, lung cancer, and lymphoma. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 851–860. [Google Scholar] [CrossRef]
- Finkelman, B.S.; Putt, M.; Wang, T.; Wang, L.; Narayan, H.; Domchek, S.; DeMichele, A.; Fox, K.; Matro, J.; Shah, P.; et al. Arginine-nitric oxide metabolites and cardiac dysfunction in patients with breast cancer. J. Am. Coll. Cardiol. 2017, 70, 152–162. [Google Scholar] [CrossRef]
- Beer, L.A.; Kossenkov, A.V.; Liu, Q.; Luning Prak, E.; Domchek, S.; Speicher, D.W.; Ky, B. Baseline immunoglobulin E levels as a marker of doxorubicin- and trastuzumab-associated cardiac dysfunction. Circ. Res. 2016, 119, 1135–1144. [Google Scholar] [CrossRef]
- Rigaud, V.O.; Ferreira, L.R.; Ayub-Ferreira, S.M.; Avila, M.S.; Brandao, S.M.; Cruz, F.D.; Santos, M.H.; Cruz, C.B.; Alves, M.S.; Issa, V.S.; et al. Circulating miR-1 as a potential biomarker of doxorubicin-induced cardiotoxicity in breast cancer patients. Oncotarget 2017, 8, 6994–7002. [Google Scholar] [CrossRef]
- Meijers, W.C.; Maglione, M.; Bakker, S.J.; Oberhuber, R.; Kieneker, L.M.; de Jong, S.; Haubner, B.J.; Nagengast, W.B.; Lyon, A.R.; van der Vegt, B.; et al. Heart failure stimulates tumor growth by circulating factors. Circulation 2018, 138, 678–691. [Google Scholar] [CrossRef]
- Jensen, B.T.; Lien, C.-Y.; Hydock, D.S.; Schneider, C.M.; Hayward, R. Exercise mitigates cardiac doxorubicin accumulation and preserves function in the rat. J. Cardiovasc. Pharmacol. 2013, 62, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.L.; Crumley, D.; McTiernan, A.; Bernstein, L.; Baumgartner, R.N.; Gilliland, F.D.; Kriska, A.; Ballard-Barbash, R. Physical activity levels before and after a diagnosis of breast carcinoma: The health, eating, activity, and lifestyle (HEAL) study. Cancer 2003, 97, 1746–1757. [Google Scholar] [CrossRef]
- Rock, C.L.; Flatt, S.W.; Newman, V.; Caan, B.J.; Haan, M.N.; Stefanick, M.L.; Faerber, S.; Pierce, J.P. Factors associated with weight gain in women after diagnosis of breast cancer. Women’s healthy eating and living study group. J. Am. Diet. Assoc. 1999, 99, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, J.; Roshan, V.D. Is short-term exercise a therapeutic tool for improvement of cardioprotection against DOX-induced cardiotoxicity? An experimental controlled protocol in rats. Asian Pac. J. Cancer Prev. 2012, 13, 4025–4030. [Google Scholar] [CrossRef]
- Aakre, K.M.; Omland, T. Physical activity, exercise and cardiac troponins: Clinical implications. Prog. Cardiovasc. Dis. 2019, 62, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Chauin, A. The Main Causes and Mechanisms of Increase in Cardiac Troponin Concentrations Other Than Acute Myocardial Infarction (Part 1): Physical Exertion, Inflammatory Heart Disease, Pulmonary Embolism, Renal Failure, Sepsis. Vasc. Health Risk Manag. 2021, 17, 601–617. [Google Scholar] [CrossRef]
- Stavroulakis, G.A.; George, K.P. Exercise-induced release of troponin. Clin. Cardiol. 2020, 43, 872–881. [Google Scholar] [CrossRef]
- Schmitz, K.H.; Courneya, K.S.; Matthews, C.; Demark-Wahnefried, W.; Galvão, D.A.; Pinto, B.M.; Irwin, M.L.; Wolin, K.Y.; Segal, R.J.; Lucia, A.; et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med. Sci. Sport. Exerc. 2010, 42, 1409–1426. [Google Scholar] [CrossRef]
- van Dalen, E.C.; van der Pal, H.J.; Kremer, L.C. Different dosage schedules for reducing cardiotoxicity in people with cancer receiving anthracycline chemotherapy. Cochrane Database Syst Rev. 2016, 3, CD005008. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef]
- Rukavitsin, O.A.; Pop, V.P. Pegylated liposomal doxorubicin (caelyx) in oncohematology: Modern aspects. Oncohematology 2008, 1–2, 75–84. [Google Scholar]
- Artamonova, E.V. Place of pegylated liposomal doxorubicin in the therapy of metastatic breast cancer. Tumors Female Reprod. Syst. 2016, 12, 35–45. [Google Scholar] [CrossRef]
- Tian, Y.; Li, S.; Song, J.; Ji, T.; Zhu, M.; Anderson, G.J.; Wei, J.; Nie, G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 2014, 35, 2383–2390. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Yang, Y.; Bai, B.; Wang, S.; Liu, M.; Sun, R.C.; Yu, T.; Chu, X.M. Potential of exosomes as diagnostic biomarkers and therapeutic carriers for doxorubicin-induced cardiotoxicity. Int. J. Biol. Sci. 2021, 17, 1328–1338. [Google Scholar] [CrossRef] [PubMed]
- Hadla, M.; Palazzolo, S.; Corona, G.; Caligiuri, I.; Canzonieri, V.; Toffoli, G.; Rizzolio, F. Exosomes increase the therapeutic index of doxorubicin in breast and ovarian cancer mouse models. Nanomedicine 2016, 11, 2431–2441. [Google Scholar] [CrossRef]
- Thakur, A.; Sidu, R.K.; Zou, H.; Alam, M.K.; Yang, M.; Lee, Y. Inhibition of Glioma Cells’ Proliferation by Doxorubicin-Loaded Exosomes via Microfluidics. Int. J. Nanomed. 2020, 15, 8331–8343. [Google Scholar] [CrossRef]
- Wei, H.; Chen, J.; Wang, S.; Fu, F.; Zhu, X.; Wu, C.; Liu, Z.; Zhong, G.; Lin, J. A Nanodrug Consisting Of Doxorubicin And Exosome Derived From Mesenchymal Stem Cells For Osteosarcoma Treatment In Vitro. Int. J. Nanomed. 2019, 14, 8603–8610. [Google Scholar] [CrossRef]
- Bagheri, E.; Abnous, K.; Farzad, S.A.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Targeted doxorubicin-loaded mesenchymal stem cells-derived exosomes as a versatile platform for fighting against colorectal cancer. Life Sci. 2020, 261, 118369. [Google Scholar] [CrossRef]
- Toffoli, G.; Hadla, M.; Corona, G.; Caligiuri, I.; Palazzolo, S.; Semeraro, S.; Gamini, A.; Canzonieri, V.; Rizzolio, F. Exosomal doxorubicin reduces the cardiac toxicity of doxorubicin. Nanomedicine 2015, 10, 2963–2971. [Google Scholar] [CrossRef]
- Schindler, C.; Collinson, A.; Matthews, C.; Pointon, A.; Jenkinson, L.; Minter, R.R.; Vaughan, T.J.; Tigue, N.J. Exosomal delivery of doxorubicin enables rapid cell entry and enhanced in vitro potency. PLoS ONE 2019, 14, e0214545. [Google Scholar] [CrossRef]
- Schloemer, N.J.; Brickler, M.; Hoffmann, R.; Pan, A.; Simpson, P.; McFadden, V.; Block, J.; Tower, R.L.; Burke, M.J. Administration of dexrazoxane improves cardiac indices in children and young adults with acute myeloid leukemia (AML) while maintaining survival outcomes. J. Pediatr. Hematol. Oncol. 2017, 39, e254–e258. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, P.; Tabone, M.-D.; Mora, J.; Morland, B.; Jones, R. Risk-benefit of dexrazoxane for preventing anthracycline-related cardiotoxicity: Re-evaluating the European labeling. Future Oncol. 2018, 14, 2663–2676. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Sung, R.Y.T.; Li, K.; Pong, N.H.; Xiang, P.; Shen, J.; Ng, P.C.; Chen, Y. Cardioprotective effect of dexrazoxane in a rat model of myocardial infarction: Anti-apoptosis and promoting angiogenesis. Int. J. Cardiol. 2011, 152, 196–201. [Google Scholar] [CrossRef]
- Xiang, P.; Deng, H.Y.; Li, K.; Huang, G.-Y.; Chen, Y.; Tu, L.; Ng, P.C.; Pong, N.H.; Zhao, H.; Zhang, L.; et al. Dexrazoxane protects against doxorubicin-induced cardiomyopathy: Upregulation of Akt and Erk phosphorylation in a rat model. Cancer Chemother. Pharmacol. 2009, 63, 343–349. [Google Scholar] [CrossRef]
- Oesterle, A.; Laufs, U.; Liao, J.K. Pleiotropic effects of statins on the cardiovascular system. Circ. Res. 2017, 120, 229–243. [Google Scholar] [CrossRef]
- Seicean, S.; Seicean, A.; Plana, J.C.; Budd, G.T.; Marwick, T.H. Effect of statin therapy on the risk for incident heart failure in patients with breast cancer receiving anthracycline chemotherapy: An observational clinical cohort study. J. Am. Coll. Cardiol. 2012, 60, 2384–2390. [Google Scholar] [CrossRef] [PubMed]
- Chotenimitkhun, R.; D’Agostino, R., Jr.; Lawrence, J.A.; Hamilton, C.A.; Jordan, J.H.; Vasu, S.; Lash, T.L.; Yeboah, J.; Herrington, D.M.; Hundley, W.G. Chronic statin administration may attenuate early anthracycline associated declines in left ventricular ejection function. Can. J. Cardiol. 2015, 31, 302–307. [Google Scholar] [CrossRef]
- Mendieta, G.; Ben-Aicha, S.; Casani, L.; Badimon, L.; Sabate, M.; Vilahur, G. Molecular pathways involved in the cardioprotective effects of intravenous statin administration during ischemia. Basic Res. Cardiol. 2019, 115, 2. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, L.; Wang, Q.; Zhou, D.; Wu, Z.; Shen, L.; Zhu, J. Atorvastatin protects cardiomyocytes from oxidative stress by inhibiting LOX-1 expression and cardiomyocyte apoptosis. Acta Biochim. Biophys. Sin. 2015, 47, 174–182. [Google Scholar] [CrossRef]
- Riad, A.; Bien, S.; Westermann, D.; Becher, P.M.; Loya, K.; Landmesser, U.; Kroemer, H.K.; Schultheiss, H.P.; Tschöpe, C. Pretreatment with statin attenuates the cardiotoxicity of Doxorubicin in mice. Cancer Res. 2009, 69, 695–699. [Google Scholar] [CrossRef]
- Beltowski, J. Statins and modulation of oxidative stress. Toxicol. Mech. Methods 2005, 15, 61–92. [Google Scholar] [CrossRef] [PubMed]
- Ünlü, S.; Nurkoç, S.G.; Sezenöz, B.; Cingirt, M.; Gülbahar, Ö.; Abacı, A. Impact of statin use on high sensitive troponin T levels with moderate exercise. Acta Cardiol. 2019, 74, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Chaulin, A.M. Review of Recent Laboratory and Experimental Data on Cardiotoxicity of Statins. J. Cardiovasc. Dev. Dis. 2022, 9, 403. [Google Scholar] [CrossRef] [PubMed]
- Ghavami, S.; Yeganeh, B.; Stelmack, G.L.; Kashani, H.H.; Sharma, P.; Cunnington, R.; Rattan, S.; Bathe, K.; Klonisch, T.; Dixon, I.M.; et al. Apoptosis, autophagy and ER stress in mevalonate cascade inhibition-induced cell death of human atrial fibroblasts. Cell Death Dis. 2012, 3, e330. [Google Scholar] [CrossRef]
- Godoy, J.C.; Niesman, I.R.; Busija, A.R.; Kassan, A.; Schilling, J.M.; Schwarz, A.; Alvarez, E.A.; Dalton, N.D.; Drummond, J.C.; Roth, D.M.; et al. Atorvastatin, but not pravastatin, inhibits cardiac Akt/mTOR signaling and disturbs mitochondrial ultrastructure in cardiac myocytes. FASEB J. 2019, 33, 1209–1225. [Google Scholar] [CrossRef]
- Nabati, M.; Janbabai, G.; Baghyari, S.; Esmaili, K.; Yazdani-Charati, J. Cardioprotective effects of Carvedilol in inhibiting doxorubicin-induced cardiotoxicity. J. Cardiovasc. Pharmacol. 2017, 69, 279–285. [Google Scholar] [CrossRef]
- Avila, M.S.; Ayub-Ferreira, S.M.; Wanderley, M.R.D.B.; Cruz, F.D.D.; Brandão, S.M.G.; Rigaud, V.O.C.; Higuchi-Dos-Santos, M.H.; Hajjar, L.A.; Filho, R.K.; Hoff, P.M.; et al. Carvedilol for Prevention of Chemotherapy-Related Cardiotoxicity: The CECCY Trial. J. Am. Coll. Cardiol. 2018, 71, 2281–2290. [Google Scholar] [CrossRef]
- Kaya, M.G.; Ozkan, M.; Gunebakmaz, O.; Akkaya, H.; Kaya, E.G.; Akpek, M.; Kalay, N.; Dikilitas, M.; Yarlioglues, M.; Karaca, H.; et al. Protective effects of nebivolol against anthracycline-induced cardiomyopathy: A randomized control study. Int. J. Cardiol. 2013, 167, 2306–2310. [Google Scholar] [CrossRef]
- Gulati, G.; Heck, S.L.; Ree, A.H.; Hoffmann, P.; Schulz-Menger, J.; Fagerland, M.W.; Gravdehaug, B.; von Knobelsdorff-Brenkenhoff, F.; Bratland, Å.; Storås, T.H.; et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): A 2 x 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur. Heart J. 2016, 37, 1671–1680. [Google Scholar] [CrossRef]
- Liu, J.; Masoudi, F.A.; Spertus, J.A.; Wang, Q.; Murugiah, K.; Spatz, E.S.; Li, J.; Li, X.; Ross, J.S.; Krumholz, H.M.; et al. Patterns of use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers among patients with acute myocardial infarction in China from 2001 to 2011: China PEACE-Retrospective AMI Study. J. Am. Heart Assoc. 2015, 4, e001343. [Google Scholar] [CrossRef]
- Cardinale, D.; Colombo, A.; Sandri, M.T.; Lamantia, G.; Colombo, N.; Civelli, M.; Martinelli, G.; Veglia, F.; Fiorentini, C.; Cipolla, C.M.; et al. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation 2006, 114, 2474–2481. [Google Scholar] [CrossRef] [PubMed]
- Burridge, P.W.; Li, Y.F.; Matsa, E.; Wu, H.; Ong, S.G.; Sharma, A.; Holmström, A.; Chang, A.C.; Coronado, M.J.; Ebert, A.D.; et al. Human induced pluripotent stem cell-derived cardiomyocytes recapitulate the predilection of breast cancer patients to doxorubicin-induced cardiotoxicity. Nat. Med. 2016, 22, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Schwach, V.; Slaats, R.H.; Passier, R. Human Pluripotent Stem Cell-Derived Cardiomyocytes for Assessment of Anticancer Drug-Induced Cardiotoxicity. Front. Cardiovasc. Med. 2020, 7, 50. [Google Scholar] [CrossRef]
- Maillet, A.; Tan, K.; Chai, X.; Sadananda, S.N.; Mehta, A.; Ooi, J.; Hayden, M.R.; Pouladi, M.A.; Ghosh, S.; Shim, W.; et al. Modeling Doxorubicin-Induced Cardiotoxicity in Human Pluripotent Stem Cell Derived-Cardiomyocytes. Sci. Rep. 2016, 6, 25333. [Google Scholar] [CrossRef] [PubMed]
- Nebigil, C.G.; Désaubry, L. Updates in Anthracycline-Mediated Cardiotoxicity. Front. Pharmacol. 2018, 9, 1262. [Google Scholar] [CrossRef] [PubMed]
- Christidi, E.; Huang, H.; Shafaattalab, S.; Maillet, A.; Lin, E.; Huang, K.; Laksman, Z.; Davis, M.K.; Tibbits, G.F.; Brunham, L.R. Variation in RARG increases susceptibility to doxorubicin-induced cardiotoxicity in patient specific induced pluripotent stem cell-derived cardiomyocytes. Sci. Rep. 2020, 10, 10363. [Google Scholar] [CrossRef] [PubMed]
- Magdy, T.; Jiang, Z.; Jouni, M.; Fonoudi, H.; Lyra-Leite, D.; Jung, G.; Romero-Tejeda, M.; Kuo, H.H.; Fetterman, K.A.; Gharib, M.; et al. RARG variant predictive of doxorubicin-induced cardiotoxicity identifies a cardioprotective therapy. Cell. Stem. Cell. 2021, 28, 2076–2089.e7. [Google Scholar] [CrossRef]
- Li, K.; Sung, R.Y.T.; Huang, W.Z.; Yang, M.; Pong, N.H.; Lee, S.M.; Chan, W.Y.; Zhao, H.; To, M.Y.; Fok, T.F.; et al. Thrombopoietin protects against in vitro and in vivo cardiotoxicity induced by doxorubicin. Circulation 2006, 113, 2211–2220. [Google Scholar] [CrossRef]
- Chan, K.Y.; Xiang, P.; Zhou, L.; Li, K.; Ng, P.C.; Wang, C.C.; Zhang, L.; Deng, H.Y.; Pong, N.H.; Zhao, H.; et al. Thrombopoietin protects against doxorubicin-induced cardiomyopathy, improves cardiac function, and reversely alters specific signalling networks. Eur. J. Heart Fail. 2011, 13, 366–376. [Google Scholar] [CrossRef]
- Chan, K.Y.Y.; Zhou, L.; Xiang, P.; Li, K.; Ng, P.C.; Wang, C.C.; Li, M.; Pong, N.H.; Tu, L.; Deng, H.; et al. Thrombopoietin improved ventricular function and regulated remodeling genes in a rat model of myocardial infarction. Int. J. Cardiol. 2013, 167, 2546–2554. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Liang, E.Y.; Zhou, L.X.; Dong, Z.L.; Liang, P.; Weng, Q.F.; Yang, M. Thrombopoietin protects H9C2 cells from excessive autophagy and apoptosis in doxorubicin-induced cardiotoxicity. Oncol. Lett. 2018, 15, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Budanov, A.V.; Karin, M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell 2008, 134, 451–460, Erratum in Cell 2009, 136, 378. [Google Scholar] [CrossRef]
- Li, R.; Huang, Y.; Semple, I.; Kim, M.; Zhang, Z.; Lee, J.H. Cardioprotective roles of sestrin 1 and sestrin 2 against doxorubicin cardiotoxicity. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H39–H48. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.J.; Tang, Y.; Zhang, J.; Wang, B.J.; Xiao, M.; Lu, G.; Li, J.; Liu, Q.; Guo, Y.; Gu, J. Cardiac SIRT1 ameliorates doxorubicin-induced cardiotoxicity by targeting sestrin 2. Redox. Biol. 2022, 52, 102310. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, Y.; Zhang, Z.; Liu, Q.; Gu, J. Cardioprotective effects of fibroblast growth factor 21 against doxorubicin-induced toxicity via the SIRT1/LKB1/AMPK pathway. Cell Death Dis. 2017, 8, e3018. [Google Scholar] [CrossRef]
- Liu, D.; Ma, Z.; Xu, L.; Zhang, X.; Qiao, S.; Yuan, J. PGC1α activation by pterostilbene ameliorates acute doxorubicin cardiotoxicity by reducing oxidative stress via enhancing AMPK and SIRT1 cascades. Aging 2019, 11, 10061–10073. [Google Scholar] [CrossRef]
- Morrison, A.; Chen, L.; Wang, J.; Zhang, M.; Yang, H.; Ma, Y.; Budanov, A.; Lee, J.H.; Karin, M.; Li, J. Sestrin2 promotes LKB1-mediated AMPK activation in the ischemic heart. FASEB J. 2015, 29, 408–417. [Google Scholar] [CrossRef]
- Wang, P.; Lan, R.; Guo, Z.; Cai, S.; Wang, J.; Wang, Q.; Li, Z.; Li, Z.; Wang, Q.; Li, J.; et al. Histone Demethylase JMJD3 Mediated Doxorubicin-Induced Cardiomyopathy by Suppressing SESN2 Expression. Front. Cell Dev. Biol. 2020, 8, 548605. [Google Scholar] [CrossRef]
- Wang, P.; Wang, L.; Lu, J.; Hu, Y.; Wang, Q.; Li, Z.; Cai, S.; Liang, L.; Guo, K.; Xie, J.; et al. SESN2 protects against doxorubicin-induced cardiomyopathy via rescuing mitophagy and improving mitochondrial function. J. Mol. Cell. Cardiol. 2019, 133, 125–137. [Google Scholar] [CrossRef]
- Eid, R.A.; Alkhateeb, M.A.; Eleawa, S.; Al-Hashem, F.H.; Al-Shraim, M.; El-Kott, A.F.; Zaki, M.S.A.; Dallak, M.A.; Aldera, H. Cardioprotective effect of ghrelin against myocardial infarction-induced left ventricular injury via inhibition of SOCS3 and activation of JAK2/STAT3 signaling. Basic Res. Cardiol. 2018, 113, 13. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.-L.; Chen, H.-L.; Wu, D.; Chen, J.-X.; Wang, X.-X.; Li, R.-L.; He, J.-H.; Mo, L.; Cen, X.; et al. Ghrelin inhibits doxorubicin cardiotoxicity by inhibiting excessive autophagy through AMPK and p38-MAPK. Biochem. Pharmacol. 2014, 88, 334–350. [Google Scholar] [CrossRef]
- Xu, Z.; Lin, S.; Wu, W.; Tan, H.; Wang, Z.; Cheng, C.; Lu, L.; Zhang, X. Ghrelin prevents doxorubicin-induced cardiotoxicity through TNF-alpha/NF-kappaB pathways and mitochondrial protective mechanisms. Toxicology 2008, 247, 133–138. [Google Scholar] [CrossRef]
- Elhadidy, M.G.; Elmasry, A.; Rabei, M.R.; Eladel, A.E. Effect of ghrelin on VEGF-B and connexin-43 in a rat model of doxorubicin-induced cardiomyopathy. J. Basic Clin. Physiol. Pharmacol. 2019, 31, 20180212. [Google Scholar] [CrossRef]
- Shati, A.A.; El-Kott, A.F. Acylated ghrelin prevents doxorubicin-induced cardiac intrinsic cell death and fibrosis in rats by restoring IL-6/JAK2/STAT3 signaling pathway and inhibition of STAT1. Naunyn Schmiedebergs Archr Pharmacol. 2019, 392, 1151–1168. [Google Scholar] [CrossRef] [PubMed]
- Shati, A.A.; Dallak, M. Acylated Ghrelin Protects the Hearts of Rats from Doxorubicin-Induced Fas/FasL Apoptosis by Stimulating SERCA2a Mediated by Activation of PKA and Akt. Cardiovasc. Toxicol. 2019, 19, 529–547. [Google Scholar] [CrossRef] [PubMed]
- Kihara, M.; Kaiya, H.; Hirai, Y.; Katayama, H.; Terao, A.; Nishikawa, M. Salmon acyl-ghrelin increases food intake and reduces doxorubicin-induced myocardial apoptosis in rats, likely by anti-oxidative activity. Peptides 2021, 137, 170471. [Google Scholar] [CrossRef] [PubMed]
- Kida, Y.; Goligorsky, M.S. Sirtuins, Cell Senescence, and Vascular Aging. Can. J. Cardiol. 2016, 32, 634–641. [Google Scholar] [CrossRef]
- Ruan, Y.; Dong, C.; Patel, J.; Duan, C.; Wang, X.; Wu, X.; Cao, Y.; Pu, L.; Lu, D.; Shen, T.; et al. SIRT1 suppresses doxorubicin-induced cardiotoxicity by regulating the oxidative stress and p38MAPK pathways. Cell. Physiol. Biochem. 2015, 35, 1116–1124. [Google Scholar] [CrossRef]
- Gu, J.; Hu, W.; Zhang, D.D. Resveratrol, a polyphenol phytoalexin, protects against doxorubicin-induced cardiotoxicity. J. Cell. Mol. Med. 2015, 19, 2324–2328. [Google Scholar] [CrossRef]
- Lou, Y.; Wang, Z.; Xu, Y.; Zhou, P.; Cao, J.; Li, Y.; Chen, Y.; Sun, J.; Fu, L. Resveratrol prevents doxorubicin-induced cardiotoxicity in H9c2 cells through the inhibition of endoplasmic reticulum stress and the activation of the Sirt1 pathway. Int. J. Mol. Med. 2015, 36, 873–880. [Google Scholar] [CrossRef]
- Zhang, C.; Feng, Y.; Qu, S.; Wei, X.; Zhu, H.; Luo, Q.; Liu, M.; Chen, G.; Xiao, X. Resveratrol attenuates doxorubicin-induced cardiomyocyte apoptosis in mice through SIRT1-mediated deacetylation of p53. Cardiovasc. Res. 2011, 90, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Terahara, N. Flavonoids in foods: A review. Nat. Prod. Commun. 2015, 10, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Syahputra, R.A.; Harahap, U.; Dalimunthe, A.; Nasution, M.P.; Satria, D. The Role of Flavonoids as a Cardioprotective Strategy against Doxorubicin-Induced Cardiotoxicity: A Review. Molecules 2022, 27, 1320. [Google Scholar] [CrossRef] [PubMed]
- Mulvihill, E.E.; Huff, M.W. Antiatherogenic properties of flavonoids: Implications for cardiovascular health. Can. J. Cardiol. 2010, 26, 17A–21A. [Google Scholar] [CrossRef] [PubMed]
- Chiva-Blanch, G.; Badimon, L. Effects of Polyphenol Intake on Metabolic Syndrome: Current Evidences from Human Trials. Oxidative Med. Cell. Longev. 2017, 2017, 5812401. [Google Scholar] [CrossRef]
- Treml, J.; Šmejkal, K. Flavonoids as Potent Scavengers of Hydroxyl Radicals. Compr. Rev. Food Sci. Food Saf. 2016, 15, 720–738. [Google Scholar] [CrossRef]
- Zaragozá, C.; Monserrat, J.; Mantecón, C.; Villaescusa, L.; Zaragozá, F.; Álvarez-Mon, M. Antiplatelet activity of flavonoid and coumarin drugs. Vascul. Pharmacol. 2016, 87, 139–149. [Google Scholar] [CrossRef]
- Vazhappilly, C.G.; Ansari, S.A.; Al-Jaleeli, R.; Al-Azawi, A.M.; Ramadan, W.S.; Menon, V.; Hodeify, R.; Siddiqui, S.S.; Merheb, M.; Matar, R.; et al. Role of flavonoids in thrombotic, cardiovascular, and inflammatory diseases. Inflammopharmacology 2019, 27, 863–869. [Google Scholar] [CrossRef]
- Ojeda, D.; Jiménez-Ferrer, E.; Zamilpa, A.; Herrera-Arellano, A.; Tortoriello, J.; Alvarez, L. Inhibition of angiotensin convertin enzyme (ACE) activity by the anthocyanins delphinidin- and cyanidin-3-O-sambubiosides from Hibiscus sabdariffa. J. Ethnopharmacol. 2010, 127, 7–10. [Google Scholar] [CrossRef]
- Bjelogrlic, S.K.; Radic, J.; Jovic, V.; Radulovic, S. Activity of d,l-alpha-tocopherol (vitamin E) against cardiotoxicity induced by doxorubicin and doxorubicin with cyclophosphamide in mice. Basic Clin. Pharmacol. Toxicol. 2005, 97, 311–319. [Google Scholar] [CrossRef]
- Puri, A.; Maulik, S.K.; Ray, R.; Bhatnagar, V. Electrocardiographic and biochemical evidence for the cardioprotective effect of vitamin E in doxorubicin-induced acute cardiotoxicity in rats. Eur. J. Pediatr. Surg. 2005, 15, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Hadi, N.; Yousif, N.G.; Al-amran, F.G.; Huntei, N.K.; Mohammad, B.I.; Ali, S.J. Vitamin E and telmisartan attenuates doxorubicin induced cardiac injury in rat through down regulation of inflammatory response. BMC Cardiovasc. Disord. 2012, 12, 63. [Google Scholar] [CrossRef] [PubMed]
- Harishkumar, R.; Selvaraj, C.I. Lotusine, an alkaloid from Nelumbo nucifera (Gaertn.), attenuates doxorubicin-induced toxicity in embryonically derived H9c2 cells. Vitr. Cell. Dev. Biol. Anim. 2020, 56, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.B.; Leung, K.T.; Poon, E.N. Mitochondrial-Targeted Therapy for Doxorubicin-Induced Cardiotoxicity. Int. J. Mol. Sci. 2022, 23, 1912. [Google Scholar] [CrossRef] [PubMed]


| Risk Factor | Score |
|---|---|
| Previous cardiovascular disease: | |
| - Heart failure or cardiomyopathy | Very high |
| - Severe heart valve disease | High |
| - Myocardial infarction or prior coronary revascularization | High |
| - Stable angina pectoris | High |
| - Baseline left ventricular ejection fraction < 50% | High |
| - Borderline left ventricular ejection fraction (50–54%) | Moderate |
| Cardiac biomarker levels (if available): | |
| - Increased concentration of cardiac troponins | Moderate |
| - Elevated levels of natriuretic peptides | Moderate |
| Demographic and cardiovascular risk factors: | |
| - Age > 80 | High |
| - Age 65–79 | Moderate |
| - Hypertension | Moderate |
| - Diabetes mellitus | Moderate |
| - Chronic kidney disease | Moderate |
| Previous cancer chemotherapy and radiotherapy: | |
| - Previous treatment with anthracyclines | High |
| - Previous radiotherapy to the breast area | High |
| - Previous chemotherapy with non-anthracyclines | Moderate |
| Lifestyle-related risk factors: | |
| - Smoking (current or long-term history) | Moderate |
| - Obesity (body mass index > 30) | Moderate |
| Baseline Cardiovascular Risk Assessment (in Accordance with [13]) | Cardiomarkers | During Anthracycline Chemotherapy | Following Anthracycline Chemotherapy |
|---|---|---|---|
| Low risk | - Natriuretic peptides (BNP/NT-proBNP) - Cardiac troponins | - Baseline - Before 5th cycle during treatment (optional) | - 12 months after final cycle |
| Medium risk | - Natriuretic peptides (BNP/NT-proBNP) - Cardiac troponins | - Baseline - Before 5th cycle - Before every cycle (optional) | - 12 months after final cycle |
| High risk | - Natriuretic peptides (BNP/NT-proBNP) - Cardiac troponins | - Baseline - Before cycles 2, 4, and 6 - Before every cycle (optional) | - 3 and/or 6 months after final cycle - 12 months after final cycle |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaulin, A.M. The Essential Strategies to Mitigate Cardiotoxicity Caused by Doxorubicin. Life 2023, 13, 2148. https://doi.org/10.3390/life13112148
Chaulin AM. The Essential Strategies to Mitigate Cardiotoxicity Caused by Doxorubicin. Life. 2023; 13(11):2148. https://doi.org/10.3390/life13112148
Chicago/Turabian StyleChaulin, Aleksey Michailovich. 2023. "The Essential Strategies to Mitigate Cardiotoxicity Caused by Doxorubicin" Life 13, no. 11: 2148. https://doi.org/10.3390/life13112148
APA StyleChaulin, A. M. (2023). The Essential Strategies to Mitigate Cardiotoxicity Caused by Doxorubicin. Life, 13(11), 2148. https://doi.org/10.3390/life13112148






