Innovative Biosensing Approaches for Swift Identification of Candida Species, Intrusive Pathogenic Organisms
Abstract
1. Introduction
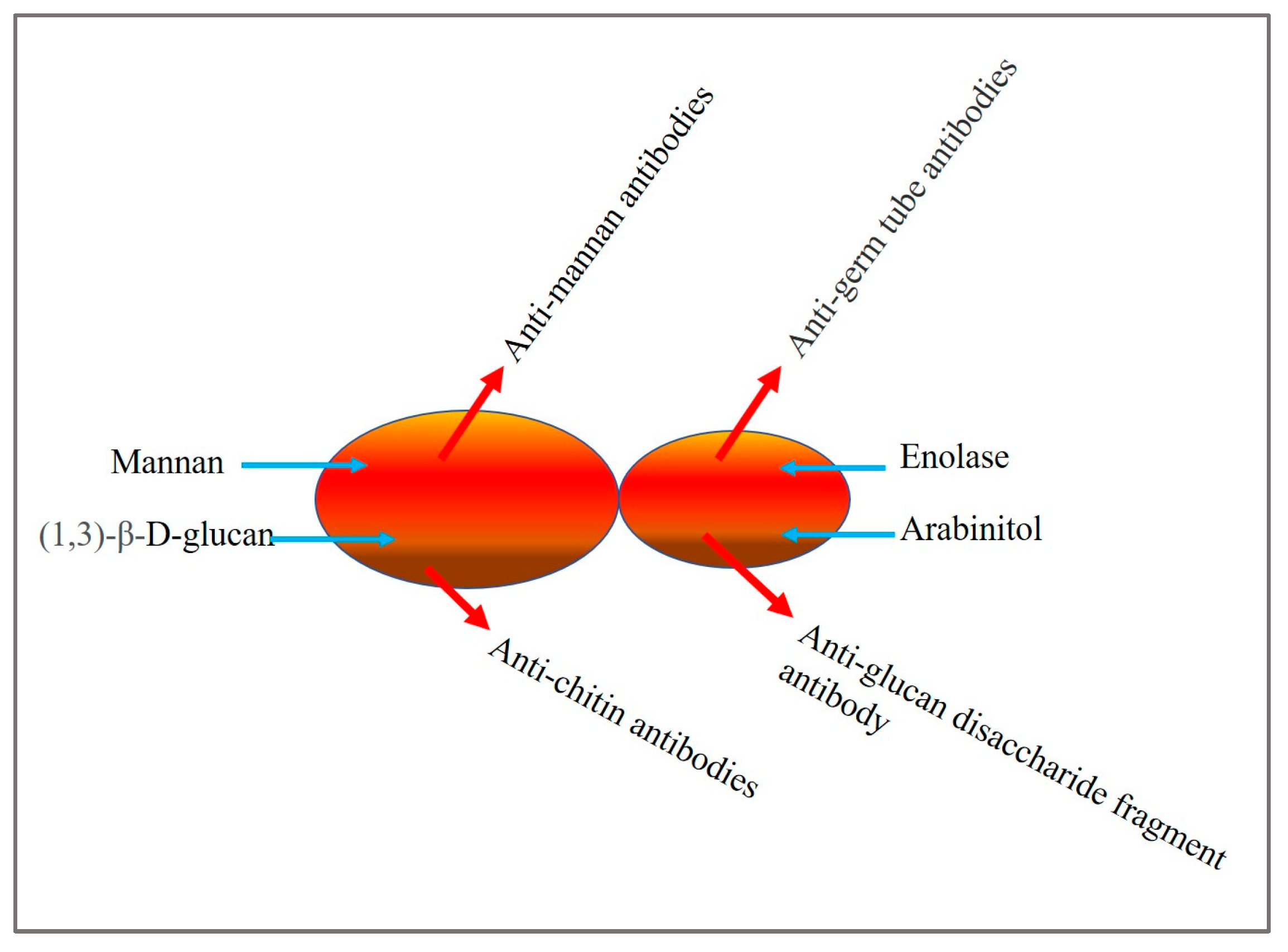
2. Candida Biomarkers
3. Conventional Diagnostic Approaches
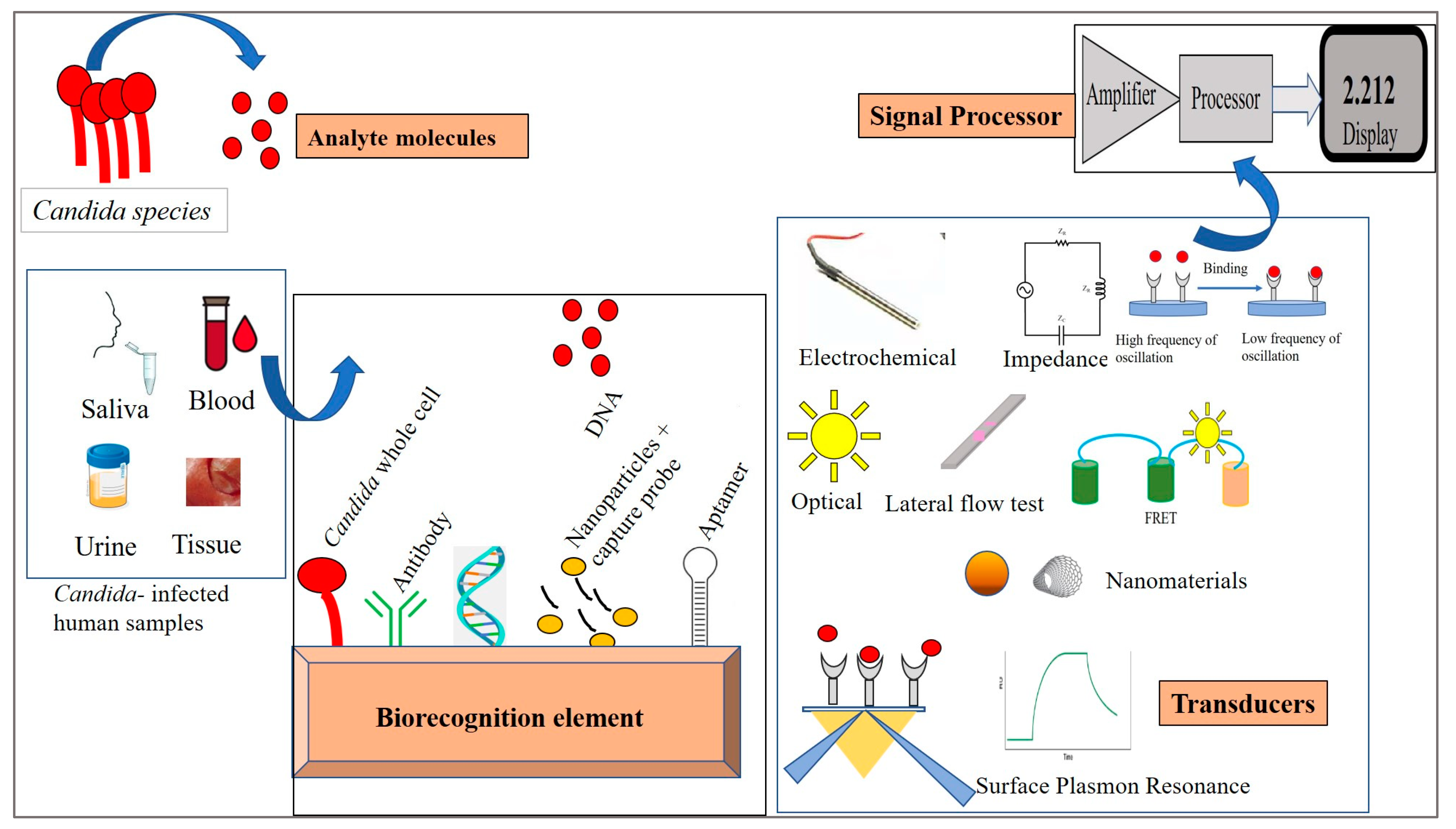
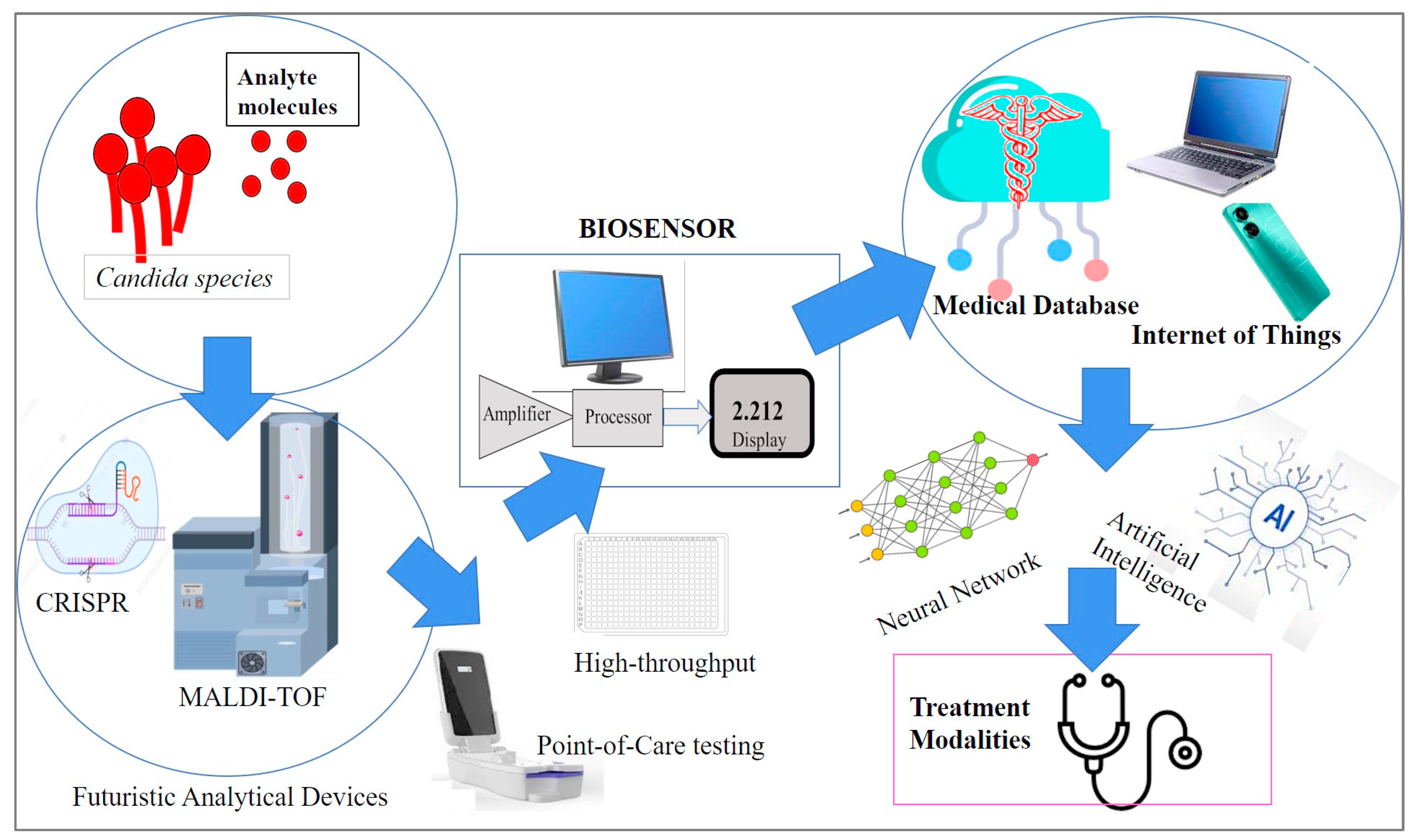
4. Biosensors—Design and Properties
5. Immobilization Techniques
6. Biosensors for Candida Detection
6.1. Electrochemical Biosensor
6.2. Piezoelectric Biosensor
6.3. Optical Biosensor
6.4. Surface Plasmon Resonance (SPR)-Based Biosensor
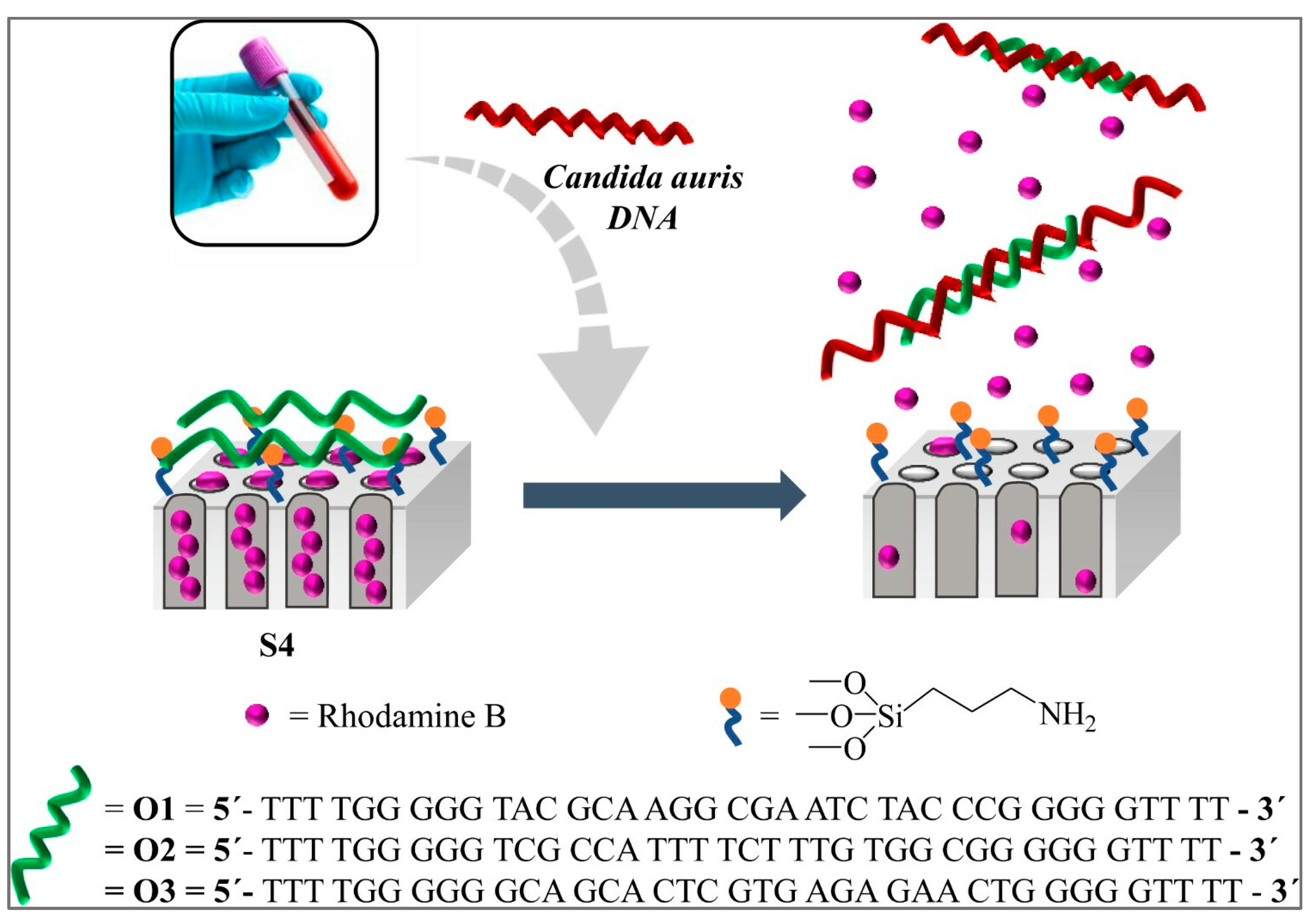
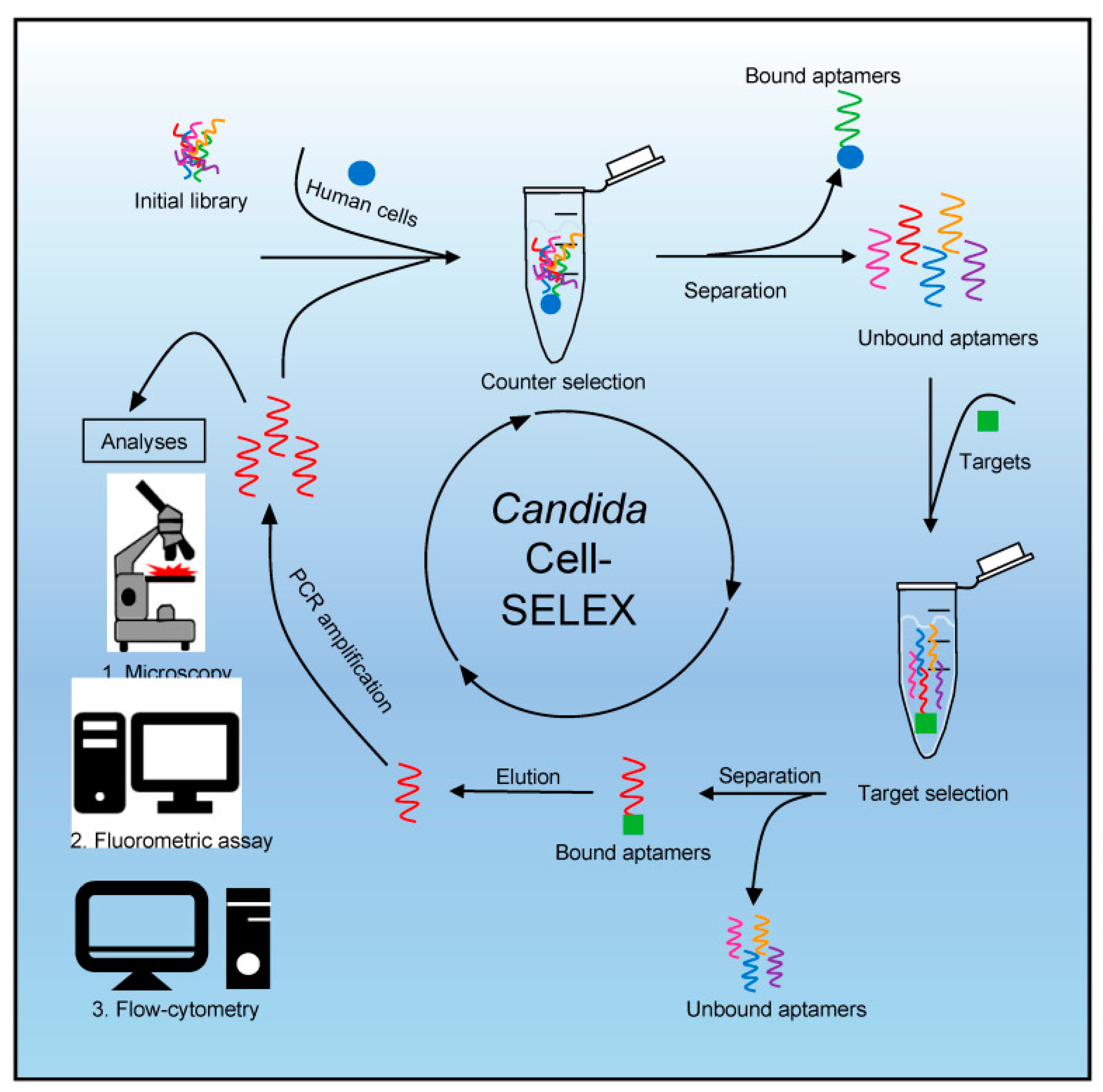
6.5. Nucleic Acid-Based Biosensors
6.6. Nanomaterial-Based Biosensor
7. Emerging Biosensor Methods for Candida
8. Future Perspectives and Directions
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fisher, M.C.; Denning, D.W. The WHO fungal priority pathogens list as a game-changer. Nat. Rev. Microbiol. 2023, 21, 211–212. [Google Scholar] [CrossRef] [PubMed]
- Fungal Disease Frequency—Gaffi. Gaffi—Global Action for Fungal Infections. Published 31 May 2013. Available online: https://gaffi.org/why/fungal-disease-frequency/ (accessed on 20 August 2023).
- Turner, S.A.; Butler, G. The Candida Pathogenic Species Complex. Cold Spring Harb. Perspect. Med. 2014, 4, a019778. [Google Scholar] [CrossRef] [PubMed]
- Sherrington, S.L.; Sorsby, E.; Mahtey, N.; Kumwenda, P.; Lenardon, M.D.; Brown, I.; Ballou, E.R.; MacCallum, D.M.; Hall, R.A. Adaptation of Candida albicans to environmental pH induces cell wall remodelling and enhances innate immune recognition. PLoS Pathog. 2017, 13, e1006403. [Google Scholar] [CrossRef] [PubMed]
- Canela, H.M.S.; Cardoso, B.; Vitali, L.H.; Coelho, H.C.; Martinez, R.; Ferreira, M.E.d.S. Prevalence, virulence factors and antifungal susceptibility of Candida spp. isolated from bloodstream infections in a tertiary care hospital in Brazil. Mycoses 2018, 61, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Li, M.; Mao, Z.; Deng, A.; Lv, W.; Huang, L.; Zhong, H.; Yang, H.; Zhang, L.; Liao, Q.; et al. An Integrated and Multi-Target Nucleic Acid Isothermal Analysis System for Rapid Diagnosis of Vulvovaginal Candidiasis. Biosensors 2023, 13, 559. [Google Scholar] [CrossRef]
- Vergidis, P.; Clancy, C.J.; Shields, R.K.; Park, S.Y.; Wildfeuer, B.N.; Simmons, R.L.; Nguyen, M.H. Intra-Abdominal Candidiasis: The Importance of Early Source Control and Antifungal Treatment. PLoS ONE 2016, 11, e0153247. [Google Scholar] [CrossRef]
- Mamali, V.; Siopi, M.; Charpantidis, S.; Samonis, G.; Tsakris, A.; Vrioni, G. Increasing Incidence and Shifting Epidemiology of Candidemia in Greece: Results from the First Nationwide 10-Year Survey. J. Fungi 2022, 8, 116. [Google Scholar] [CrossRef]
- Wan Ismail, W.N.A.; Jasmi, N.; Khan, T.M.; Hong, Y.H.; Neoh, C.F. The Economic Burden of Candidemia and Invasive Candidiasis: A Systematic Review. Value Health Reg. Issues 2020, 21, 5358. [Google Scholar] [CrossRef]
- Koehler, P.; Stecher, M.; Cornely, O.A.; Koehler, D.; Vehreschild, M.J.G.T.; Bohlius, J.; Wisplinghoff, H.; Vehreschild, J.J. Morbidity and mortality of candidaemia in Europe: An epidemiologic meta-analysis. Clin. Microbiol. Infect. 2019, 25, 1200–1212. [Google Scholar] [CrossRef]
- Lockhart, S.R.; Etienne, K.A.; Vallabhaneni, S.; Farooqi, J.; Chowdhary, A.; Govender, N.P.; Colombo, A.L.; Calvo, B.; Cuomo, C.A.; Desjardins, C.A.; et al. Simultaneous Emergence of Multidrug-Resistant Candida auris on 3 Continents Confirmed by Whole-Genome Sequencing and Epidemiological Analyses. Clin. Infect. Dis. 2017, 64, 134–140. [Google Scholar] [CrossRef]
- Hussain, K.K.; Malavia, D.; Johnson, E.M.; Littlechild, J.; Winlove, C.P.; Vollmer, F.; Gow, N.A.R. Biosensors and Diagnostics for Fungal Detection. J. Fungi 2020, 6, 349. [Google Scholar] [CrossRef] [PubMed]
- Al-Baqsami, Z.F.; Ahmad, S.; Khan, Z. Antifungal drug susceptibility, molecular basis of resistance to echinocandins and molecular epidemiology of fluconazole resistance among clinical Candida glabrata isolates in Kuwait. Sci. Rep. 2020, 10, 6238. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.E.; Bicanic, T. Drug Resistance and Novel Therapeutic Approaches in Invasive Candidiasis. Front. Cell Infect. Microbiol. 2021, 11, 759408. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Bing, J.; Hu, T.; Ennis, C.L.; Nobile, C.J.; Huang, G. Candida auris: Epidemiology, biology, antifungal resistance, and virulence. PLoS Pathog. 2020, 16, e1008921. [Google Scholar] [CrossRef]
- Ibrahim, A.U.; Al-Turjman, F.; Sa’id, Z.; Ozsoz, M. Futuristic CRISPR-based biosensing in the cloud and internet of things era: An overview. Multimed. Tools Appl. 2022, 81, 35143–35171. [Google Scholar] [CrossRef]
- Wang, J.; Xu, B.; Shi, L.; Zhu, L.; Wei, X. Prospects and Challenges of AI and Neural Network Algorithms in MEMS Microcantilever Biosensors. Processes 2022, 10, 1658. [Google Scholar] [CrossRef]
- Nugaeva, N.; Gfeller, K.Y.; Backmann, N.; Lang, H.P.; Düggelin, M.; Hegner, M. Micromechanical cantilever array sensors for selective fungal immobilization and fast growth detection. Biosens. Bioelectron. 2005, 21, 849–856. [Google Scholar] [CrossRef]
- Sendid, B.; Poirot, J.L.; Tabouret, M.; Bonnin, A.; Caillot, D.; Camus, D.; Poulain, D. Combined detection of mannanaemia and anti-mannan antibodies as a strategy for the diagnosis of systemic infection caused by pathogenic Candida species. J. Med. Microbiol. 2002, 51, 433–442. [Google Scholar] [CrossRef]
- García-Ruiz, J.C.; del Carmen Arilla, M.; Regúlez, P.; Quindós, G.; Alvarez, A.; Pontón, J. Detection of antibodies to Candida albicans germ tubes for diagnosis and therapeutic monitoring of invasive candidiasis in patients with hematologic malignancies. J. Clin. Microbiol. 1997, 35, 3284–3287. [Google Scholar] [CrossRef]
- Walsh, T.J.; Hathorn, J.W.; Sobel, J.D.; Merz, W.G.; Sanchez, V.; Maret, S.M.; Buckley, H.R.; Pfaller, M.A.; Schaufele, R.; Sliva, C.; et al. Detection of Circulating Candida Enolase by Immunoassay in Patients with Cancer and Invasive Candidiasis. N. Engl. J. Med. 1991, 324, 1026–1031. [Google Scholar] [CrossRef]
- Christensson, B.; Sigmundsdottir, G.; Larsson, L. D-arabinitol—A marker for invasive candidiasis. Med. Mycol. 1999, 37, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Sendid, B.; Dotan, N.; Nseir, S.; Savaux, C.; Vandewalle, P.; Standaert, A.; Zerimech, F.; Guery, B.P.; Dukler, A.; Colombel, J.F.; et al. Antibodies against Glucan, Chitin, and Saccharomyces cerevisiae Mannan as New Biomarkers of Candida albicans Infection That Complement Tests Based on C. albicans Mannan. Clin. Vaccine Immunol. 2008, 15, 1868–1877. [Google Scholar] [CrossRef] [PubMed]
- Afshar, P.; Larijani, L.V.; Rouhanizadeh, H. A comparison of conventional rapid methods in diagnosis of superficial and cutaneous mycoses based on KOH, Chicago sky blue 6B and calcofluor white stains. Iran. J. Microbiol. 2018, 10, 433–440. [Google Scholar] [PubMed]
- Hu, L.; Zhou, P.; Zhao, W.; Hua, H.; Yan, Z. Fluorescence staining vs. routine KOH smear for rapid diagnosis of oral candidiasis-A diagnostic test. Oral. Dis. 2020, 26, 941–947. [Google Scholar] [CrossRef]
- Sabino, R.; Wiederhold, N. Diagnosis from Tissue: Histology and Identification. J. Fungi 2022, 8, 505. [Google Scholar] [CrossRef] [PubMed]
- Guarner, J.; Brandt, M.E. Histopathologic Diagnosis of Fungal Infections in the 21st Century. Clin. Microbiol. Rev. 2011, 24, 247–280. [Google Scholar] [CrossRef]
- Dichtl, K.; Seybold, U.; Wagener, J. Serological biomarkers of candidemia: A retrospective evaluation of three assays. Infection 2019, 47, 217–224. [Google Scholar] [CrossRef]
- Ruan, H.; Xu, W.; Xia, M.; Ma, Z.; Fu, S.; Chen, X. Analysis of Clinical Characteristics and Diagnostic Value of Fungal Serology in Patients with Invasive Candidiasis. Open J. Med. Microbiol. 2020, 10, 222–232. [Google Scholar] [CrossRef]
- Clancy, C.; Nguyen, M.H. Non-Culture Diagnostics for Invasive Candidiasis: Promise and Unintended Consequences. J. Fungi 2018, 4, 27. [Google Scholar] [CrossRef]
- Sande, M.G.; Rodrigues, J.L.; Ferreira, D.; Silva, C.J.; Rodrigues, L.R. Novel Biorecognition Elements against Pathogens in the Design of State-of-the-Art Diagnostics. Biosensors 2021, 11, 418. [Google Scholar] [CrossRef]
- Bibikova, M.V.; Ivanitskaia, L.P.; Nikishina, V.G.; Kuznetsova, S.M. Characteristics of the substrate specificity of the crude endogenous beta-lactamases of gram-negative bacteria. Antibiotiki 1983, 28, 566–573. [Google Scholar] [PubMed]
- Polat, E.O.; Cetin, M.M.; Tabak, A.F.; Bilget Güven, E.; Uysal, B.Ö.; Arsan, T.; Kabbani, A.; Hamed, H.; Gül, S.B. Transducer Technologies for Biosensors and Their Wearable Applications. Biosensors 2022, 12, 385. [Google Scholar] [CrossRef] [PubMed]
- Tetyana, P.; Morgan Shumbula, P.; Njengele-Tetyana, Z. Biosensors: Design, Development and Applications. In Nanopores; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Asal, M.; Özen, Ö.; Şahinler, M.; Baysal, H.T.; Polatoğlu, İ. An overview of biomolecules, immobilization methods and support materials of biosensors. Sens. Rev. 2019, 39, 377–386. [Google Scholar] [CrossRef]
- Yi, S.; Dai, F.; Zhao, C.; Si, Y. A reverse micelle strategy for fabricating magnetic lipase-immobilized nanoparticles with robust enzymatic activity. Sci. Rep. 2017, 7, 9806. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Simon, B.; Campas, M.; Marty, J.L. Biomolecule Immobilization in Biosensor Development: Tailored Strategies Based on Affinity Interactions. Protein Pept. Lett. 2008, 15, 757–763. [Google Scholar] [CrossRef]
- Ribeiro, K.L.; Frías, I.A.M.; Silva, A.G.; Lima-Neto, R.G.; Sá, S.R.; Franco, O.L.; Oliveira, M.D.L.; Andrade, C.A.S. Impedimetric clavmo peptide-based sensor differentiates ploidy of Candida species. Biochem. Eng. J. 2021, 167, 107918. [Google Scholar] [CrossRef]
- Dutta, P.; Lu, Y.J.; Hsieh, H.Y.; Lee, T.Y.; Lee, Y.T.; Cheng, C.M.; Fan, Y.J. Detection of Candida albicans Using a Manufactured Electrochemical Sensor. Micromachines 2021, 12, 166. [Google Scholar] [CrossRef]
- Muramatsu, H.; Kajiwara, K.; Tamiya, E.; Karube, I. Piezoelectric immuno sensor for the detection of Candida albicans microbes. Anal. Chim. Acta. 1986, 188, 257–261. [Google Scholar] [CrossRef]
- Cai, Z.; Kwak, D.H.; Punihaole, D.; Hong, Z.; Velankar, S.S.; Liu, X.; Asher, S.A. A Photonic Crystal Protein Hydrogel Sensor for Candida albicans. Angew. Chem. Int. Ed. 2015, 54, 13036–13040. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, L.; Lu, Y.; Chen, C.; Wang, K.; Zhang, L. Recombinase Polymerase Amplification Assay with Lateral Flow Strips for Rapid Detection of Candidiasis Due to Candida parapsilosis. Curr. Microbiol. 2023, 80, 217. [Google Scholar] [CrossRef]
- Liu, L.; Li, J.; Dong, J.; Sisak, D.; Baerlocher, C.; McCusker, L.B. Application of surface plasmon resonance biosensor for the detection of Candida albicans. JPN J. Appl. Phys. 2016, 55, 02BE03. [Google Scholar] [CrossRef]
- Farooq, S.; Neves, W.W.; Pandoli, O.; Del Rosso, T.; de Lima, L.M.; Dutra, R.F.; de Araujo, R.E. Engineering a plasmonic sensing platform for Candida albicans antigen identification. J. Nanophotonics 2018, 12, 1. [Google Scholar] [CrossRef]
- Sá, S.R.; Silva Junior, A.G.; Lima-Neto, R.G.; Andrade, C.A.S.; Oliveira, M.D.L. Lectin-based impedimetric biosensor for differentiation of pathogenic Candida species. Talanta 2020, 220, 121375. [Google Scholar] [CrossRef] [PubMed]
- Neely, L.A.; Audeh, M.; Phung, N.A.; Min, M.; Suchocki, A.; Plourde, D.; Blanco, M.; Demas, V.; Skewis, L.R.; Anagnostou, T.; et al. T2 Magnetic Resonance Enables Nanoparticle-Mediated Rapid Detection of Candidemia in Whole Blood. Sci. Transl. Med. 2013, 5, 182ra54. [Google Scholar] [CrossRef] [PubMed]
- Ribes, À.; Aznar, E.; Santiago-Felipe, S.; Xifre-Perez, E.; Tormo-Mas, M.Á.; Pemán, J.; Marsal, L.F.; Martínez-Máñez, R. Selective and Sensitive Probe Based in Oligonucleotide-Capped Nanoporous Alumina for the Rapid Screening of Infection Produced by Candida albicans. ACS Sens. 2019, 4, 1291–1298. [Google Scholar] [CrossRef]
- Zahra, Q.U.A.; Khan, Q.A.; Luo, Z. Advances in Optical Aptasensors for Early Detection and Diagnosis of Various Cancer Types. Front. Oncol. 2021, 11, 632165. [Google Scholar] [CrossRef]
- Su, X.; Ren, R.; Wu, Y.; Li, S.; Ge, C.; Liu, L.; Xu, Y. Study of biochip integrated with microelectrodes modified by poly-dopamine-co-chitosan composite gel for separation, enrichment and detection of microbes in the aerosol. Biosens. Bioelectron. 2021, 176, 112931. [Google Scholar] [CrossRef]
- Xu, Y.; Gu, F.; Hu, S.; Wu, Y.; Wu, C.; Deng, Y.; Gu, B.; Chen, Z.; Yang, Y. A cell wall-targeted organic-inorganic hybrid nano-catcher for ultrafast capture and SERS detection of invasive fungi. Biosens. Bioelectron. 2023, 228, 115173. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, X.; Zhou, Y.; Lu, J.; Yu, H.; Li, S. Establishment and application of loop-mediated isothermal amplification coupled with nanoparticle-based lateral flow biosensor (LAMP-LFB) for visual and rapid diagnosis of Candida albicans in clinical samples. Front. Bioeng. Biotechnol. 2022, 10, 1025083. [Google Scholar] [CrossRef]
- Zhao, F.; Niu, L.; Yan, L.; Nong, J.; Wang, C.; Wang, J.; Gao, N.; Zhu, X.; Wu, L.; Zheng, F.; et al. Establishment and Application of Multiple Cross Displacement Amplification Coupled With Lateral Flow Biosensor (MCDA-LFB) for Visual and Rapid Detection of Candida albicans in Clinical Samples. Front. Cell Infect. Microbiol. 2019, 9, 102. [Google Scholar] [CrossRef]
- Guedes, P.H.G.; Brussasco, J.G.; Moço, A.C.R.; Moraes, D.D.; Flauzino, J.M.R.; Luz, L.F.G.; Almeida, M.T.G.; Soares, M.M.C.N.; Oliveira, R.J.; Madurro, J.M.; et al. Ninhydrin as a novel DNA hybridization indicator applied to a highly reusable electrochemical genosensor for Candida auris. Talanta 2021, 235, 122694. [Google Scholar] [CrossRef] [PubMed]
- Zopf, D.; Pittner, A.; Dathe, A.; Grosse, N.; Csáki, A.; Arstila, K.; Toppari, J.J.; Schott, W.; Dontsov, D.; Uhlrich, G.; et al. Plasmonic Nanosensor Array for Multiplexed DNA-based Pathogen Detection. ACS Sens. 2019, 4, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Kneißle, K.; Krämer, M.; Kissmann, A.K.; Xing, H.; Müller, F.; Amann, V.; Noschka, R.; Gottschalk, K.E.; Bozdogan, A.; Andersson, J.; et al. A Polyclonal SELEX Aptamer Library Allows Differentiation of Candida albicans, C. auris and C. parapsilosis Cells from Human Dermal Fibroblasts. J. Fungi 2022, 8, 856. [Google Scholar] [CrossRef]
- Hassan, R.Y.A.; El-Attar, R.O.; Hassan, H.N.A.; Ahmed, M.A.; Khaled, E. Carbon nanotube-based electrochemical biosensors for determination of Candida albicans’s quorum sensing molecule. Sens. Actuators B Chem. 2017, 244, 565–570. [Google Scholar] [CrossRef]
- Doğan, Ö.; Inkaya, A.Ç.; Gülmez, D.; Uzun, Ö.; Akova, M.; Arikan Akdağli, S. PNA-FISH Yönteminin Kan Kültürlerinden İzole Edilen Candida Türlerinin Direkt Tanımlanması ve Antifungal Tedavi Planına Olası Etki Yönünden Değerlendirilmesi. Mikrobiyoloji Bul. 2016, 50, 580–589. [Google Scholar] [CrossRef][Green Version]
- Barbosa, V.B.; Rodrigues, C.F.; Cerqueira, L.; Miranda, J.M.; Azevedo, N.F. Microfluidics combined with fluorescence in situ hybridization (FISH) for Candida spp. detection. Front. Bioeng. Biotechnol. 2022, 10, 987669. [Google Scholar] [CrossRef]
- Busser, F.D.; Coelho, V.C.; Fonseca, C.D.; Del Negro, G.M.; Shikanai-Yasuda, M.A.; Lopes, M.H.; Magri, M.M.; Freitas, V.L. A Real Time PCR strategy for the detection and quantification of Candida albicans in human blood. Rev. Inst. Med. Trop. 2020, 62, e9. [Google Scholar] [CrossRef]
- Kwasny, D.; Tehrani, S.; Almeida, C.; Schjødt, I.; Dimaki, M.; Svendsen, W. Direct Detection of Candida albicans with a Membrane Based Electrochemical Impedance Spectroscopy Sensor. Sensors 2018, 18, 2214. [Google Scholar] [CrossRef]
- Kumaraswamy Naik, L.; Shetty, P.; Krishna Prasad, M.; Karnaker, V.; Shroff, S.; Madathil, L. Fluorescence of Candida in diagnosis of oral candidiasis. Indian J. Dent. Res. 2016, 27, 618. [Google Scholar] [CrossRef]
- Yao, Y.; Shi, L.; Zhang, C.; Sun, H.; Wu, L. Application of fungal fluorescent staining in oral candidiasis: Diagnostic analysis of 228 specimens. BMC Microbiol. 2019, 19, 96. [Google Scholar] [CrossRef]
- Camarca, A.; Varriale, A.; Capo, A.; Pennacchio, A.; Calabrese, A.; Giannattasio, C.; Murillo Almuzara, C.; D’Auria, S.; Staiano, M. Emergent Biosensing Technologies Based on Fluorescence Spectroscopy and Surface Plasmon Resonance. Sensors 2021, 21, 906. [Google Scholar] [CrossRef] [PubMed]
- Harun-Ur-Rashid, M.; Foyez, T.; Jahan, I.; Pal, K.; Bin Imran, A. Rapid diagnosis of COVID-19 via nano-biosensor-implemented biomedical utilization: A systematic review. RSC Adv. 2022, 12, 9445–9465. [Google Scholar] [CrossRef] [PubMed]
- Eghtedar Nejad, E.; Ghasemi Nejad Almani, P.; Mohammadi, M.A.; Salari, S. Molecular identification of Candida isolates by Real-time PCR-high-resolution melting analysis and investigation of the genetic diversity of Candida species. J. Clin. Lab. Anal. 2020, 34, e23444. [Google Scholar] [CrossRef]
- Avni, T.; Leibovici, L.; Paul, M. PCR Diagnosis of Invasive Candidiasis: Systematic Review and Meta-Analysis. J. Clin. Microbiol. 2011, 49, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.; Yilmaz, T.; Cohen, S.; Beyhan, S.; Argun, A.A. A novel biosensor for ultrasensitive detection of fungal genes. Biosens. Bioelectron. 2023, 222, 114986. [Google Scholar] [CrossRef] [PubMed]
- Kasai, M.; Francesconi, A.; Petraitiene, R.; Petraitis, V.; Kelaher, A.M.; Kim, H.S.; Meletiadis, J.; Sein, T.; Bacher, J.; Walsh, T.J. Use of Quantitative Real-Time PCR To Study the Kinetics of Extracellular DNA Released from Candida albicans, with Implications for Diagnosis of Invasive Candidiasis. J. Clin. Microbiol. 2006, 44, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Asadzadeh, M.; Ahmad, S.; Al-Sweih, N.; Khan, Z. Rapid and Accurate Identification of Candida albicans and Candida dubliniensis by Real-Time PCR and Melting Curve Analysis. Med. Princ. Pract. 2018, 27, 543–548. [Google Scholar] [CrossRef]
- Jiang, X.; Dong, D.; Bian, L.; Zou, D.; He, X.; Ao, D.; Yang, Z.; Huang, S.; Liu, N.; Liu, W.; et al. Rapid Detection of Candida albicans by Polymerase Spiral Reaction Assay in Clinical Blood Samples. Front. Microbiol. 2016, 7, 916. [Google Scholar] [CrossRef]
- Uthayakumar, D.; Sharma, J.; Wensing, L.; Shapiro, R.S. CRISPR-Based Genetic Manipulation of Candida Species: Historical Perspectives and Current Approaches. Front. Genome Ed. 2021, 2, 606281. [Google Scholar] [CrossRef]
- Dubey, A.K.; Kumar Gupta, V.; Kujawska, M.; Orive, G.; Kim, N.Y.; Li, C.Z.; Kumar Mishra, Y.; Kaushik, A. Exploring nano-enabled CRISPR-Cas-powered strategies for efficient diagnostics and treatment of infectious diseases. J. Nanostructure Chem. 2022, 12, 833–864. [Google Scholar] [CrossRef]
- Villamizar, R.A.; Maroto, A.; Rius, F.X. Improved detection of Candida albicans with carbon nanotube field-effect transistors. Sens. Actuators B Chem. 2009, 136, 451–457. [Google Scholar] [CrossRef]
- Pla, L.; Santiago-Felipe, S.; Tormo-Mas, M.Á.; Ruiz-Gaitán, A.; Pemán, J.; Valentín, E.; Sancenón, F.; Aznar, E.; Martínez-Máñez, R. Oligonucleotide-capped nanoporous anodic alumina biosensor as diagnostic tool for rapid and accurate detection of Candida auris in clinical samples. Emerg. Microbes Infect. 2021, 10, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Clancy, C.J.; Nguyen, M.H. Diagnosing Invasive Candidiasis. J. Clin. Microbiol. 2018, 56, 10–128. [Google Scholar] [CrossRef] [PubMed]
- Alenichev, M.K.; Levin, A.D.; Yushina, A.A.; Kostrikin, E.S.; Lebedin, Y.; Andreeva, I.P.; Grigorenko, V.; Krylov, V.B.; Nifantiev, N. Nano-biosensor based on the combined use of the dynamic and static light scattering for Aspergillus galactomannan analysis. Sens. Biosensing Res. 2022, 35, 100475. [Google Scholar] [CrossRef]
- Licker, M.; Anghel, A.; Moldovan, R.; Hogea, E.; Muntean, D.; Horhat, F.; Seclaman, E.; Tamas, L.; Anghel, M.; Baditoiu, L. Genotype-phenotype correlation in multiresistant Escherichia coli and Klebsiella pneumoniae strains isolated in Western Romania. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1888–1894. [Google Scholar]
- Guna Serrano, M.R.; Larrosa Escartín, N.; Marín Arriaza, M.; Rodríguez Díaz, J.C. Microbiological diagnosis of bacteraemia and fungaemia: Blood cultures and molecular methods. Enfermedades Infecc. Y Microbiol. Clínica 2019, 37, 335–340. [Google Scholar] [CrossRef]
- Harris, D.M.; Hata, D.J. Rapid identification of bacteria and Candida using pna-fish from blood and peritoneal fluid cultures: A retrospective clinical study. Ann. Clin. Microbiol. Antimicrob. 2013, 12, 2. [Google Scholar] [CrossRef]
- Schell, W.A.; Benton, J.L.; Smith, P.B.; Poore, M.; Rouse, J.L.; Boles, D.J.; Johnson, M.D.; Alexander, B.D.; Pamula, V.K.; Eckhardt, A.E.; et al. Evaluation of a digital microfluidic real-time PCR platform to detect DNA of Candida albicans in blood. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 2237–2245. [Google Scholar] [CrossRef]
- Delavy, M.; Dos Santos, A.R.; Heiman, C.M.; Coste, A.T. Investigating Antifungal Susceptibility in Candida Species with MALDI-TOF MS-Based Assays. Front. Cell Infect. Microbiol. 2019, 9, 19. [Google Scholar] [CrossRef]
- Bader, O.; Weig, M.; Taverne-Ghadwal, L.; Lugert, R.; Groß, U.; Kuhns, M. Improved clinical laboratory identification of human pathogenic yeasts by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin. Microbiol. Infect. 2011, 17, 1359–1365. [Google Scholar] [CrossRef]
- Segrelles-Calvo, G.; de SAraújo, G.R.; Llopis-Pastor, E.; Carrillo, J.; Hernández-Hernández, M.; Rey, L.; Melean, N.R.; Escribano, I.; Antón, E.; Zamarro, C.; et al. Candida spp. co-infection in COVID-19 patients with severe pneumonia: Prevalence study and associated risk factors. Respir. Med. 2021, 188, 106619. [Google Scholar] [CrossRef]
- Horhat, F.G.; Rogobete, A.F.; Papurica, M.; Sandesc, D.; Tanasescu, S.; Dumitrascu, V.; Licker, M.; Nitu, R.; Cradigati, C.A.; Sarandan, M.; et al. The Use of Lipid Peroxidation Expression as a Biomarker for the Molecular Damage in the Critically Ill Polytrauma Patient. Clin. Lab. 2016, 62, 1601–1607. [Google Scholar] [CrossRef]
- Thomas-Rüddel, D.O.; Schlattmann, P.; Pletz, M.; Kurzai, O.; Bloos, F. Risk Factors for Invasive Candida Infection in Critically Ill Patients: A Systematic Review and Meta-analysis. Chest 2022, 161, 345–355. [Google Scholar] [CrossRef]
- Rahimizadeh, K.; Zahra, Q.U.A.; Chen, S.; Le, B.T.; Ullah, I.; Veedu, R.N. Nanoparticles-assisted aptamer biosensing for the detection of environmental pathogens. Environ Res. 2023, 238 Pt 1, 117123. [Google Scholar] [CrossRef] [PubMed]
- Zahra, Q.U.A.; Luo, Z.; Ali, R.; Khan, M.I.; Li, F.; Qiu, B. Advances in Gold Nanoparticles-Based Colorimetric Aptasensors for the Detection of Antibiotics: An Overview of the Past Decade. Nanomaterials 2021, 11, 840. [Google Scholar] [CrossRef] [PubMed]
- Zahra, Q.U.A.; Ullah, S.; Shahzad, F.; Qiu, B.; Fang, X.; Ammar, A.; Luo, Z.; Zaidi, S. MXene-based aptasensors: Advances, challenges, and prospects. Prog. Mater. Sci. 2022, 129, 100967. [Google Scholar] [CrossRef]
- Zahra, Q.U.A.; Mohsan, S.A.H.; Shahzad, F.; Qamar, M.; Qiu, B.; Luo, Z.; Zaidi, S.A. Progress in smartphone-enabled aptasensors. Biosens. Bioelectron. 2022, 215, 114509. [Google Scholar] [CrossRef]
- Muntean, D.; Horhat, F.-G.; Bădițoiu, L.; Dumitrașcu, V.; Bagiu, I.-C.; Horhat, D.-I.; Coșniță, D.A.; Krasta, A.; Dugăeşescu, D.; Licker, M. Multidrug-Resistant Gram-Negative Bacilli: A Retrospective Study of Trends in a Tertiary Healthcare Unit. Medicina 2018, 54, 92. [Google Scholar] [CrossRef]
- Qureshi, R.; Irfan, M.; Ali, H.; Khan, A.; Nittala, A.S.; Ali, S.; Shah, A.; Gondal, T.M.; Sadak, F.; Shah, Z.; et al. Artificial Intelligence and Biosensors in Healthcare and its Clinical Relevance: A Review. IEEE Access. 2023, 11, 61600–61620. [Google Scholar] [CrossRef]
- Mylonakis, E.; Clancy, C.J.; Ostrosky-Zeichner, L.; Garey, K.W.; Alangaden, G.J.; Vazquez, J.A.; Groeger, J.S.; Judson, M.A.; Vinagre, Y.M.; Heard, S.O.; et al. T2 magnetic resonance assay for the rapid diagnosis of candidemia in whole blood: A clinical trial. Clin. Infect. Dis. 2015, 60, 892–899. [Google Scholar] [CrossRef]
- Otero, F.; Magner, E. Biosensors-Recent Advances and Future Challenges in Electrode Materials. Sensors 2020, 20, 3561. [Google Scholar] [CrossRef] [PubMed]

| Biosensor Type | Sensor Material | Candida Species | Sample | Sensitivity | Reference |
|---|---|---|---|---|---|
| Electrochemical impedance | Electropolymerized poly(thiophene acetic acid) (PTAA) and amino-functionalized TiO2 nanoparticles | C. albicans, C. tropicalis, C. krusei, and C. glabrata | Culture | 2 and 3 CFU mL−¹ | [38] |
| Electrochemical impedance | Personal glucose meter | C. albicans | Culture, urine, serum, blood | 10 CFU/ml | [39] |
| Piezoelectric immunosensor | Piezoelectric crystal | C. albicans | Culture | 106–5 × 108 cells cm−3 | [40] |
| Optical | 2D arrays of photonic crystals | C. albicans | Culture | 32 CFU/mL | [41] |
| Loop-mediated isothermal amplification (LAMP) method | Nucleic acid | C. albicans, C. parapsilosis, C. glabrata, and C. tropicalis | Vaginal Swabs | <2 CFU/reaction | [6] |
| Lateral flow strip | DNA | C. parapsilosis | Clinical samples | 5.0 × 102 copies/50 µL | [42] |
| Surface plasmon resonance (SPR) | Antibody | C. albicans | Culture | 106 cells/mL | [43] |
| SPR | Monoclonal antibody-conjugated AgNPs | C. albicans | C. albicans antigens | 50 ng/mL | [44] |
| Impedance | Lectin-modified AuNPs | C. albicans, C. krusei, C. parapsilosis, and C. tropicalis | Culture | 102 to 106 CFU/mL | [45] |
| Nanoparticle-mediated immunosensor | Single-walled carbon nanotubes (SWCNTs) | C. albicans , C. parapsilosis, C. krusei, C. tropicalis, and C. glabrata | Whole blood | 1–2 CFU/mL without any false positives | [46] |
| Nanoporous anodic alumina nanogate |
DNA/ oligonucleotides | C. auris | Blood | 6 CFU/mL | [47] |
| T2 magnetic resonance assay | Magnetic resonance | C. albicans, C. glabrata, C. krusei, C. tropicalis, and C. parapsilosis | Whole blood | <1 CFU/mL | [48] |
| Electrical impedance | Poly-dopamine-co-chitosan composite gel-modified copper sheet microelectrodes | C. albicans | Culture | 99.9% | [49] |
| Surface-enhanced Raman spectroscopy | Magnetic nanoparticles -N-isopropylacrylamide-acrylic acid-caspofungin | Candida species | Clinical samples | 102 cells/mL | [50] |
| LAMP-LFB | Nanoparticles and DNA | C. albicans | Isolated from clinical samples | 1 fg | [51] |
| LFB | AuNPs | C. albicans | Isolated from clinical samples | 200 fg | [52] |
| Genosensor | Ninhydrin-DNA | C. auris | Human urine enriched with C. auris gDNA | 4.5 pg μL−1 | [53] |
| Plasmonic optical nanosensor | Arrays of DNA sequence-functionalized gold nanoparticles | Candida | DNA target sequence | 60 nM | [54] |
| Fluorescence and flow cytometry | SELEX Aptamer library | C. parapsilosis, C. auris, and C. albicans | Culture | 0.2 ng of DNA and ~75% of the Candida cells | [55] |
| Electrochemical | MWCNTs and crown ether, 12-crown-4-ether | Candida species | Culture | 1 μg/mL tryptophol detection | [56] |
| Yeast Traffic Light PNA-FISH | Fluorescence in situ hybridization | C. tropicalis, C. albicans, C. parapsilosis, C. krusei, C. glabrata | Blood | 82.6% positive results | [57] |
| Microfluidic hydrodynamic cell trapping and epifluorescence | PNA_FISH | C. tropicalis | Culture, artificially contaminated urine sample | Error 5.38–10.75% | [58] |
| Q-PCR | Ribosomal RNA gene complex | C. albicans | Blood | 0.2 CFU/µL | [59] |
| Electrochemical impedance spectroscopy sensor | Polycarbonate membrane | C. albicans | Culture | [60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorenzo-Villegas, D.L.; Gohil, N.V.; Lamo, P.; Gurajala, S.; Bagiu, I.C.; Vulcanescu, D.D.; Horhat, F.G.; Sorop, V.B.; Diaconu, M.; Sorop, M.I.; et al. Innovative Biosensing Approaches for Swift Identification of Candida Species, Intrusive Pathogenic Organisms. Life 2023, 13, 2099. https://doi.org/10.3390/life13102099
Lorenzo-Villegas DL, Gohil NV, Lamo P, Gurajala S, Bagiu IC, Vulcanescu DD, Horhat FG, Sorop VB, Diaconu M, Sorop MI, et al. Innovative Biosensing Approaches for Swift Identification of Candida Species, Intrusive Pathogenic Organisms. Life. 2023; 13(10):2099. https://doi.org/10.3390/life13102099
Chicago/Turabian StyleLorenzo-Villegas, Dionisio Lorenzo, Namra Vinay Gohil, Paula Lamo, Swathi Gurajala, Iulia Cristina Bagiu, Dan Dumitru Vulcanescu, Florin George Horhat, Virgiliu Bogdan Sorop, Mircea Diaconu, Madalina Ioana Sorop, and et al. 2023. "Innovative Biosensing Approaches for Swift Identification of Candida Species, Intrusive Pathogenic Organisms" Life 13, no. 10: 2099. https://doi.org/10.3390/life13102099
APA StyleLorenzo-Villegas, D. L., Gohil, N. V., Lamo, P., Gurajala, S., Bagiu, I. C., Vulcanescu, D. D., Horhat, F. G., Sorop, V. B., Diaconu, M., Sorop, M. I., Oprisoni, A., Horhat, R. M., Susan, M., & MohanaSundaram, A. (2023). Innovative Biosensing Approaches for Swift Identification of Candida Species, Intrusive Pathogenic Organisms. Life, 13(10), 2099. https://doi.org/10.3390/life13102099








