Differential Expression of Serum Proinflammatory Cytokine TNF-α and Genetic Determinants of TNF-α, CYP2C19*17, miR-423 Genes and Their Effect on Coronary Artery Disease Predisposition and Progression
Abstract
:1. Introduction
2. Methodology
2.1. Study Population
2.1.1. Patient Selection Criteria
2.1.2. Healthy Controls
2.1.3. Genomic DNA Extraction
2.1.4. Genotyping of TNF-α rs1800629 G>A, miR-423rs6505162C>A and CYP2C19*17-rs12248560 C>T was Determined by ARMS-PCR Using Tetra-Primers
2.1.5. Statistical Analysis
2.2. Estimation of Serum Expression of TNF-α
3. Results
3.1. Demographic Features of CAD Patients
3.2. Lipid Biomarkers
3.3. Hardy–Weinberg Equilibrium for Genotype Distributions and Allele Frequencies
3.4. Frequency, Distribution, and Association of Genotypes and Alleles of TNF-α G>A, CYP2C19*17—C>T, and miR-423 C>A between CAD Patient and Controls
3.4.1. Statistical Analysis to Estimate the Association of TNF-α rs1800629 G>A Genotypes with CAD Patient Susceptibility
3.4.2. Statistical Analysis to Estimate the Association of the CYP2C19*17—rs12248560 C>T Genotypes with the CAD Patient Susceptibility
3.4.3. Statistical Analysis to Estimate the Association of the miR-423 rs6505162 C>A Genotypes with CAD Patient Susceptibility
3.5. Logistic Regression to Estimate the Association of TNF-α rs1800629 G>A Genotypes with CAD Patient Susceptibility
3.6. Logistic Regression to Estimate the Association of CYP2C19*17 (rs12248560) C>T Genotypes with CAD Patient Susceptibility
3.7. Logistic Regression to Estimate the Association of the miR-423 rs6505162 C>A Genotypes with CAD Patient Susceptibility
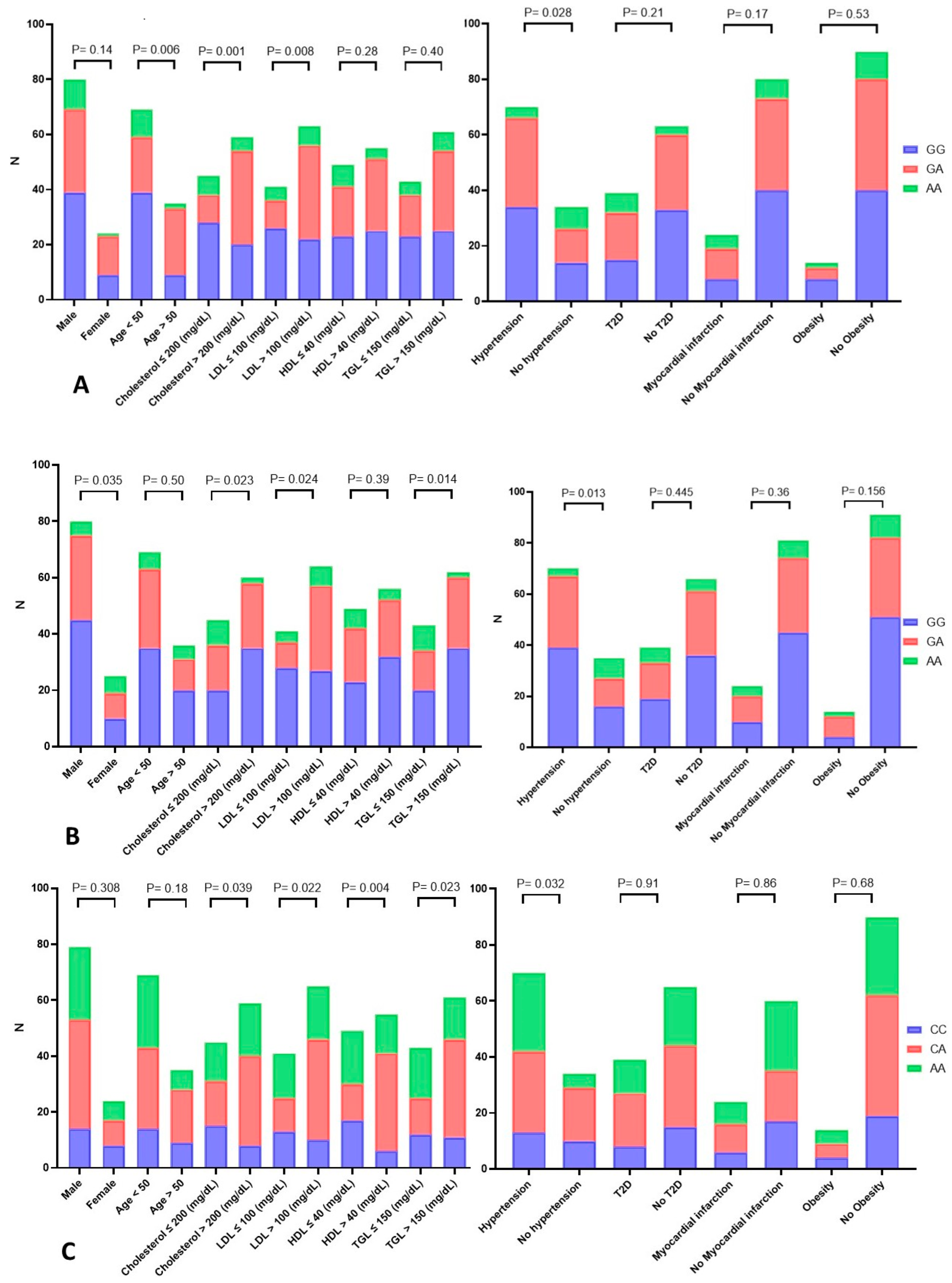
3.8. Statistical Analysis to Estimate the Association between the TNF-α rs1800629 G>A Genotypes and Demographic and Clinical Variables of CAD Patients
3.9. Statistical Analysis to Estimate the Association between the CYP2C19*17 (rs12248560) C>T Genotypes and Demographic and Clinical Variables of CAD Patients
3.10. Statistical Analysis to Estimate the Association between miR-423 rs6505162 C>A Genotypes and Demographic and Clinical Variables of CAD Patients
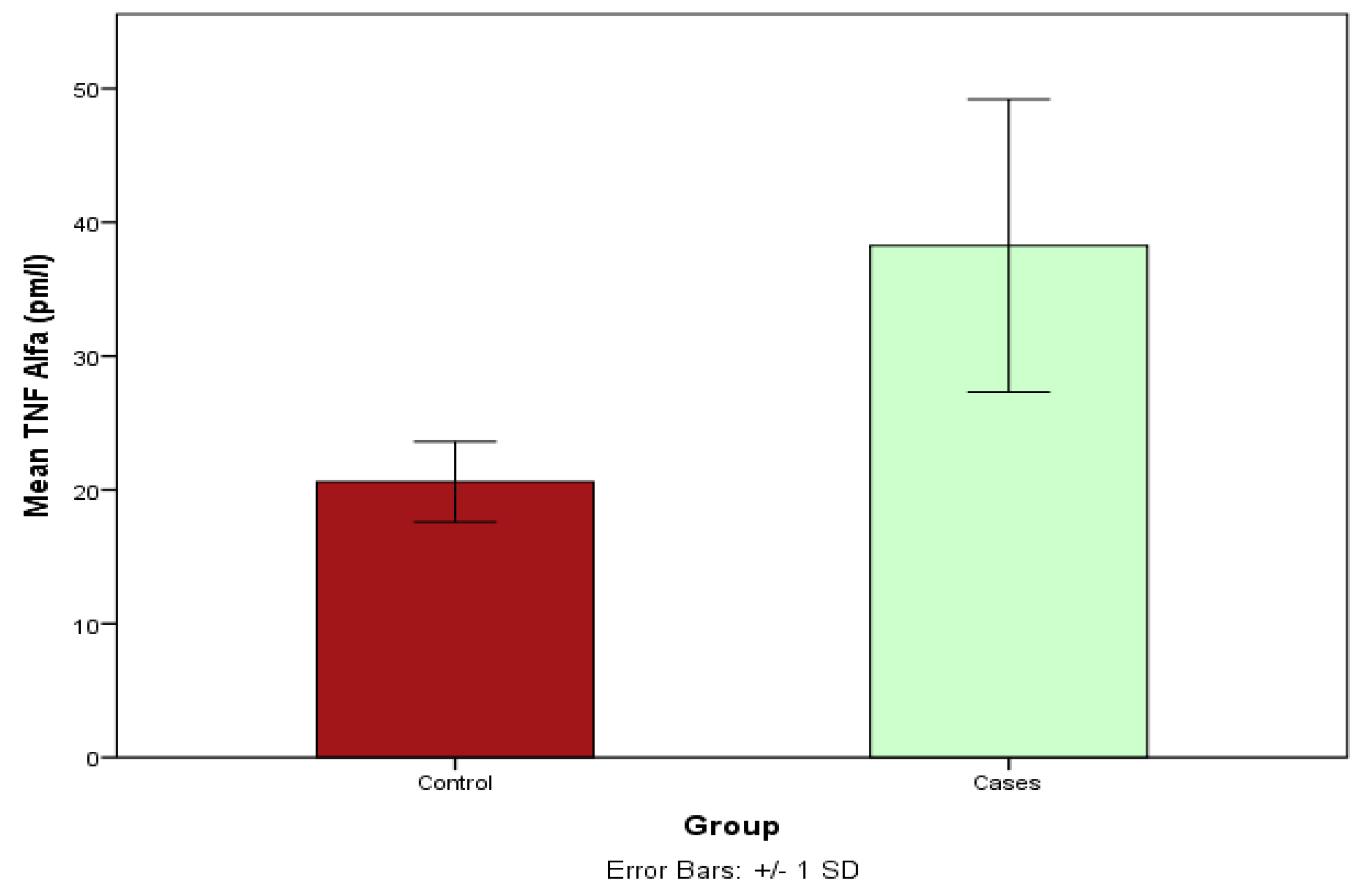
3.11. Estimation of the TNA Serum Levels between Cases and Controls Using ELISA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report from the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef] [PubMed]
- Rolski, F.; Blyszczuk, P. Complexity of TNF-alpha Signaling in Heart Disease. J. Clin. Med. 2020, 9, 3267. [Google Scholar] [CrossRef] [PubMed]
- Freeman, A.M.; Raman, S.V.; Aggarwal, M.; Maron, D.J.; Bhatt, D.L.; Parwani, P.; Osborne, J.; Earls, J.P.; Min, J.K.; Bax, J.J.; et al. Integrating Coronary Atherosclerosis Burden and Progression with Coronary Artery Disease Risk Factors to Guide Therapeutic Decision Making. Am. J. Med. 2023, 136, 260–269.e7. [Google Scholar] [CrossRef] [PubMed]
- Henein, M.Y.; Vancheri, S.; Longo, G.; Vancheri, F. The Role of Inflammation in Cardiovascular Disease. Int. J. Mol. Sci. 2022, 23, 12906. [Google Scholar] [CrossRef] [PubMed]
- Engelen, S.E.; Robinson, A.J.B.; Zurke, Y.-X.; Monaco, C. Therapeutic strategies targeting inflammation and immunity in atherosclerosis: How to proceed? Nat. Rev. Cardiol. 2022, 19, 522–542. [Google Scholar] [CrossRef]
- Wong, N.D.; Budoff, M.J.; Ferdinand, K.; Graham, I.M.; Michos, E.D.; Reddy, T.; Shapiro, M.D.; Toth, P.P. Atherosclerotic cardiovascular disease risk assessment: An American Society for Preventive Cardiology clinical practice statement. Am. J. Prev. Cardiol. 2022, 10, 100335. [Google Scholar]
- Jang, D.I.; Lee, A.-H.; Shin, H.-Y.; Song, H.-R.; Park, J.-H.; Kang, T.-B.; Lee, S.-R.; Yang, S.-H. The Role of Tumor Necrosis Factor Alpha (TNF-alpha) in Autoimmune Disease and Current TNF-alpha Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef] [PubMed]
- Fioranelli, M.; Bottaccioli, A.G.; Bottaccioli, F.; Bianchi, M.; Rovesti, M.; Roccia, M.G. Stress and Inflammation in Coronary Artery Disease: A Review Psychoneuroendocrineimmunology-Based. Front. Immunol. 2018, 9, 2031. [Google Scholar] [CrossRef]
- Yuan, S.; Carter, P.; Bruzelius, M.; Vithayathil, M.; Kar, S.; Mason, A.M.; Lin, A.; Burgess, S.; Larsson, S.C. Effects of tumour necrosis factor on cardiovascular disease and cancer: A two-sample Mendelian randomization study. EBioMedicine 2020, 59, 102956. [Google Scholar] [CrossRef]
- Guengerich, F.P. Mechanisms of Cytochrome P450-Catalyzed Oxidations. ACS Catal. 2018, 8, 10964–10976. [Google Scholar] [CrossRef]
- Elfaki, I.; Mir, R.; Almutairi, F.M.; Duhier, F.M.A. Cytochrome P450: Polymorphisms and Roles in Cancer, Diabetes and Atherosclerosis. Asian Pac. J. Cancer Prev. 2018, 19, 2057–2070. [Google Scholar] [PubMed]
- Sarparast, M.; Dattmore, D.; Alan, J.; Lee, K.S.S. Cytochrome P450 Metabolism of Polyunsaturated Fatty Acids and Neurodegeneration. Nutrients 2020, 12, 3523. [Google Scholar] [CrossRef] [PubMed]
- Rendic, S.; Guengerich, F.P. Survey of Human Oxidoreductases and Cytochrome P450 Enzymes Involved in the Metabolism of Xenobiotic and Natural Chemicals. Chem. Res. Toxicol. 2015, 28, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Zanger, U.M.; Schwab, M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Zolfaghari, R. Cytochrome P450s in the regulation of cellular retinoic acid metabolism. Annu. Rev. Nutr. 2011, 31, 65–87. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P. Cytochrome P450 2E1 and its roles in disease. Chem. Biol. Interact. 2020, 322, 109056. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Mucino-Olmos, E.A.; Vázquez-Jiménez, A.; López-Esparza, D.E.; Maldonado, V.; Valverde, M.; Resendis-Antonio, O. MicroRNAs Regulate Metabolic Phenotypes during Multicellular Tumor Spheroids Progression. Front. Oncol. 2020, 10, 582396. [Google Scholar] [CrossRef]
- Annese, T.; Tamma, R.; De Giorgis, M.; Ribatti, D. microRNAs Biogenesis, Functions and Role in Tumor Angiogenesis. Front. Oncol. 2020, 10, 581007. [Google Scholar] [CrossRef]
- Rizzacasa, B.; Morini, E.; Mango, R.; Vancheri, C.; Budassi, S.; Massaro, G.; Maletta, S.; Macrini, M.; D’annibale, S.; Romeo, F.; et al. MiR-423 is differentially expressed in patients with stable and unstable coronary artery disease: A pilot study. PLoS ONE 2019, 14, e0216363. [Google Scholar] [CrossRef]
- Vulf, M.; Shunkina, D.; Komar, A.; Bograya, M.; Zatolokin, P.; Kirienkova, E.; Gazatova, N.; Kozlov, I.; Litvinova, L. Analysis of miRNAs Profiles in Serum of Patients with Steatosis and Steatohepatitis. Front. Cell Dev. Biol. 2021, 9, 736677. [Google Scholar] [CrossRef]
- Jalal, M.M.; Mir, R.; Hamadi, A.; Altayar, M.A.; Elfaki, I.; Barnawi, J.; Alkayyal, A.A.; Amr, M.; Hadeel, J.; Moawadh, M.S.; et al. Association of Genetic and Allelic Variants of Von Willebrand Factor (VWF), Glutathione S-Transferase and Tumor Necrosis Factor Alpha with Ischemic Stroke Susceptibility and Progression in the Saudi Population. Life 2023, 13, 1200. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.M.; Mir, R.; Alghamdi, M.A.A.; Wani, J.I.; Elfaki, I.; Sabah, Z.U.; Alhujaily, M.; Jeelani, M.; Marakala, V.; Alharthi, M.H.; et al. Potential impact of GCK, MIR-196A-2 and MIR-423 gene abnormalities on the development and progression of type 2 diabetes mellitus in Asir and Tabuk regions of Saudi Arabia. Mol. Med. Rep. 2022, 25, 162. [Google Scholar] [CrossRef] [PubMed]
- Imadeldin Elfaki, R.M.; Tayeb, F.J.; Barnawi, J.; Alalawy, A.I.; Mirghani, H.; Algehainy, N.; Alshammari, S.E.; Dabla, P.K. Pharmacogenetics of CYP2C19*17: Functional and Clinical Implications of CYP2C19*17—rs12248560 (c.-806C>T) in the Development of Type 2 Diabetes. Biomed. Pharmacol. J. 2022, 15, 1897–1904. [Google Scholar] [CrossRef]
- Albar, H.M.; Alahmdi, R.A.; Almedimigh, A.A.; Shaik, R.A.; Ahmad, M.S.; Almutairi, A.B.; Alghuyaythat, W.K.Z.; Alaoufi, S.Y.; Aloyari, W.O.; Alanazi, R.M.S. Prevalence of coronary artery disease and its risk factors in Majmaah City, Kingdom of Saudi Arabia. Front. Cardiovasc. Med. 2022, 9, 943611. [Google Scholar] [CrossRef] [PubMed]
- Tcheandjieu, C.; Zhu, X.; Hilliard, A.T.; Clarke, S.L.; Napolioni, V.; Ma, S.; Lee, K.M.; Fang, H.; Chen, F.; Lu, Y.; et al. Large-scale genome-wide association study of coronary artery disease in genetically diverse populations. Nat. Med. 2022, 28, 1679–1692. [Google Scholar] [CrossRef] [PubMed]
- Ladha, F.A.; Stitzel, M.L.; Hinson, J.T. From GWAS Association to Function: Candidate Gene Screening within Insulin Resistance-Associated Genomic Loci Using a Preadipocyte Differentiation Model. Circ. Res. 2020, 126, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Ramseyer, V.D.; Garvin, J.L. Tumor necrosis factor-alpha: Regulation of renal function and blood pressure. Am. J. Physiol. Renal Physiol. 2013, 304, F1231–F1242. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Cheng, Y.; Usa, K.; Liu, Y.; Baker, M.A.; Mattson, D.L.; He, Y.; Wang, N.; Liang, M. Renal Tumor Necrosis Factor alpha Contributes to Hypertension in Dahl Salt-Sensitive Rats. Sci. Rep. 2016, 6, 21960. [Google Scholar] [CrossRef]
- Fuchs, F.D.; Whelton, P.K. High Blood Pressure and Cardiovascular Disease. Hypertension 2020, 75, 285–292. [Google Scholar] [CrossRef]
- Ghareeb, D.; Abdelazem, A.S.; Hussein, E.M.; Al-Karamany, A.S. Association of TNF-alpha-308 G>A (rs1800629) polymorphism with susceptibility of metabolic syndrome. J. Diabetes Metab. Disord. 2021, 20, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Tarahomi, T.; Singh, L.; Bollampally, M.; Heydari-Kamjani, M.; Kesselman, M.M. Cardiovascular Risk Associated with TNF Alpha Inhibitor Use in Patients with Rheumatoid Arthritis. Cureus 2021, 13, e17938. [Google Scholar] [CrossRef] [PubMed]
- Tousoulis, D.; Oikonomou, E.; Economou, E.K.; Crea, F.; Kaski, J.C. Inflammatory cytokines in atherosclerosis: Current therapeutic approaches. Eur. Heart J. 2016, 37, 1723–1732. [Google Scholar] [CrossRef] [PubMed]
- Clark, P.R.; Kim, R.K.; Pober, J.S.; Kluger, M.S. Tumor necrosis factor disrupts claudin-5 endothelial tight junction barriers in two distinct NF-kappaB-dependent phases. PLoS ONE 2015, 10, e0120075. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Mao, M.; Nian, F. The Effect of TNF-alpha on CHD and the Relationship between TNF-alpha Antagonist and CHD in Rheumatoid Arthritis: A Systematic Review. Cardiol. Res. Pract. 2022, 2022, 6192053. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, S.; Zhang, H.; Hill, M.A.; Zhang, C.; Park, Y. Interaction of IL-6 and TNF-alpha contributes to endothelial dysfunction in type 2 diabetic mouse hearts. PLoS ONE 2017, 12, e0187189. [Google Scholar]
- Nicolaou, A.; Zhao, Z.; Northoff, B.H.; Sass, K.; Herbst, A.; Kohlmaier, A.; Chalaris, A.; Wolfrum, C.; Weber, C.; Steffens, S.; et al. Adam17 Deficiency Promotes Atherosclerosis by Enhanced TNFR2 Signaling in Mice. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 247–257. [Google Scholar] [CrossRef]
- Liu, S.; Wang, C.; Guo, J.; Yang, Y.; Huang, M.; Li, L.; Wang, Y.; Qin, Y.; Zhang, M. Serum Cytokines Predict the Severity of Coronary Artery Disease without Acute Myocardial Infarction. Front. Cardiovasc. Med. 2022, 9, 896810. [Google Scholar] [CrossRef]
- Payan, M.; Tajik, N.; Rouini, M.R.; Ghahremani, M.H. Genotype and allele frequency of CYP2C19*17 in a healthy Iranian population. Med. J. Islam. Repub. Iran 2015, 29, 269. [Google Scholar]
- Gaio, V.; Nunes, B.; Fernandes, A.; Mendonça, F.; Correia, F.H.; Beleza, A.; Gil, A.P.; Bourbon, M.; Vicente, A.; Dias, C.M.; et al. Genetic variation at the CYP2C19 gene associated with metabolic syndrome susceptibility in a South Portuguese population: Results from the pilot study of the European Health Examination Survey in Portugal. Diabetol. Metab. Syndr. 2014, 6, 23. [Google Scholar] [CrossRef]
- Bai, Y.; Huang, R.; Wan, L.; Zhao, R. Association between CYP2C19 gene polymorphisms and lipid metabolism in Chinese patients with ischemic stroke. J. Int. Med. Res. 2020, 48, 300060520934657. [Google Scholar] [CrossRef]
- Ariyanti, R.; Besral, B. Dyslipidemia Associated with Hypertension Increases the Risks for Coronary Heart Disease: A Case-Control Study in Harapan Kita Hospital, National Cardiovascular Center, Jakarta. J. Lipids 2019, 2019, 2517013. [Google Scholar] [CrossRef] [PubMed]
- Frak, W.; Wojtasińska, A.; Lisińska, W.; Młynarska, E.; Franczyk, B.; Rysz, J. Pathophysiology of Cardiovascular Diseases: New Insights into Molecular Mechanisms of Atherosclerosis, Arterial Hypertension, and Coronary Artery Disease. Biomedicines 2022, 10, 1938. [Google Scholar] [CrossRef] [PubMed]
- Cai, N.; Li, C.; Gu, X.; Zeng, W.; Zhong, J.; Liu, J.; Zeng, G.; Zhu, J.; Hong, H. CYP2C19 loss-of-function is associated with increased risk of hypertension in a Hakka population: A case-control study. BMC Cardiovasc. Disord. 2023, 23, 185. [Google Scholar] [CrossRef] [PubMed]
- Peng J, Luo F, Ruan G, Peng R, Li X: Hypertriglyceridemia and atherosclerosis. Lipids Health Dis. 2017, 16, 233. [CrossRef] [PubMed]
- Casula, M.; Colpani, O.; Xie, S.; Catapano, A.L.; Baragetti, A. HDL in Atherosclerotic Cardiovascular Disease: In Search of a Role. Cells 2021, 10, 1869. [Google Scholar] [CrossRef] [PubMed]
- Poznyak, A.V.; Sadykhov, N.K.; Kartuesov, A.G.; Borisov, E.E.; Melnichenko, A.A.; Grechko, A.V.; Orekhov, A.N. Hypertension as a risk factor for atherosclerosis: Cardiovascular risk assessment. Front. Cardiovasc. Med. 2022, 9, 959285. [Google Scholar] [CrossRef]
- Jha, C.K.; Mir, R.; Elfaki, I.; Khullar, N.; Rehman, S.; Javid, J.; Banu, S.; Chahal, S.M.S. Potential Impact of MicroRNA-423 Gene Variability in Coronary Artery Disease. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 67–74. [Google Scholar] [CrossRef]
- Morales-Pison, S.; Jara, L.; Carrasco, V.; Gutiérrez-Vera, C.; Reyes, J.M.; Gonzalez-Hormazabal, P.; Carreño, L.J.; Tapia, J.C.; Contreras, H.R. Genetic Variation in MicroRNA-423 Promotes Proliferation, Migration, Invasion, and Chemoresistance in Breast Cancer Cells. Int. J. Mol. Sci. 2021, 23, 380. [Google Scholar] [CrossRef]
- Tijsen, A.J.; Creemers, E.E.; Moerland, P.D.; de Windt, L.J.; van der Wal, A.C.; Kok, W.E.; Pinto, Y.M. MiR423-5p as a circulating biomarker for heart failure. Circ. Res. 2010, 106, 1035–1039. [Google Scholar] [CrossRef]
- Yang, W.; Wang, J.; Chen, Z.; Chen, J.; Meng, Y.; Chen, L.; Chang, Y.; Geng, B.; Sun, L.; Dou, L.; et al. NFE2 Induces miR-423-5p to Promote Gluconeogenesis and Hyperglycemia by Repressing the Hepatic FAM3A-ATP-Akt Pathway. Diabetes 2017, 66, 1819–1832. [Google Scholar] [CrossRef]
- Wang, T.; Li, M.; Zeng, T.; Hu, R.; Xu, Y.; Xu, M.; Zhao, Z.; Chen, Y.; Wang, S.; Lin, H.; et al. Association Between Insulin Resistance and Cardiovascular Disease Risk Varies According to Glucose Tolerance Status: A Nationwide Prospective Cohort Study. Diabetes Care 2022, 45, 1863–1872. [Google Scholar] [CrossRef]
- Li, W.; Jin, K.; Luo, J.; Xu, W.; Wu, Y.; Zhou, J.; Wang, Y.; Xu, R.; Jiao, L.; Wang, T.; et al. NF-kappaB and its crosstalk with endoplasmic reticulum stress in atherosclerosis. Front. Cardiovasc. Med. 2022, 9, 988266. [Google Scholar] [CrossRef]

| Amplification-Refractory Mutation System Primers of TNF-α rs1800629 G>A Genotyping | Ref | ||||
|---|---|---|---|---|---|
| TNF-α FI | (A allele) | 5′-AGTTGGGGACACGCAAGCATGAAGGATA-3′ | 60 °C | 154 bp | [22] |
| TNF-α RI | (G allele) | 5′-TAGGACCCTGGAGGCTAGACCCCGTACC-3′ | 224 bp | ||
| TNF-α Fo | 5′-ACCCAAACACAGGCCTCAGGACTCAACA-3′ | 323 bp | |||
| TNF-α Ro | 5′-TGGAGGCAATAGCTTTTGAGGGGCAGGA-3′ | ||||
| Amplification-Refractory Mutation System Primers of miR-423 rs6505162 C>A Genotyping | |||||
| miR-423 FI | (A allele) | 5′-TGAGGCCCCTCAGTCTTGCTTCCCAA-3′ | 62 °C | 228 bp | [23] |
| miR-423 RI | (C allele) | 5′-CAAGCGGGGAGAAACTCAAGCGCGAGG-3′ | 160 bp | ||
| miR-423 Fo | 5′-TTTTCCCGGATGGAAGCCCGAAGTTTGA-3′ | 336 bp | |||
| miR-423 Ro | 5′-TTTTGCGGCAACGTATACCCCAATTTCC-3′ | ||||
| Amplification-Refractory Mutation System Primers of CYP2C19*17-rs12248560 C>T Genotyping | |||||
| CYP2C19*17 FI | T allele | 5′-TTTTTCAAATTTGTGTCTTCTGTTCTCAAATT-3′ | 56 °C | 227 bp | [24] |
| CYP2C19*17 RI | C allele | 5′-GCGCATTATCTCTTACATCAGAGCTG-3′ | 292 bp | ||
| CYP2C19*17 Fo | 5′-GAGATCAGCTCTTCCTTCAGTTACAC-3′ | 462 bp | |||
| CYP2C19*17 Ro | 5′-CACCTTTACCATTTAACCCCCTAAAAA-3′ | ||||
| n = 104 | % | n = 114 | % | |
|---|---|---|---|---|
| Male | 80 | 76.92 | 74 | 64.91 |
| Female | 24 | 23.07 | 40 | 35.08 |
| Age < 50 | 69 | 66.34 | 64 | 56.14 |
| Age > 50 | 35 | 33.65 | 50 | 43.85 |
| Lipid Biomarkers | ||||
| HDL ≤ 40 (mg/dL) | 49 | 47.11 | ||
| HDL > 40 (mg/dL) | 55 | 52.88 | ||
| Cholesterol ≤ 200 (mg/dL) | 45 | 43.26 | ||
| Cholesterol > 200 (mg/dL) | 59 | 56.73 | ||
| TGL ≤ 150 (mg/dL) | 43 | 41.34 | ||
| TGL > 150 (mg/dL) | 61 | 58.65 | ||
| LDL ≤ 100 (mg/dL) | 41 | 39.42 | ||
| LDL > 100 (mg/dL) | 63 | 60.57 | ||
| Clinical Complications | ||||
| Hypertension | 70 | 67.30 | ||
| No hypertension | 34 | 32.70 | ||
| T2D | 39 | 37.5 | ||
| No T2D | 65 | 62.5 | ||
| Myocardial infarction | 24 | 23.07 | ||
| No Myocardial infarction | 80 | 76.92 | ||
| Obesity | 14 | 13.46 | ||
| No Obesity | 90 | 86.53 | ||
| Group | Mean ± Std. | p-Value | |
|---|---|---|---|
| Age | Cases | 35.33 ± 14.31 | 0.090 |
| Control | 39.36 ± 12.20 | ||
| FBG (mmol/L) | Cases | 7.66 ± 2.34 | <0.05 |
| Controls | 6.69 ± 2.10 | ||
| Lipid profile | |||
| Cholesterol | Cases | 129.22 ± 10.11 | <0.00012 |
| Controls | 110.31 ± 8.01 | ||
| VLDL | Cases | 40.09 ± 7.01 | <0.00013 |
| Controls | 27.98 ± 5.58 | ||
| Triglyceride | Cases | 151.16 ± 15.01 | <0.00012 |
| Controls | 125.70 ± 8.99 | ||
| HDL | Cases | 33.90 ± 8.81 | <0.00013 |
| Controls | 28.27 ± 5.10 | ||
| LDL | Cases | 185.70 ± 33.37 | <0.00012 |
| Controls | 118.83 ± 33.37 | ||
| Potential Association of the TNF-α rs1800629 G>A Genotypes with CAD | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Subjects | n = 208 | GG | GA | AA | Df | X2 | G | A | p-Value |
| CAD | 104 | 48 (46.15%) | 44 (42.30%) | 12 (11.53%) | 2 | 10.7 | 0.67 | 0.33 | 0.006 |
| Controls | 104 | 70 (67.30%) | 29 (27.88%) | 5 (4.80%) | 0.81 | 0.19 | |||
| Potential Association of the CYP2C19*17—rs12248560 C>T Genotypes with CAD | |||||||||
| Subjects | n = 209 | CC | CT | TT | Df | X2 | C | T | p-Value |
| Cases | 105 | 55 (52.88%) | 39 (37.5%) | 11 (10.47%) | 2 | 7.98 | 0.71 | 0.29 | 0.018 |
| Controls | 104 | 73 (70.19%) | 27 (25.96%) | 04 (3.84%) | 0.84 | 0.16 | |||
| Potential Association of the miR-423 rs6505162 C>A Genotypes with CAD | |||||||||
| Subjects | n = 218 | CC | CA | AA | Df | X2 | C | A | p-Value |
| Cases | 104 | 23 (22.11%) | 48 (46.15%) | 33 (31.73%) | 2 | 6.0 | 0.46 | 0.54 | 0.049 |
| Controls | 114 | 32 (28.07%) | 62 (54.38%) | 20 (17.54%) | 0.55 | 0.45 | |||
| Genotypes | Healthy Controls (n = 104) | CAD Cases (n = 104) | Odd Ratio OR (95% CI) | Risk Ratio RR (95% CI) | p-Value |
|---|---|---|---|---|---|
| Codominant model | |||||
| TNF-α—GG | 70 | 48 | 1 (ref.) | 1 (ref.) | |
| TNF-α—GA | 29 | 44 | 2.21 (1.2197 to 4.0138) | 1.49 (1.0847 to 2.0557) | 0.009 |
| TNF-α—AA | 05 | 12 | 3.12 (1.1580 to 10.578) | 2.1 (0.9514 to 4.2761) | 0.026 |
| Dominant model | |||||
| TNF-α—GG | 70 | 48 | 1 (ref.) | 1 (ref.) | |
| TNF-α (GA + AA) | 34 | 56 | 2.40 (1.3685 to 4.2159) | 1.57 (1.1583 to 2.1289) | 0.002 |
| Recessive model | |||||
| TNF-α—(GA + GG) | 99 | 92 | 1 (ref.) | 1 (ref.) | |
| TNF-α—AA | 05 | 12 | 2.58 (0.8760 to 7.6142) | 1.76 (0.8760 to 7.6142) | 0.085 |
| Allele | |||||
| TNF-α—G | 169 | 140 | 1 (ref.) | 1 (ref.) | |
| TNF-α—A | 39 | 68 | 2.10 (1.3381 to 3.3107) | 1.50 (1.1455 to 1.9656) | 0.0013 |
| Over dominant model | |||||
| TNF-α—(GG + AA) | 75 | 60 | 1 (ref.) | 1 (ref.) | |
| TNF-α (GA) | 29 | 44 | 1.89 (1.063 to 3.383) | 1.30 (1.0152 to 1.9265) | 0.030 |
| Genotypes | Healthy Controls | CAD Cases | OR (95% CI) | Risk Ratio (RR) | p-Value |
|---|---|---|---|---|---|
| (n = 104) | (n = 105) | ||||
| Codominant model | |||||
| CYP2C19*17—CC | 73 | 55 | 1 (ref.) | 1 (ref.) | |
| CYP2C19*17—CT | 27 | 39 | 1.91 (1.0493 to 3.5028) | 1.39 (1.0056 to 1.9326) | 0.030 |
| CYP2C19*17—TT | 04 | 11 | 3.65 (1.1030 to 12.078) | 2.13 (0.9117 to 5.0167) | 0.034 |
| Dominant model | |||||
| CYP2C19*17—CC | 73 | 55 | 1 (ref.) | 1 (ref.) | |
| CYP2C19*17—(CT + TT) | 31 | 50 | 2.14 (1.2124 to 3.7799) | 1.49 (1.2124 to 3.7799) | 0.0087 |
| Recessive model | |||||
| CYP2C19*17—(CC + CT) | 100 | 94 | 1 (ref.) | 1 (ref.) | |
| CYP2C19*17—TT | 04 | 11 | 2.92 (0.9003 to 9.5067) | 1.93 (0.8260 to 4.5236) | 0.074 |
| Allele | |||||
| CYP2C19*17—C | 173 | 149 | 1 (ref.) | 1 (ref.) | |
| CYP2C19*17—T | 35 | 61 | 2.02 (1.2650 to 3.2371) | 1.47 (1.1106 to 1.9554) | 0.003 |
| Over dominant model | |||||
| CYP2C19*17—CC + TT | 77 | 94 | 1 (ref.) | 1 (ref.) | |
| CYP2C19*17—CT | 27 | 66 | 2.0 (1.1673 to 3.4349) | 1.55 (1.0839 to 2.2194) | 0.0117 |
| Genotypes | Healthy Controls (n = 114) | CAD Cases (n = 104) | OR (95% CI) | Risk Ratio (RR) | p-Value |
|---|---|---|---|---|---|
| Codominant inheritance model | |||||
| MicroR-423—CC | 32 | 23 | 1 (ref.) | 1 (ref.) | |
| MicroR-423—CA | 62 | 48 | 1.07 (0.5595 to 2.0737) | 1.03 (0.7818 to 1.3630) | 0.82 |
| MicroR-423—AA | 20 | 33 | 2.29 (1.0611 to 4.9667) | 1.54 (1.0611 to 4.9667) | 0.034 |
| Dominant inheritance model | |||||
| MicroR-423—CC | 32 | 23 | 1 (ref.) | 1 (ref.) | |
| MicroR-423—(CA + AA) | 82 | 81 | 1.37 (0.7411 to 2.5485) | 1.15 (0.8819 to 1.5167) | 0.31 |
| Recessive inheritance model | |||||
| MicroR-423—(CC + CA) | 94 | 71 | 1 (ref.) | 1 (ref.) | |
| MicroR-423—AA | 20 | 33 | 2.18 (1.1574 to 4.1230) | 1.50 (1.0424 to 2.1865) | 0.029 |
| Allele | |||||
| MicroR-423—C | 126 | 94 | 1 (ref.) | 1 (ref.) | |
| MicroR-423—A | 102 | 114 | 1.49 (1.0268 to 2.1858) | 1.21 (1.0116 to 1.4541) | 0.036 |
| Over dominant Inheritance model | |||||
| MicroR-423—CC + AA | 52 | 56 | 1 (ref.) | 1 (ref.) | |
| MicroR-423—CA | 62 | 48 | 0.71 (0.4217 to 1.2255) | 0.85 (0.6616 to 1.1031) | 0.225 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almassabi, R.F.; Mir, R.; Javid, J.; AbuDuhier, F.M.; Almotairi, R.; Alhelali, M.H.; Algehainy, N.; Alsaedi, B.S.O.; Albalawi, S.O.; Elfaki, I. Differential Expression of Serum Proinflammatory Cytokine TNF-α and Genetic Determinants of TNF-α, CYP2C19*17, miR-423 Genes and Their Effect on Coronary Artery Disease Predisposition and Progression. Life 2023, 13, 2142. https://doi.org/10.3390/life13112142
Almassabi RF, Mir R, Javid J, AbuDuhier FM, Almotairi R, Alhelali MH, Algehainy N, Alsaedi BSO, Albalawi SO, Elfaki I. Differential Expression of Serum Proinflammatory Cytokine TNF-α and Genetic Determinants of TNF-α, CYP2C19*17, miR-423 Genes and Their Effect on Coronary Artery Disease Predisposition and Progression. Life. 2023; 13(11):2142. https://doi.org/10.3390/life13112142
Chicago/Turabian StyleAlmassabi, Rehab F., Rashid Mir, Jamsheed Javid, Faisel M. AbuDuhier, Reema Almotairi, Marwan H. Alhelali, Naseh Algehainy, Basim S. O. Alsaedi, Salem Owaid Albalawi, and Imadeldin Elfaki. 2023. "Differential Expression of Serum Proinflammatory Cytokine TNF-α and Genetic Determinants of TNF-α, CYP2C19*17, miR-423 Genes and Their Effect on Coronary Artery Disease Predisposition and Progression" Life 13, no. 11: 2142. https://doi.org/10.3390/life13112142
APA StyleAlmassabi, R. F., Mir, R., Javid, J., AbuDuhier, F. M., Almotairi, R., Alhelali, M. H., Algehainy, N., Alsaedi, B. S. O., Albalawi, S. O., & Elfaki, I. (2023). Differential Expression of Serum Proinflammatory Cytokine TNF-α and Genetic Determinants of TNF-α, CYP2C19*17, miR-423 Genes and Their Effect on Coronary Artery Disease Predisposition and Progression. Life, 13(11), 2142. https://doi.org/10.3390/life13112142






