Septicemic Outbreak in A Rainbow Trout Intensive Aquaculture System: Clinical Finds, Etiological Agents, and Predisposing Factors
Abstract
1. Introduction
2. Material and Methods
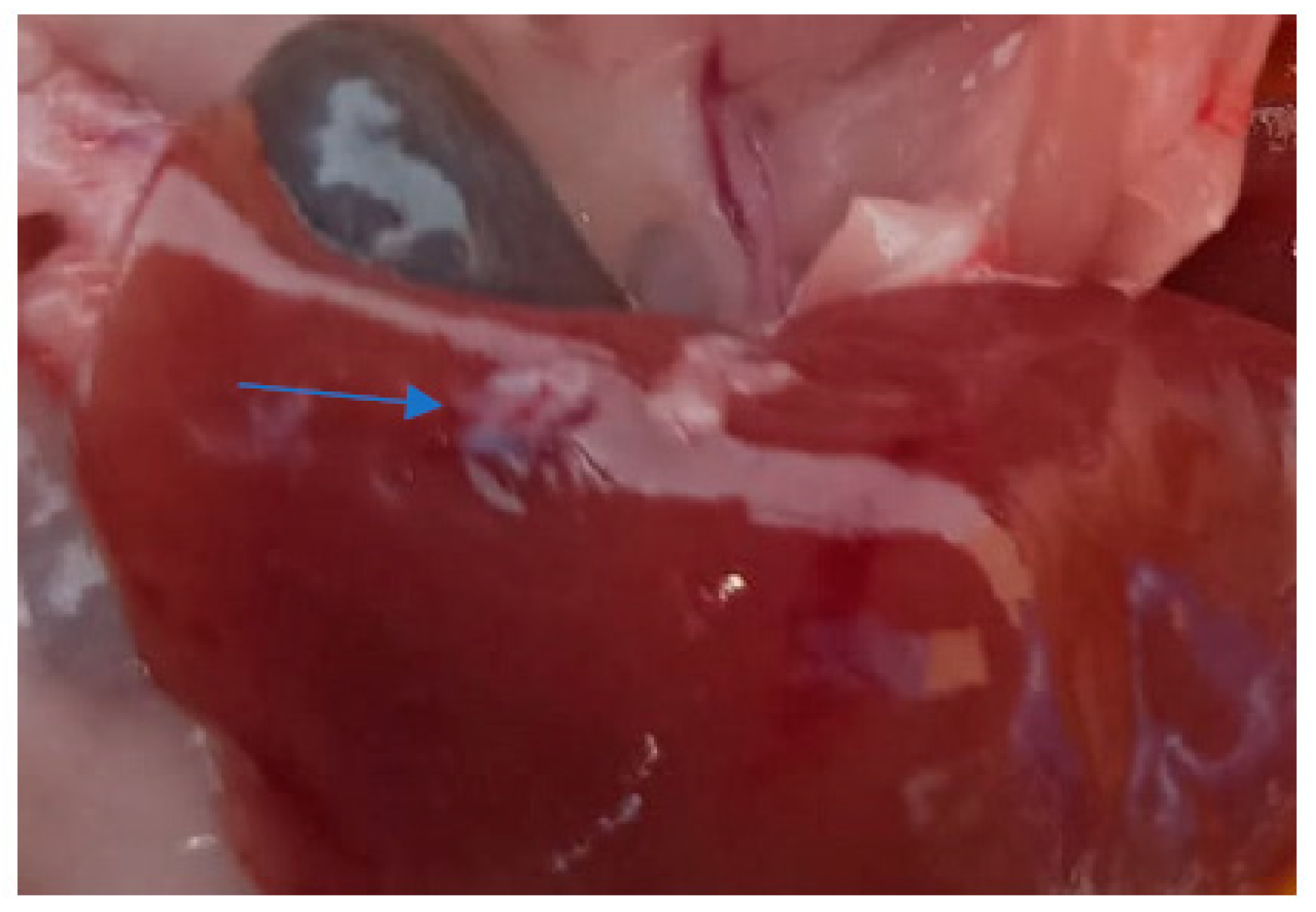
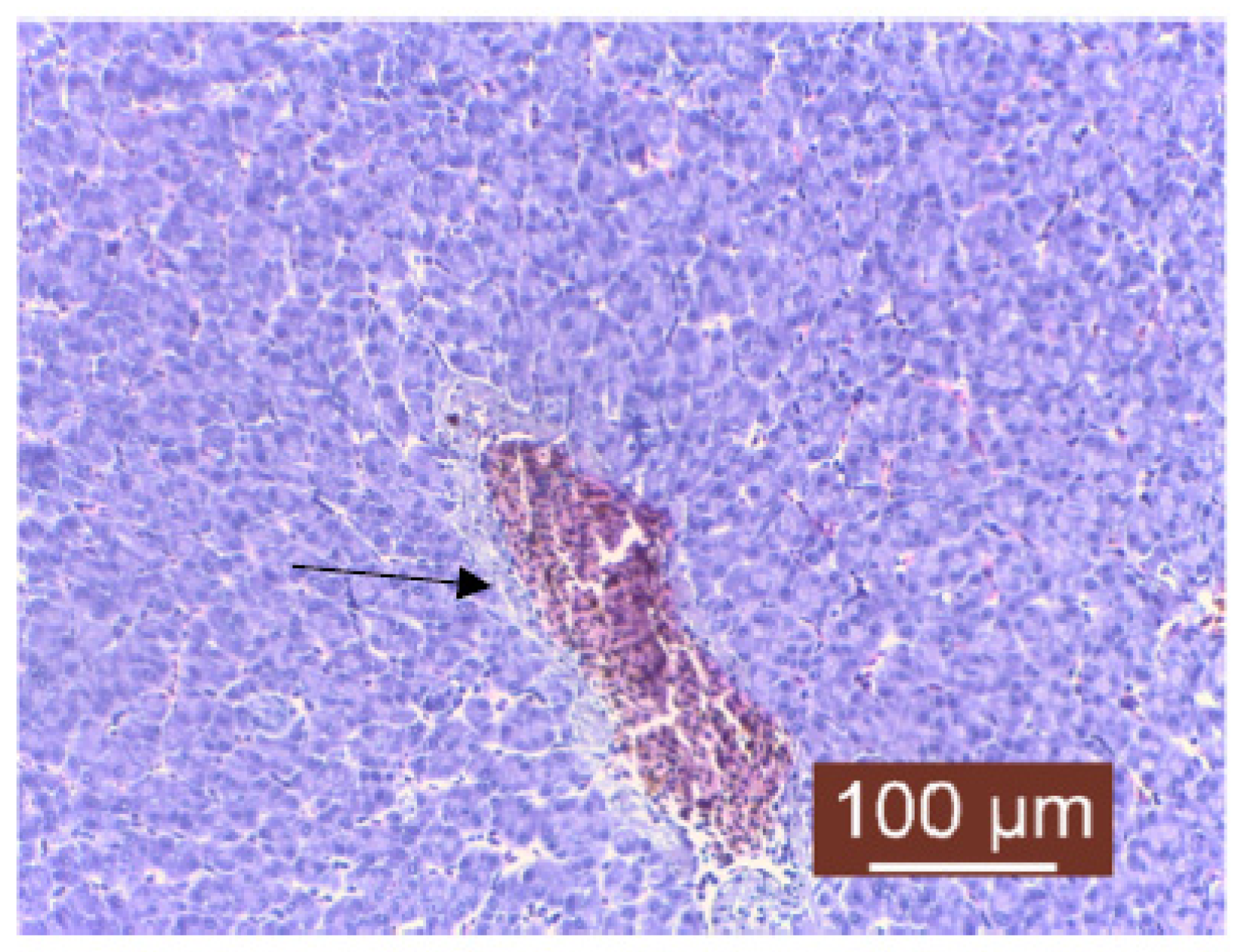
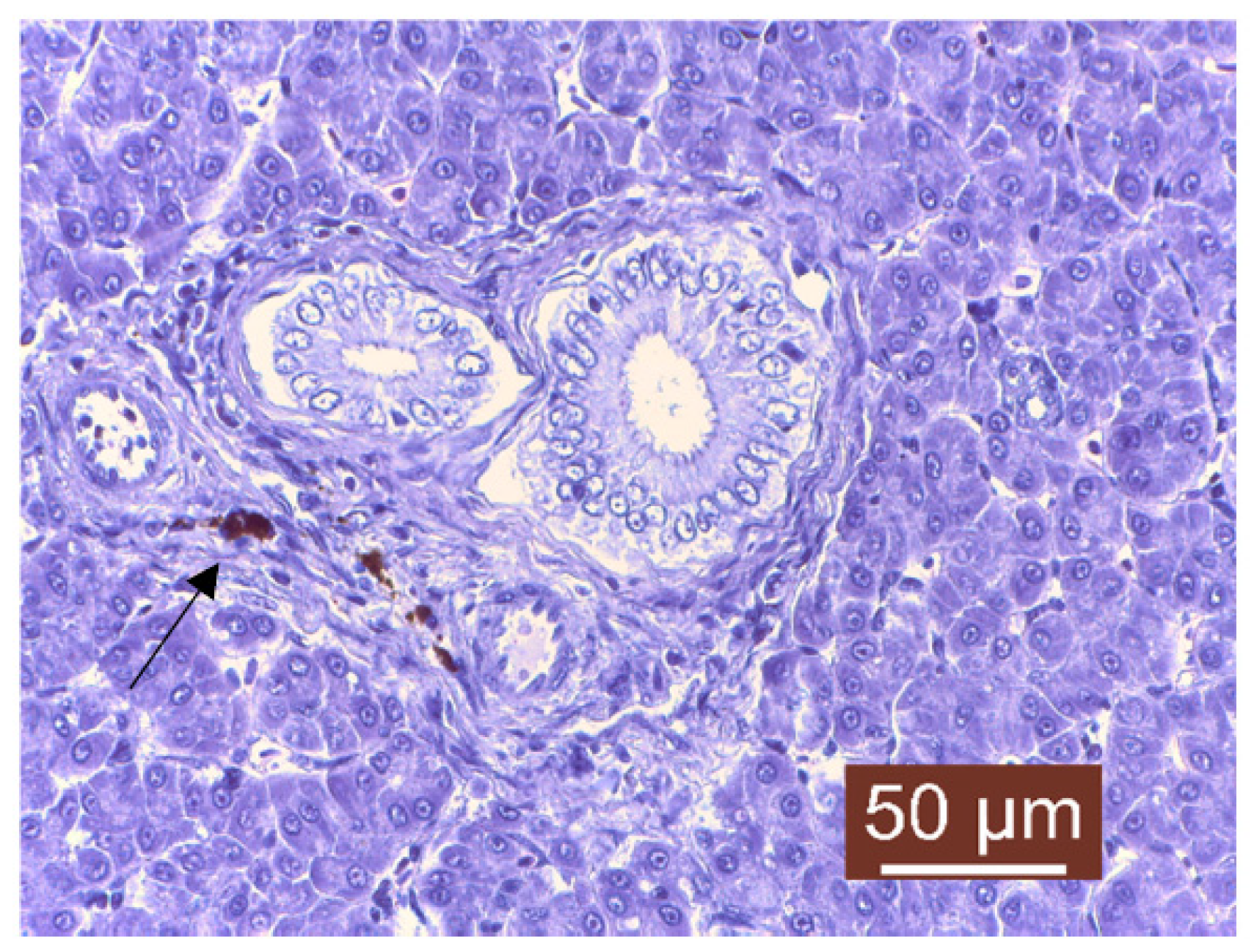
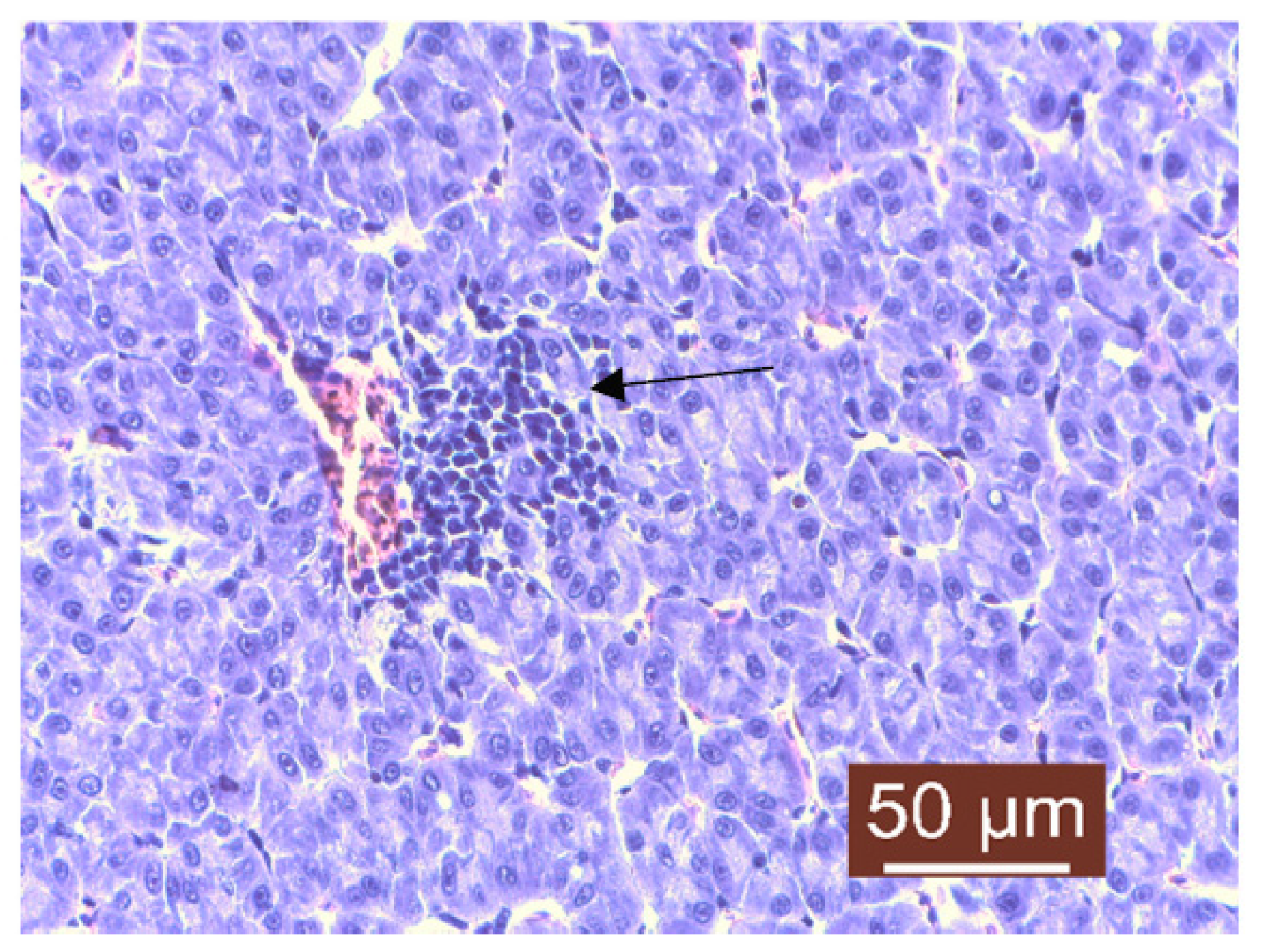
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO. Towards Blue Transformation. In Brief to The State of World Fisheries and Aquaculture 2022; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Torrissen, O.; Olsen, R.E.; Toresen, R.; Hemre, G.I.; Tacon, A.C.J.; Asche, F.; Hardy, R.W.; Lall, S. Atlantic Salmon (Salmo salar): The “Super-Chicken” of the Sea? Rev. Fish. Sci. 2011, 19, 257–278. [Google Scholar] [CrossRef]
- Eurofish International Organization. Eurofish Magazine Issue 3. 2023. Available online: https://eurofish.dk/member-countries/romania/ (accessed on 6 October 2023).
- National Agency for Fisheries and Aquaculture, Romania. Available online: https://www.anpa.ro/ (accessed on 6 October 2023).
- Meyer, F.P. Aquaculture Disease and Health Management. J. Anim. Sci. 1991, 69, 4201–4208. [Google Scholar] [CrossRef] [PubMed]
- Totoiu, A.; Radu, G.; Nenciu, M.I.; Patriche, N.I. Overview of Health Status of The Main Romanian Black Sea Coast Fish. J. Environ. Prot. Ecol. 2018, 19, 1591–1602. [Google Scholar]
- Houghton, J. Global Warming. Rep. Prog. Phys. 2005, 68, 1343. [Google Scholar] [CrossRef]
- Alfonso, S.; Gesto, M.; Sadoul, B. Temperature increase and its effects on fish stress physiology in the context of global warming. J. Fish Biol. 2021, 98, 1496–1508. [Google Scholar] [CrossRef]
- Marcogliese, D. The impact of climate change on the parasites and infectious diseases of aquatic animals. Rev. Sci. Tech. 2008, 27, 467–484. [Google Scholar] [CrossRef]
- Yavuzcan Yildiz, H.; Robaina, L.; Pirhonen, J.; Mente, E.; Domínguez, D.; Parisi, G. Fish Welfare in Aquaponic Systems: Its Relation to Water Quality with an Emphasis on Feed and Faeces—A Review. Water 2017, 9, 13. [Google Scholar] [CrossRef]
- Hadfield, C.A. Clinical Guide to Fish Medicine; Wiley-Blackwell: Hoboken, NJ, USA, 2021; ISBN 9781119259817. [Google Scholar]
- Bjornn, T.; Reiser, D. Habitat requirements of salmonids in streams. In Influences of Forest and Rangeland Management on Salmonids Fishes and Their Habitat; American Fisheries Society Special Publication: Washington, DC, USA, 1991; Volume 19, pp. 83–138. [Google Scholar]
- Muneer, J.; AlObaid, A.; Ullah, R.; Rehman, K.U.; Erinle, K.O. Appraisal of toxic metals in water, bottom sediments and fish of fresh water lake. J. King Saud Univ.-Sci. 2022, 34, 101685. [Google Scholar] [CrossRef]
- Bhagat, R.K.; Barat, S. Water quality parameters for the culture of Rainbow Trout, Oncorhyncus mykiss (Walbaum) in the raceways of Kathmandu, Nepal. Our Nat. 2015, 13, 50–57. [Google Scholar] [CrossRef]
- Boyd, C.E.; Tucker, C.S. Water Quality and Pond Soil Analyses for Aquaculture; Springer: New York, NY, USA, 1992; ISBN 978-1-4613-7469-5. [Google Scholar]
- Cocean, I.; Diaconu, M.; Cocean, A.; Postolachi, C.; Gurlui, S. Landfill Waste Fire Effects Over Town Areas Under Rainwaters. IOP Conf. Ser. Mater. Sci. Eng. 2020, 877, 012048. [Google Scholar] [CrossRef]
- Cocean, I.; Cocean, A.; Iacomi, F.; Gurlui, S. City water pollution by soot-surface-active agents revealed by FTIR spectroscopy. Appl. Surf. Sci. 2020, 499, 142487. [Google Scholar] [CrossRef]
- Garofalide, S.; Postolachi, C.; Cocean, A.; Cocean, G.; Motrescu, I.; Cocean, I.; Munteanu, B.S.; Prelipceanu, M.; Gurlui, S.; Leontie, L. Saharan Dust Storm Aerosol Characterization of the Event (9 to 13 May 2020) over European AERONET Sites. Atmosphere 2022, 13, 493. [Google Scholar] [CrossRef]
- Bartkova, S. Aeromonas Salmonicida—Epidemiology, Whole Genome Sequencing, Detection and In Vivo Imaging; National Veterinary Institute, Technical University of Denmark: Frederiksberg, NC, USA, 2016. [Google Scholar]
- Rui, J.; Li-Ping, C.; Jin-Liang, D.; Qin, H.; Zheng-Yan, G.; Galina, J.; Pao, X.; Guo-Jun, Y. Effects of High-Fat Diet on Steatosis, Endoplasmic Reticulum Stress and Autophagy in Liver of Tilapia (Oreochromis niloticus). Front. Mar. Sci. 2020, 7, 363. [Google Scholar] [CrossRef]
- Gingerich, W.H.; Weber, L.J. Hepatic toxicology of fishes. In Aquatic Toxicology; Raven Press: New York, NY, USA, 1982; pp. 55–105. [Google Scholar]
- Gül, Ş.; Belge-Kurutaş, E.; Yıldız, E.; Şahan, A.; Doran, F. Pollution correlated modifications of liver antioxidant systems and histopathology of fish (Cyprinidae) living in Seyhan Dam Lake, Turkey. Environ. Int. 2004, 30, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.C.; Baumgartner, W.A.; Blazer, V.S.; Camus, A.C.; Engelhardt, J.A.; Fournie, J.W.; Frasca, S.; Groman, D.B.; Kent, M.L.; Khoo, L.H.; et al. Nonlesions, misdiagnoses, missed diagnoses, and other interpretive challenges in fish histopathology studies: A guide for investigators, authors, reviewers, and readers. Toxicol Pathol. 2015, 43, 297–325. [Google Scholar] [CrossRef]
- Moccia, R.D.; Hung, S.S.O.; Slinger, S.J.; Ferguson, H.W. Effect of oxidized fish oil, vitamin E and ethoxyquin on the histopathology and haematology of rainbow trout. J. Fish Dis. 1984, 7, 269–282. [Google Scholar] [CrossRef]
- Khan, R.A.; Nag, K. Estimation of Hemosiderosis in Seabirds and Fish Exposed to Petroleum. Bull. Environ. Contam. Toxicol. 1993, 50, 125–131. [Google Scholar] [CrossRef]
- Thiyagarajah, A.; Hartley, W.R.; Abdelghani, A. Hepatic hemosiderosis in buffalo fish (Ictiobus spp.). Mar. Environ. Res. 1998, 46, 203–207. [Google Scholar] [CrossRef]
- Bernet, D.; Schmidt, H.; Meier, W.; Burkhardt-Holm, P.; Wahli, T. Proposal for a histopathological assessment protocol. J. Fish Dis. 1999, 22, 25–34. [Google Scholar] [CrossRef]
- Smith, S.A. Fish Diseases and Medicine; CRC Press: Boca Raton, FL, USA, 1954; ISBN 9781498727877. [Google Scholar]
- Braunbeck, T.; Gorge, G.; Storch, V.; Nagel, R. Hepatic steatosis in zebra fish (Brachydanio rerio) induced by long-term exposure to gamma-hexachlorocyclohexane. Ecotoxicol. Environ. Saf. 1990, 19, 355–374. [Google Scholar] [CrossRef]
- Sonila, K.; Qarri, F.; Pranvera, L.; Bekteshi, L. The effect of physico-chemical parameters and nutrients on fish growth in Narta lagoon, Albania. J. Hyg. Eng. Des. 2015, 639, 32–496. [Google Scholar]
- Stone, N.M.; Hugh, K. Understanding Your Fish Pond Water Analysis Report. Thomforde University of Arkansas at Pine Bluff, United States Department of Agriculture, and County Governments Cooperating; University of Arkansas Cooperative Extension Service Printing Services: Fayetteville, NC, USA, 2004. [Google Scholar]
- Sano, H. The role of pH on the acute toxicity of sulfite in water. Water Res. 1976, 10, 139–142. [Google Scholar] [CrossRef]
- Malhotra, N.; Uapipatanakul, B.; Huang, J.C.; Chen, K.H.; Hsiao, C.D. Review of Copper and Copper Nanoparticle Toxicity in Fish. Nanomaterials 2020, 10, 1126. [Google Scholar] [CrossRef]
- Boyd, C.E. Total Alkalinity and Total Hardnes. 2007. Available online: https://www.aquaculturealliance.org/ (accessed on 6 October 2023).
- Miller, F.A.; Wilkins, C.H. Infrared Spectra and Characteristic Frequencies of Inorganic Ions. Their Use in Qualitative Analysis. Anal. Chem. 1952, 24, 1253–1294. [Google Scholar] [CrossRef]
- Pretsch, E.; Buhlmann, P.; Badertscher, M. Structure Determination of Organic Compounds. In Tables of Spectral Data, 4th ed.; Revised and Enlarged Edition; Springer: Berlin, Germany, 2009; ISBN 978-3-540-93810-1. [Google Scholar]
- Cocean, A.; Postolachi, C.; Cocean, G.; Bulai, G.; Munteanu, B.S.; Cimpoesu, N.; Cocean, I.; Gurlui, S. The Origin and PhysicoChemical Properties of Some Unusual Earth Rock Fragments. Appl. Sci. 2022, 12, 983. [Google Scholar] [CrossRef]
- Cocean, A.; Cocean, G.; Diaconu, M.; Garofalide, S.; Husanu, F.; Munteanu, B.S.; Cimpoesu, N.; Motrescu, I.; Puiu, I.; Postolachi, C.; et al. Nano-Biocomposite Materials Obtained from Laser Ablation of Hemp Stalks for Medical Applications and Potential Component in New Solar Cells. Int. J. Mol. Sci. 2023, 24, 3892. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, M.; Meheut, M.; Delon, L.; Poirier, M.; Pierre, M.; Le Roux, C.; François, M. Infrared spectroscopic study of the synthetic Mg–Ni talc series. Phys. Chem. Miner. 2018, 45, 843–854. [Google Scholar] [CrossRef]
- Cocean, A.; Cocean, I.; Cimpoesu, N.; Cocean, G.; Cimpoesu, R.; Postolachi, C.; Popescu, V.; Gurlui, S. Laser Induced Method to Produce Curcuminoid-Silanol Thin Films for Transdermal Patches Using Irradiation of Turmeric Target. Appl. Sci. 2021, 11, 4030. [Google Scholar] [CrossRef]
- Hussien, S.S. Biosynthesis, Extraction and Characterization of Extracellular Polymeric Substances (EPSs) from Aspergillus clavatus. Adv. Environ. Stud. 2019, 3, 216–228. [Google Scholar]
- van den Boom, A.F.J.; Pujari, S.P.; Bannani, F.; Driss, H.; Zuilhof, H. Fast room-temperature functionalization of silicon nanoparticles using alkyl silanols. Faraday Discuss. 2020, 222, 82–94. [Google Scholar] [CrossRef]
- Healy, B.; Cooney, S.; O’Brien, S.; Iversen, C.; Whyte, P.; Nally, J.; Callanan, J.J.; Fanning, S. Cronobacter (Enterobacter sakazakii): An Opportunistic Foodborne Pathogen. Foodborne Pathog. Dis. 2010, 7, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Sekar, V.T.; Santiago, T.C.; Vijayan, K.K.; Alavandi, S.V.; Raj, V.S.; Rajan, J.J.; Sanjuktha, M.; Kalaimani, N. Involvement of Enterobacter cloacae in the mortality of the fish. Mugil cephalus. Lett. Appl. Microbiol. 2008, 46, 667–672. [Google Scholar] [CrossRef]
- Mezzatesta, M.; Gona, F.; Stefani, S. Enterobacter cloacae complex: Clinical impact and emerging antibiotic resistance. Future Microbiol. 2012, 7, 887–902. [Google Scholar] [CrossRef] [PubMed]
- Annavajhala, M.K.; Gomez-Simmonds, A.; Uhlemann, A.-C. Multidrug-Resistant Enterobacter cloacae Complex Emerging as a Global, Diversifying Threat. Front. Microbiol. 2019, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.V.; Oliveira, M.C.; Pelli, A. Disease Infection by Enterobacteriaceae Family in Fishes: A Review. J. Microbiol. Exp. 2017, 4, 00128. [Google Scholar] [CrossRef]
- Wiklund, T.; Bylund, T. Pseudomonas anguilliseptica as a pathogen of salmonid fish in Finland. Dis. Aquat. Org. 1990, 8, 13–19. [Google Scholar] [CrossRef]
- Haruo, S.; Sugita, H.; Tanaka, K.; Yoshinami, M.; Deguchi, Y. Distribution of Aeromonas Species in the Intestinal Tracts of River Fish. Appl. Environ. Microbiol. 1995, 61, 4128–4130. [Google Scholar]
- Cahill, M.M. Bacterial flora of fishes: A review. Microb. Ecol. 1990, 19, 21–41. [Google Scholar] [CrossRef]
- Trust, T.J. Pathogenesis of infectious diseases of fish. Annu. Rev. Microbiol. 1986, 40, 479–502. [Google Scholar] [CrossRef]
- Inglis, V.; Roberts, R.J.; Bromage, N.R. Bacterial diseases of fish. In Blackwell Scientific Publications; Wiley: Hoboken, NJ, USA, 1993; ISBN 978-0-632-03497-0. [Google Scholar]
- Harikrishnan, R.; Balasundaram, C. Modern Trends in Aeromonas hydrophila Disease Management with Fish. Rev. Fish. Sci. 2005, 13, 281–320. [Google Scholar] [CrossRef]
- Reynolds, W.W.; Covert, J.B.; Casterlln, M.E. Febrile responses of goldfish Carassius auratus (L.) to Aeromonas hydrophila and to Escherichia coli endotoxin. J. Fish Dis. 1978, 1, 271–273. [Google Scholar] [CrossRef]
- Rodríguez, R.; Inglis, V.; Millar, S.D. Survival of Escherichia coli in the Intestine of Fish. Aquac. Res. 1997, 28, 257–264. [Google Scholar] [CrossRef]
- Newman, J.T.; Consenza, B.J.; Buck, J.D. Aerobic microflora of the bluefish (Pomatonnis saltatrix) intestine. J. Fish. Res. 1972, 29, 333–336. [Google Scholar] [CrossRef]
- Ogbondeminu, F.S. The occurrence and distribution of enteric bacteria in fish and water of tropical aquaculture ponds in Nigeria. J. Aquacukure Trop. 1993, 8, 61–66. [Google Scholar]
- Brett, J.R. Implications and assessments of environmental stress in the investigation of fish power problems. In MacMillam, H.R. Lectures; University of British Columbia: Vancouver, BC, Canada, 1958. [Google Scholar]










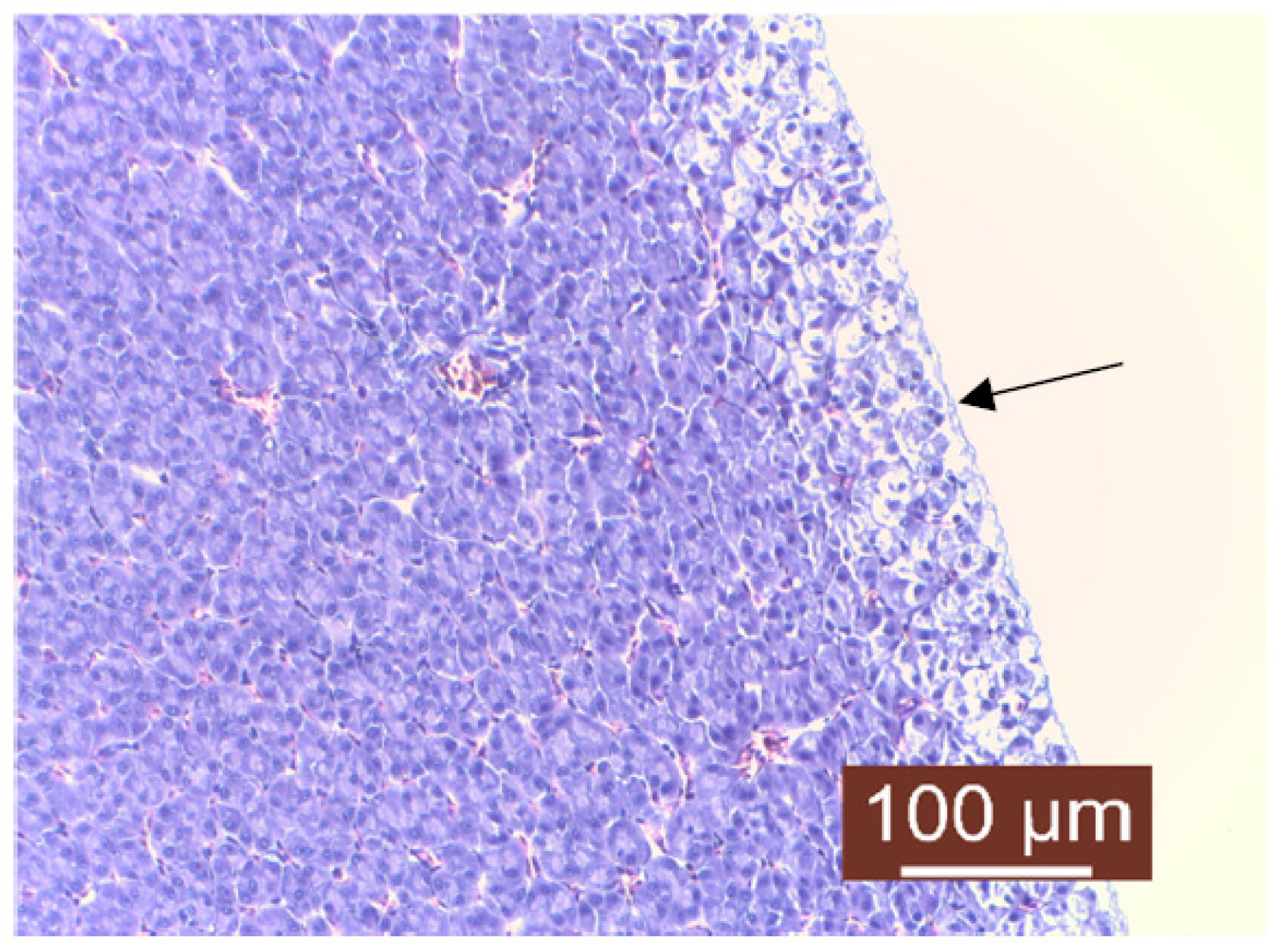
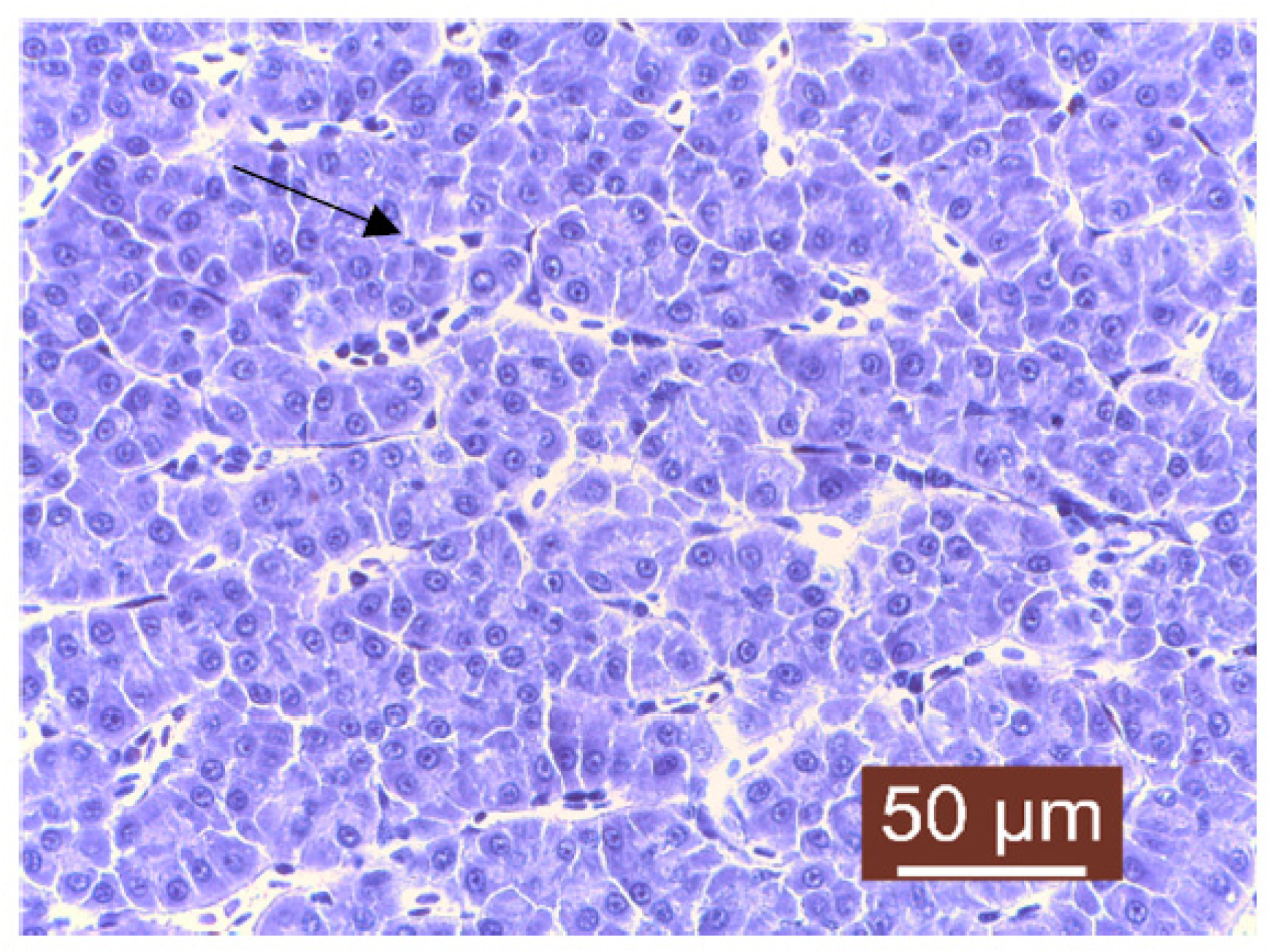
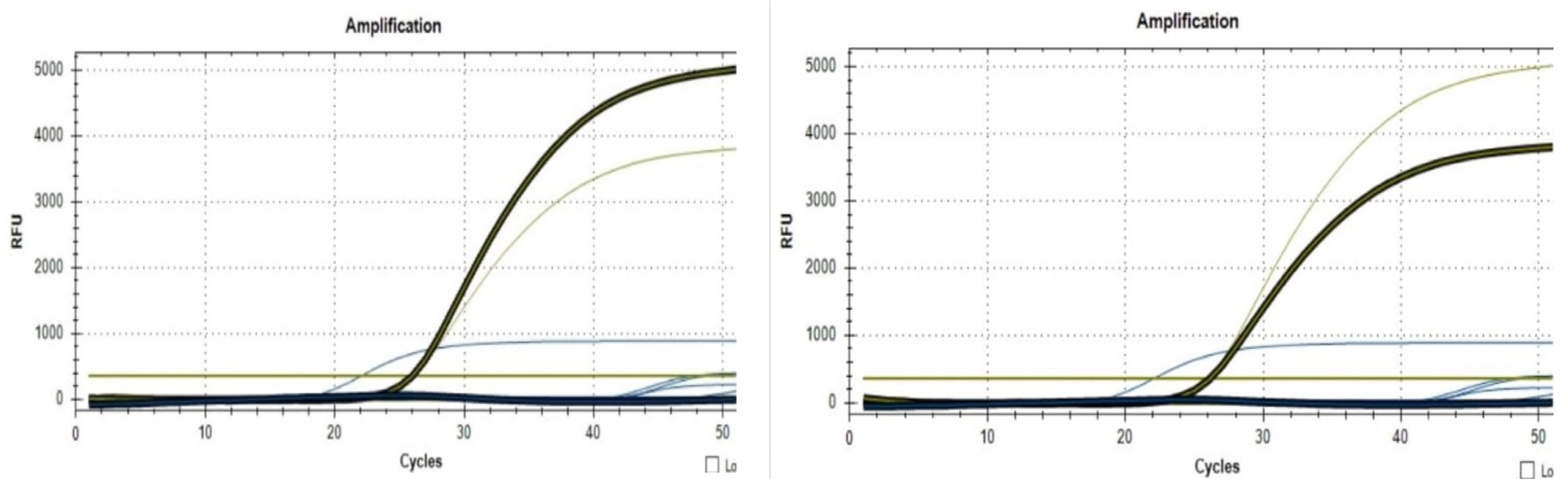
| pH | |||
|---|---|---|---|
| * Water Source | 23 September 2022 | 1. Nonhomogeneous on a vertical column: 6.7–7.6 (from the top towards the bottom) 2. After homogenization: 6.2 | DESIRABLE RANGE 6.5–9.5 |
| 28 March 2023 | 1.Nonhomogeneous on a vertical column: 7.7–6.9 (from the top towards the bottom) 2.After homogenization: 6.8 | ACCEPTABLE RANGE 5.5–10.0 | |
| ** Basin Water | 23 September 2022 | Homogeneous: 6.2 | REFERENCE [31] |
| 28 March 2023 | Homogeneous: 7.9 | ||
| Analysis Name | Water Source * | Basin Water ** | Desirable Range | Acceptable Range | Reference | ||
|---|---|---|---|---|---|---|---|
| 23 September 2022 | 28 March 2023 | 23 September 2022 | 28 March 2023 | ||||
| NH4− | 10 mg/L | <10 mg/L | 10 mg/L | 20 mg/L | 0–1 mg/L | <4 mg/L | [30] |
| Total hardness | 25 °D/444 mg/L (>4.5 mol/m3) | 25 °D/444 mg/L (>4.5 mol/m3) | 25 °D/444 mg/L (>4.5 mol/m3) | 25 °D/444 mg/L (>4.5 mol/m3) | 50–150 mg/L | Above 10 mg/L | [34] |
| SO32− | 5 mg/L | 10 mg/L | 5 mg/L | 10 mg/L | <64 mg/L | <64 mg/L | [32] |
| PO43− | 10–15 mg/L | 10 mg/L | 10–15 mg/L | 10 mg/L | 1 mg/L | 1 mg/L | [31] |
| Fe2+/3+ | 2 mg/L | 10 mg/L | 10 mg/l | 10 mg/L | <0.1 mg/L for fry, less than 1.0 mg/L for most fish (Ferric iron) | <0.1 mg/L for fry, less than 1.0 mg/L for most fish (Ferric iron) | [31] |
| Cu+/2+ | <0.1 mg/L | <0.1 mg/L | <0.1 mg/L | <0.1 mg/L | <0.1 mg/L | <0.1 mg/L | [33] |
| Dissolved oxygen (DO) | Not measured | 8.3–8.5 mg/L | 6–15 mg/L | 6–15 mg/L | [11] | ||
| Temperature | 10–11 °C | 11.6 °C–11.9 °C | 7–18 °C | <22 °C | [11] | ||
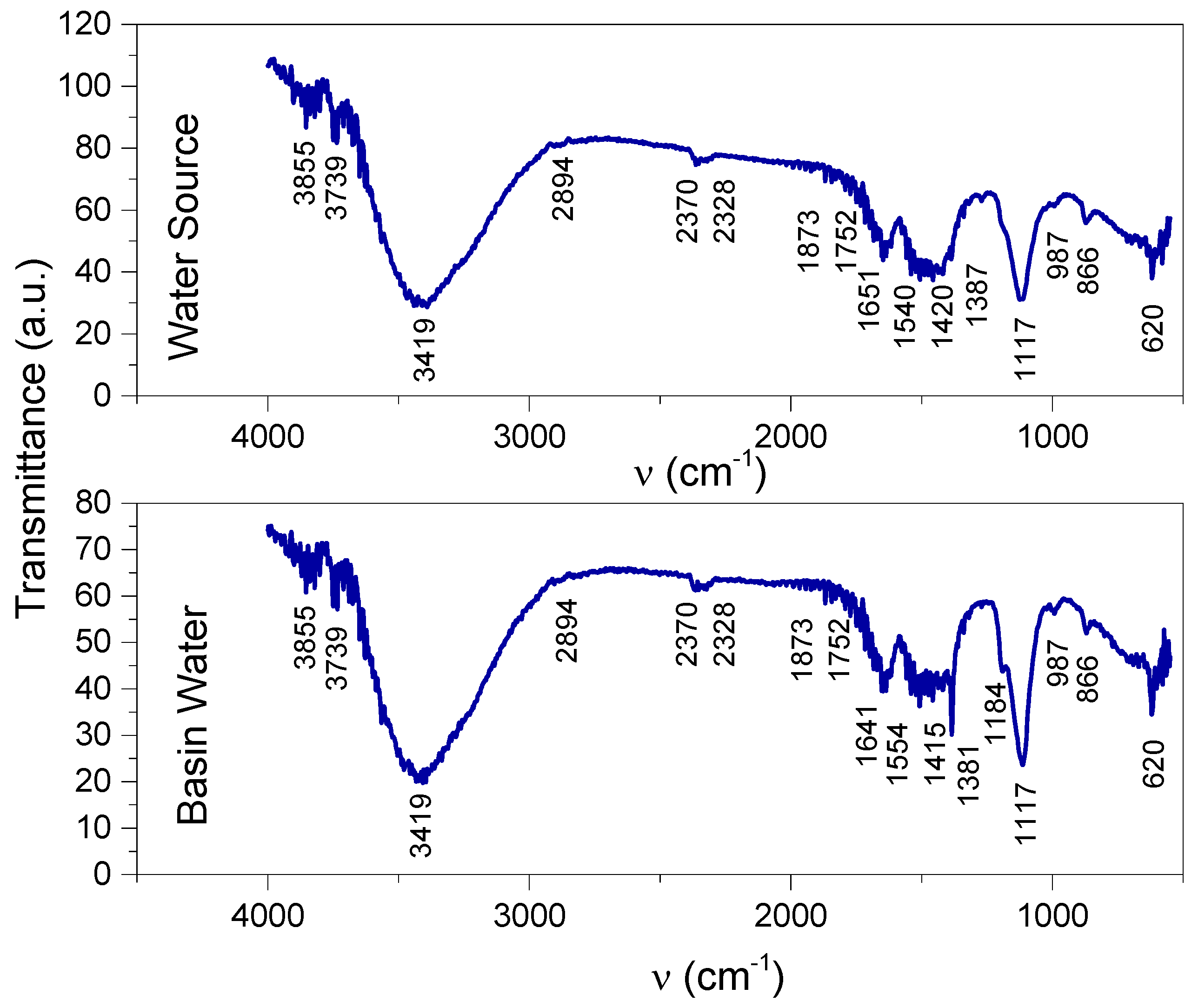
| Vibrational Bands (cm−1) | Functional Groups and References [16,17,35,36,37,38,39,40,41,42] | |
|---|---|---|
| Basin Water | Water Source | |
| 3855 | 3855 | OH that is free in alcohols OH in carboxylic acid, known to be typical carbohydrates OH in adsorbed/absorbed water or which is present as MgOH; CaOH |
| 3739 | 3739 | OH as SiOH (silanol); FeOH; MgOH; CaOH |
| 3419 | 3419 | OH that is free and bonded in alcohols NH in primary amines (including hetero-aromatic such as pyrrol), amides, aminoacids, and amino salts (NH4−) Interference with CH aromatic (the wide peak overlaps with the vibrational range of CH aromatic of 3080–3030) |
| 2894 | 2894 | CH aliphatic |
| 2370 | 2370 | Adsorbed CO2 |
| 2328 | 2328 | N=O stretching in nitrites -N+=N stretching in diazo compounds |
| 1873–1752 | 1873–1752 | C=O stretching in carbonates CO32− |
| 1641 | 1651 | CH bending in aromatic compounds NO2 stretching asymmetrically in nitrates C=O stretching in aldehydes, amides, carboxylic acids |
| 1554–1415 | 1540–1420 | NO stretching in nitro compounds and nitrates CNO2 in aromatic nitro compounds SO2 stretching asymmetrically in sulfones, sulfoxides, and sulfites (COO)- stretching asymmetrically and symmetrically in carboxylic acids and carboxylates NH2, NH3+, NH2+, NH+ bendings in amines, amides, amino salts |
| 1381 | 1387 | Medium: CH bending in aldehydes; CNO2 in aliphatic nitro compounds Sharp, strong, small molecules; NO2 stretching symmetrically in nitrates |
| 1182 | - | SO2 stretching symmetrically in sulfones, sulfoxides, and sulfites P=O stretching in phosphates and organics R3P=O |
| 1117 | 1117 | C-NO in aliphatic nitroso compounds SO2 stretching symmetrically in sulfones, sulfoxides, and sulfites SiO stretching |
| 987 | 987 | PO stretching in phosphates |
| 866 | 866 | NO stretching in nitrates CNO in aromatic nitroso compounds |
| 620 | 620 | SiC stretching in silanol terminal groups in organic compounds |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bălbărău, A.; Ivanescu, L.M.; Martinescu, G.; Rîmbu, C.M.; Acatrinei, D.; Lazar, M.; Cocean, I.; Gurlui, S.; Cocean, A.; Miron, L. Septicemic Outbreak in A Rainbow Trout Intensive Aquaculture System: Clinical Finds, Etiological Agents, and Predisposing Factors. Life 2023, 13, 2083. https://doi.org/10.3390/life13102083
Bălbărău A, Ivanescu LM, Martinescu G, Rîmbu CM, Acatrinei D, Lazar M, Cocean I, Gurlui S, Cocean A, Miron L. Septicemic Outbreak in A Rainbow Trout Intensive Aquaculture System: Clinical Finds, Etiological Agents, and Predisposing Factors. Life. 2023; 13(10):2083. https://doi.org/10.3390/life13102083
Chicago/Turabian StyleBălbărău, Adrian, Larisa Maria Ivanescu, Gabriela Martinescu, Cristina Mihaela Rîmbu, Dumitru Acatrinei, Mircea Lazar, Iuliana Cocean, Silviu Gurlui, Alexandru Cocean, and Liviu Miron. 2023. "Septicemic Outbreak in A Rainbow Trout Intensive Aquaculture System: Clinical Finds, Etiological Agents, and Predisposing Factors" Life 13, no. 10: 2083. https://doi.org/10.3390/life13102083
APA StyleBălbărău, A., Ivanescu, L. M., Martinescu, G., Rîmbu, C. M., Acatrinei, D., Lazar, M., Cocean, I., Gurlui, S., Cocean, A., & Miron, L. (2023). Septicemic Outbreak in A Rainbow Trout Intensive Aquaculture System: Clinical Finds, Etiological Agents, and Predisposing Factors. Life, 13(10), 2083. https://doi.org/10.3390/life13102083








