Enhancing Coccinella Beetle Biological Pest Control via a Floral Approach in Cucumber Greenhouse
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Location
2.2. Insect Rearing, Release, and Sampling
2.3. Greenhouse
2.4. Data Analysis
3. Results
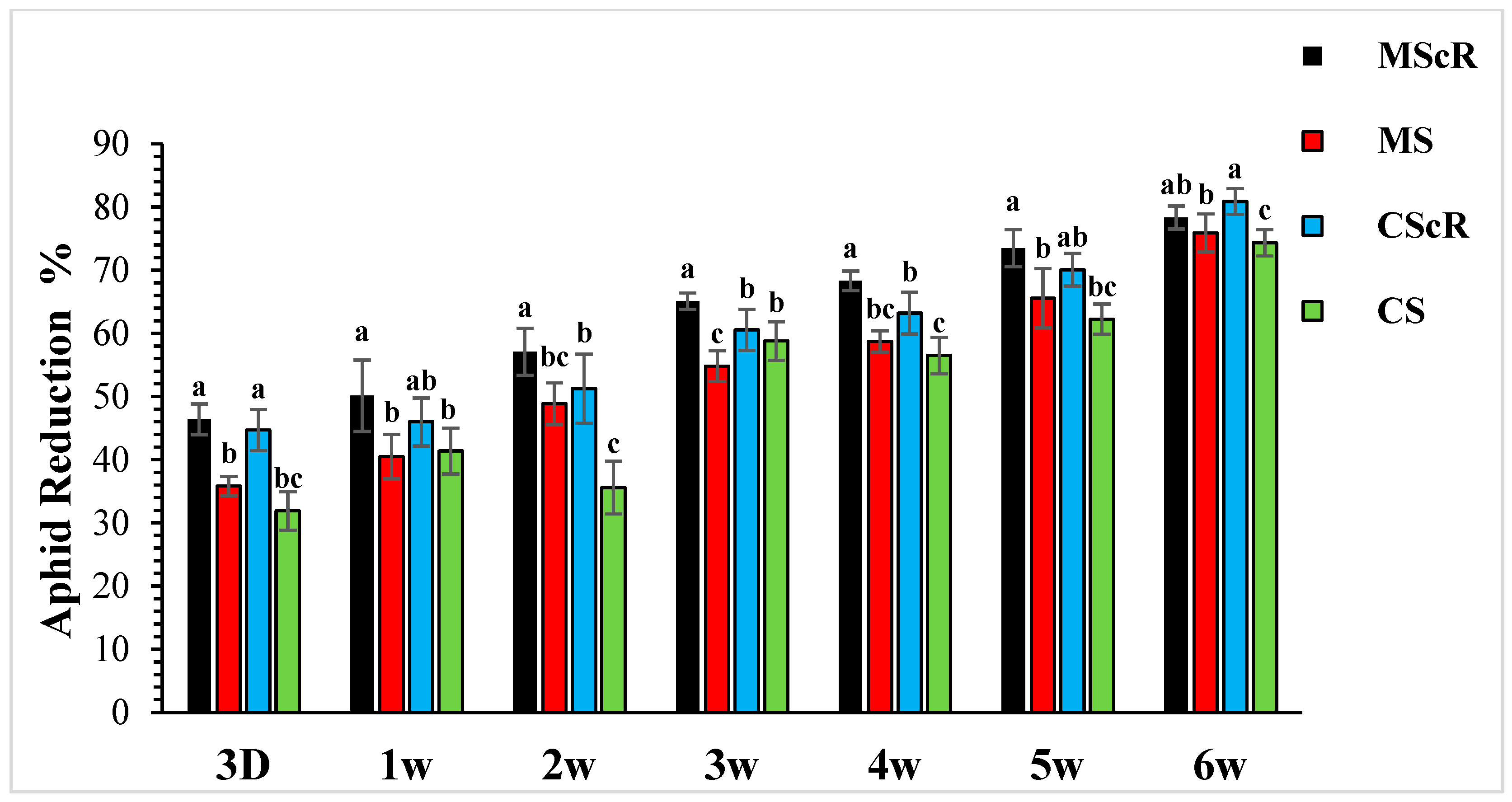
3.1. Aphid Performance in the Greenhouse
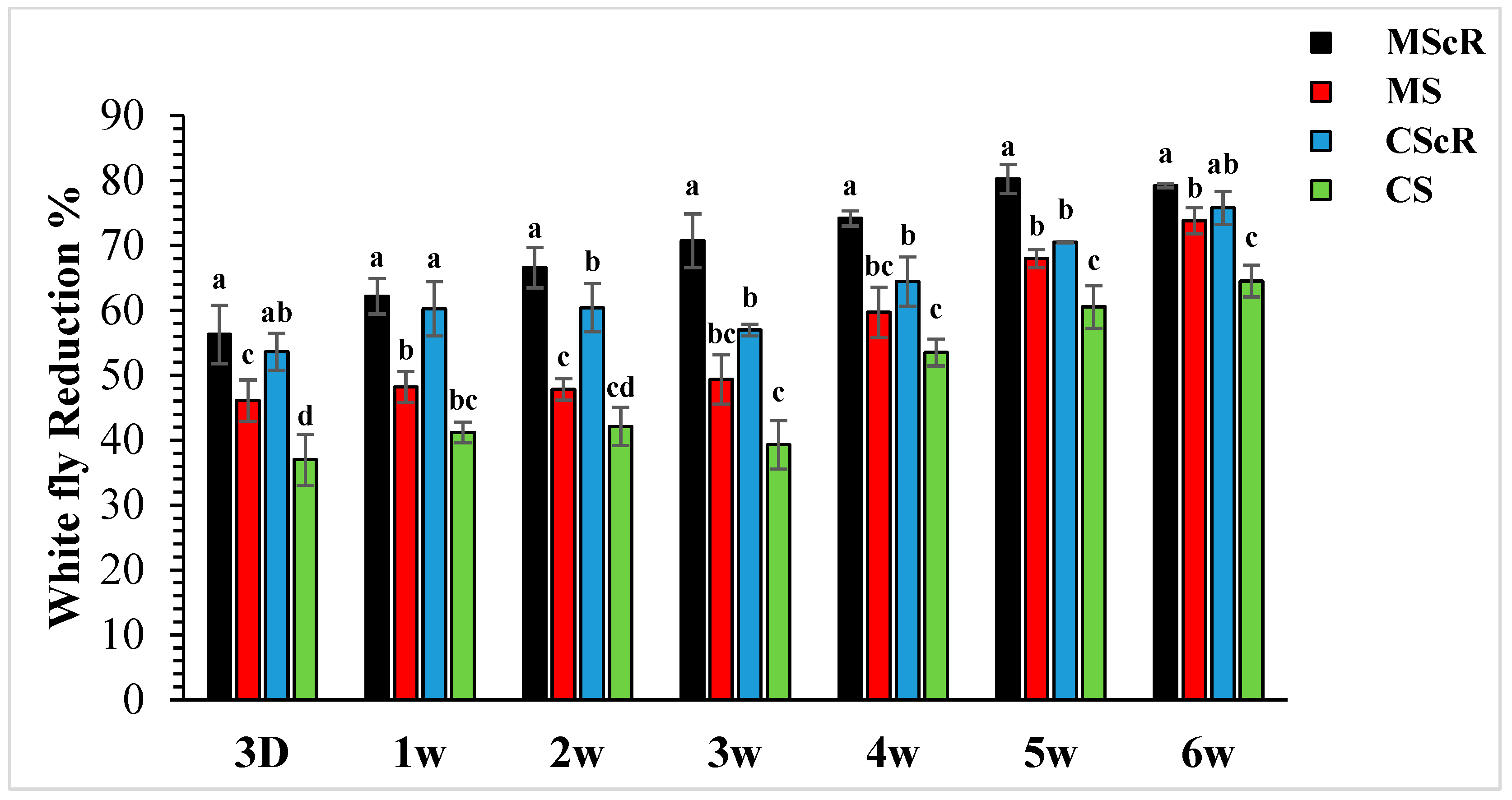
3.2. Whitefly Performance in the Greenhouse
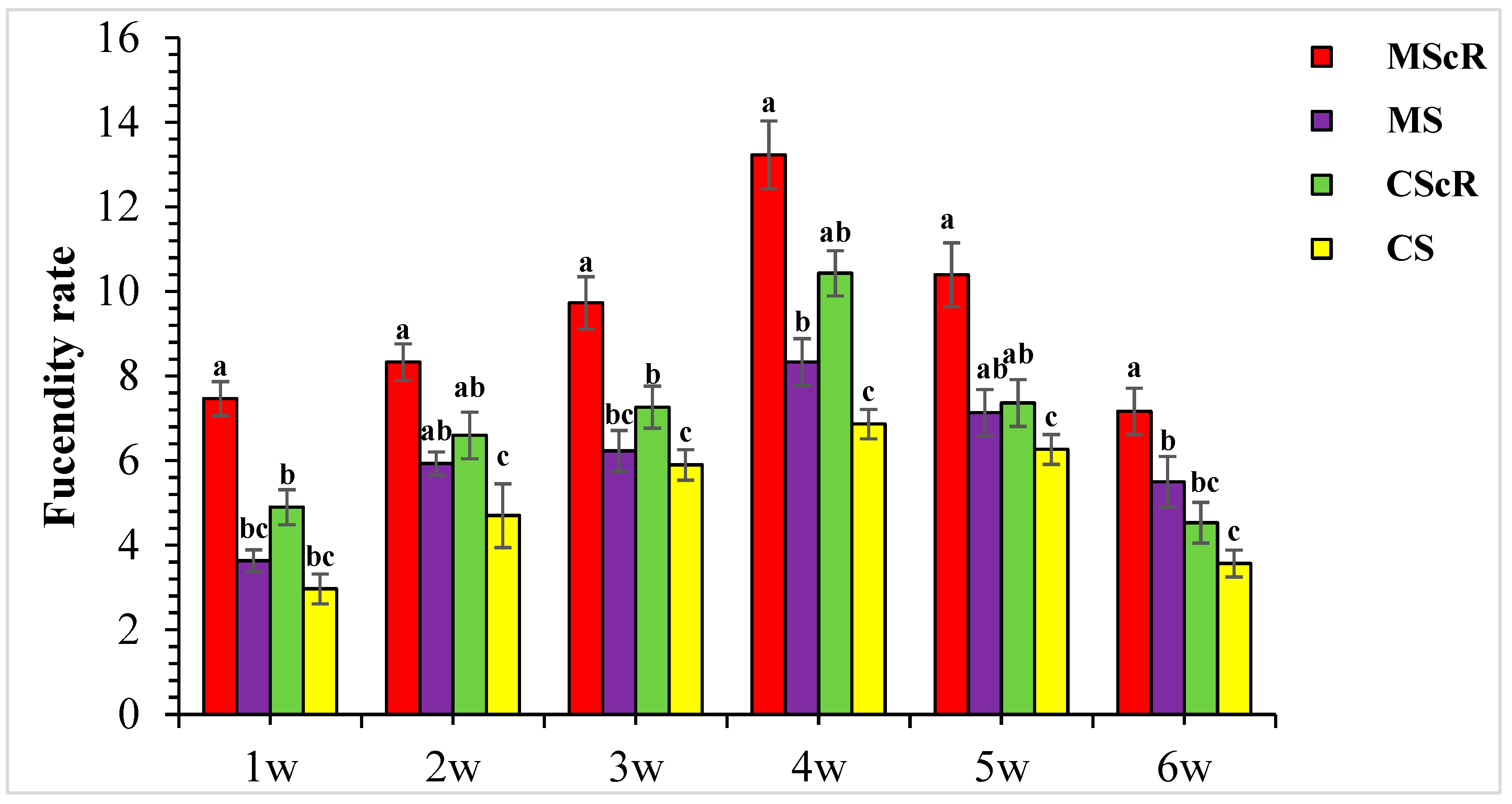
3.3. Fecundity Performance on Floral Plant
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Godfray, H.C.J. Food and biodiversity. Science 2011, 333, 1231–1232. [Google Scholar] [CrossRef] [PubMed]
- Kremen, C.; Williams, N.M.; Thorp, R.W. Crop pollination from native bees at risk from agricultural intensification. Proc. Natl. Acad. Sci. USA 2002, 99, 16812–16816. [Google Scholar] [CrossRef] [PubMed]
- Phalan, B.; Onial, M.; Balmford, A.; Green, R.E.; Godfray, H.C.J.; Tilman, D.; Green, R.E.; Cornell, S.J.; Scharlemann, J.P.W.; Balmford, A.; et al. Reconciling food production and biodiversity conservation: Land sharing and land sparing compared. Science 2011, 333, 1289–1291. [Google Scholar] [CrossRef] [PubMed]
- Wehner, T.C. Cucumbers, watermelon, squash and other cucurbits. Encycl. Food Cult. 2007, 474–479. [Google Scholar]
- Ene, C.O.; Ogbonna, P.E.; Agbo, C.U.; Chukwudi, U.P. Studies of phenotypic and genotypic variation in sixteen cucumber genotypes. Chil. J. Agric. Res. 2016, 76, 307–313. [Google Scholar] [CrossRef]
- Van Lenteren, J.C.; Bolckmans, K.; Kohl, J.; Ravensberg, W.J.; Urbaneja, A. Biological control using invertebrates and microorganisms: Plenty of new opportunities. BioControl 2018, 63, 39–59. [Google Scholar] [CrossRef]
- Omkar, P.A. Predaceous coccinellids in India: Predator-prey catalogue. Orient. Insects 2004, 38, 27–61. [Google Scholar]
- Solangi, B.K.; Lohar, M.K.; Lanjar, A.G.; Mahar, M.D. Biology of zigzag beetle, Menochilus sexmaculatus Fab (Coccinellidae: Coleoptera) on mustard aphid Lipaphis erysimi Kalt. Sarhad J. Agric. 2005, 21, 261–264. [Google Scholar]
- Jagadish, K.S.; Jayaramaiah, M.; Shivayogeshwara, B. Bioefficacy of three promising predators on Myzus nicotianae Blackman (Homoptera: Aphididae). J. Biopestic. 2010, 3, 62–67. [Google Scholar]
- Eilenberg, J.; Hajek, A.; Lomer, C. Suggestions for unifying the terminology in biological control. BioControl 2001, 46, 387–400. [Google Scholar] [CrossRef]
- Bale, J.S.; van Lenteren, J.C.; Bigler, F. Biological control and sustainable food production. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008, 363, 761–776. [Google Scholar] [CrossRef]
- Landis, D.A.; Wratten, S.D.; Gurr, G.M. Habitat Management to Conserve Natural Enemies of Arthropod Pests in Agriculture. Annu. Rev. Entomol. 2000, 45, 175–201. [Google Scholar] [CrossRef] [PubMed]
- Letourneau, D.K.; Armbrecht, I.; Rivera, B.S.; Lerma, J.M.; Carmona, E.J.; Daza, M.C.; Escobar, S.; Galindo, V.; Gutiérrez, C.; López, S.D.; et al. Does plant diversity benefit agroecosystems? A synthetic review. Ecol. Appl. 2011, 21, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Morandin, L.A.; Long, R.F.; Kremen, C. Hedgerows enhance beneficial insects on adjacent tomato fields in an intensive agricultural landscape. Agric. Ecosyst. Environ. 2014, 189, 164–170. [Google Scholar] [CrossRef]
- Blaauw, B.R.; Isaacs, R. Flower plantings increase wild bee abundance and the pollination services provided to a pollination dependent crop. J. Appl. Ecol. 2014, 51, 890–898. [Google Scholar] [CrossRef]
- Blaauw, B.R.; Isaacs, R. Wildfower plantings enhance the abundance of natural enemies and their services in adjacent blueberry fields. Biol. Control 2015, 91, 94–103. [Google Scholar] [CrossRef]
- Van Lenteren, J.C. The state of commercial augmentative biological control: Plenty of natural enemies, but a frustrating lack of uptake. BioControl 2012, 57, 1–20. [Google Scholar] [CrossRef]
- Fiedler, A.K.; Landis, D.A.; Wratten, S.D. Maximizing ecosystem services from conservation biological control: The role of habitat management. Biol. Control 2008, 45, 254–271. [Google Scholar] [CrossRef]
- Frank, T. Density of adult hoverflies (Dipt., Syrphidae) in sown weed strips and adjacent fields. J. Appl. Entomol. 1999, 123, 351–355. [Google Scholar] [CrossRef]
- Sutherland, J.P.; Sullivan, M.S.; Poppy, G.M. Distribution and abundance of aphidophagous hoverflies (Diptera: Syrphidae) in wildflower patches and field margin habitats. Agric. For. Entomol. 2001, 3, 57–64. [Google Scholar] [CrossRef]
- Pontin, D.R.; Wade, M.R.; Kehrli, P.; Wratten, S.D. Attractiveness of single and multiple species flower patches to beneficial insects in agroecosystems. Ann. Appl. Biol. 2006, 148, 39–47. [Google Scholar] [CrossRef]
- Berndt, L.A.; Wratten, S.D. Effects of alyssum flowers on the longevity, fecundity, and sex ratio of the leafroller parasitoid Dolichogenidea tasmanica. Biol. Control 2005, 32, 65–69. [Google Scholar] [CrossRef]
- Riddick, E.W. Benefts and limitations of factitious prey and artifcial diets on life parameters of predatory beetles, bugs, and lacewings: A mini-review. BioControl 2009, 54, 325–339. [Google Scholar] [CrossRef]
- Pineda, A.; Marcos-García, M.A. Introducing barley as aphid reservoir in sweet-pepper greenhouses: Effects on native and released hoverflies (Diptera, Syrphidae). Eur. J. Entomol. 2008, 105, 531–535. [Google Scholar] [CrossRef]
- Van Oystaeyen, A.; Tuyttens, E.; Boonen, S.; De Smedt, L.; Bellinkx, S.; Wäckers, F.; Pekas, A. Dual purpose: Predatory hoverflies pollinate strawberry crops and protect them against the strawberry aphid, Chaetospihon fragaefolii. Pest Manag. Sci. 2022, 78, 3051–3060. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wyckhuys, K.A.G.; Wu, K. Hoverflies provide pollination and biological pest control in greenhouse-grown horticultural crops. Front. Plant Sci. 2023, 14, 1118388. [Google Scholar] [CrossRef]
- Amoros-Jimenez, R.; Pineda, A.; Fereres, A.; Marcos-García, M.A. Feeding preferences of the aphidophagous hoverfly Sphaerophoria rueppellii affect the performance of its offspring. BioControl 2014, 59, 427–435. [Google Scholar] [CrossRef]
- Colley, M.R.; Luna, J.M. Relative attractiveness of potential beneficial insectary plants to aphidophagous hoverflies (Diptera: Syrphidae). Environ. Entomol. 2000, 20, 1054–1059. [Google Scholar] [CrossRef]
- Ambrosino, M.D.; Luna, J.M.; Jepson, P.C.; Wratten, S.D. Relative frequencies of visits to selected insectary plants by predatory hoverflies (Diptera: Syrphidae), other beneficial insects, and herbivores. Environ. Entomol. 2006, 35, 394–400. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Van Rijn, P.C.J.; Wäckers, F.L. Nectar accessibility determines fitness, flower choice and abundance of hoverflies that provide natural pest control. J. Appl. Ecol. 2016, 53, 925–933. [Google Scholar] [CrossRef]
- Winkler, K.; Wäckers, F.L.; Termorshuizen, A.J.; Lenteren, J.C. Assessing risks and benefits of floral supplements in conservation biological control. BioControl 2010, 55, 719–727. [Google Scholar] [CrossRef]
- Fitzgerald, J.D.; Solomon, M.G. Can flowering plants enhance numbers of beneficial arthropods in UK apple and pear orchards? Biocontrol Sci. Technol. 2004, 14, 291–300. [Google Scholar] [CrossRef]
- Leman, A.; Angelos, M.; Juliette, P.; Kyra, V.; Felix, W.; Gerben, J.M. Sugar and pollen supply enhances aphid control by hoverflies in strawberry. Biol. Control 2023, 186, 105347. [Google Scholar] [CrossRef]
- Riddick, E.W. Identifcation of conditions for successful aphid control by ladybirds in greenhouses. Insects 2017, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Hulle, M.; Chaubet, B.; Turpeau, E.; Simon, J.C. Encyclop’aphid: A website on aphids and their natural enemies. Entomol. Gen. 2020, 40, 97–101. [Google Scholar] [CrossRef]
- Liu, L.Z.; Dai, P.; Lv, B.; Zhang, L.S.; Du, W.M.; WAN, F.H. Interspecific competition between Encarsia sophia and E. formosa and their impacts on suppression of Trialeurodes vaporariorum. Sci. Agric. Sin. 2013, 46, 4837–4841. [Google Scholar]
- Ma, L.J.; Zhang, S.Z.; Liu, T.X. Infuences of interspecifc competition between ladybeetle Serangium japonicum and parasitoid Encarsia formosa on predation of tobacco whitefly Bemisia tabaci. J. Plant Prot. 2018, 45, 1289–1295. [Google Scholar]
- Moerkens, R.; Boonen, S.; Wackers, F.L.; Pekas, A. Aphidophagous hoverflies reduce foxglove aphid infestations and improve seed set and fruit yield in sweet pepper. Pest Manag. Sci. 2021, 77, 2690–2696. [Google Scholar] [CrossRef]
- Dib, H.; Simon, S.; Sauphanor, B.; Capowiez, Y. The role of natural enemies on the population dynamics of the rosy apple aphid, Dysaphis plantaginea Passerini (Hemiptera: Aphididae) in organic apple orchards in south-eastern France. Biol. Control 2010, 55, 97–109. [Google Scholar] [CrossRef]
- Bommarco, R.; Kleijn, D.; Potts, S.G. Ecological intensification: Harnessing ecosystem services for food security. Trends Ecol. Evol. 2013, 28, 230–238. [Google Scholar] [CrossRef] [PubMed]


| 3 Days | 1st Week | 2nd Week | 3rd Week | 4th Week | 5th Week | 6th Week | |
|---|---|---|---|---|---|---|---|
| Myzus persicae | |||||||
| F | 13.84 | 5.82 | 6.82 | 155.34 | 6.01 | 6.03 | 489.6 |
| df | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| p | 0.0059 | 0.0423 | 0.0311 | 0.0231 | 0.0398 | 0.0396 | 0.0482 |
| Bemisia tabaci | |||||||
| F | 6.95 | 8.47 | 14.63 | 12.72 | 8.86 | 9.08 | 7.70 |
| df | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| p | 0.0299 | 0.0196 | 0.0051 | 0.0073 | 0.0177 | 0.0167 | 0.0241 |
| Fecundity rate | |||||||
| F | 10.42 | 10.50 | 6.76 | 25.22 | 9.99 | 62.86 | |
| df | 2 | 2 | 2 | 2 | 2 | 2 | |
| p | 0.0233 | 0.0230 | 0.0380 | 0.0040 | 0.0251 | 0.0376 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hyder, M.; Li, Y.; Raza, M.F.; Zhang, M.; Chen, J.; Mao, J.; Bukero, A.; Zhang, L. Enhancing Coccinella Beetle Biological Pest Control via a Floral Approach in Cucumber Greenhouse. Life 2023, 13, 2080. https://doi.org/10.3390/life13102080
Hyder M, Li Y, Raza MF, Zhang M, Chen J, Mao J, Bukero A, Zhang L. Enhancing Coccinella Beetle Biological Pest Control via a Floral Approach in Cucumber Greenhouse. Life. 2023; 13(10):2080. https://doi.org/10.3390/life13102080
Chicago/Turabian StyleHyder, Moazam, Yuyan Li, Muhammad Fahad Raza, Maosen Zhang, Junjie Chen, Jianjun Mao, Aslam Bukero, and Lisheng Zhang. 2023. "Enhancing Coccinella Beetle Biological Pest Control via a Floral Approach in Cucumber Greenhouse" Life 13, no. 10: 2080. https://doi.org/10.3390/life13102080
APA StyleHyder, M., Li, Y., Raza, M. F., Zhang, M., Chen, J., Mao, J., Bukero, A., & Zhang, L. (2023). Enhancing Coccinella Beetle Biological Pest Control via a Floral Approach in Cucumber Greenhouse. Life, 13(10), 2080. https://doi.org/10.3390/life13102080







