Changes in Diversity of Silica-Scaled Chrysophytes during Lake–River–Reservoir Transition (Baikal–Angara–Irkutsk Reservoir)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Sampling and Field Work
2.3. Hydrochemistry
2.4. Investigation of Silica-Scaled Chrysophytes
2.5. Statistical Analysis
3. Results
3.1. Diversity and Distribution of Silica-Scaled Chrysophytes Depending on Water Parameters
3.2. Species with Specific Morphology and Undetermined Species
4. Discussion
4.1. Change in Species Composition and Diversity of Silica-Scaled Chrysophytes from Lake Baikal to Irkutsk Reservoir
4.2. Distribution of Silica-Scaled Chrysophytes Depending on the Water Parameters
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Resende, N.d.S.; dos Santos, J.B.O.; Josué, I.I.P.; Barros, N.O.; Cardoso, S.J. Comparing spatio-temporal dynamics of functional and taxonomic diversity of phytoplankton community in tropical Cascading Reservoirs. Front. Environ. Sci. 2022, 10, 903180. [Google Scholar] [CrossRef]
- Reynolds, C.S. The ecology of phytoplankton. In Ecology, Biodiversity and Conservation; Cambridge University Press: Cambridge, UK, 2006; 535p. [Google Scholar] [CrossRef]
- Padisák, J.; Crossetti, L.O.; Naselli-Flores, L. Use and misuse in the application of the phytoplankton functional classification: A critical review with updates. Hydrobiologia 2009, 621, 1–19. [Google Scholar] [CrossRef]
- Kouassi, B.A.T.; Komoé, K.; N’guessan, K.R. Relationships between phytoplankton structure and environmental variables in an African tropical reservoir. Int. J. Sci. Res. 2023, 10, 356–362. [Google Scholar]
- Padisák, J.; Borics, G.; Grigorszky, I.; Soróczki-Pintér, É. Use of phytoplankton assemblages for monitoring ecological status of lakes within the water framework directive: The assemblage index. Hydrobiologia 2006, 553, 1–14. [Google Scholar] [CrossRef]
- Crossetti, L.O.; de M. Bicudo, C.E. Phytoplankton as a monitoring tool in a tropical urban shallow reservoir (Garças Pond): The assemblage index application. Hydrobiologia 2008, 610, 161–173. [Google Scholar] [CrossRef]
- Nogueira, M.; Ferrareze, M.; Moreira, M.; Gouvêa, R. Phytoplankton assemblages in a reservoir Cascade of a large tropical—Subtropical river (SE, Brazil). Braz. J. Biol. 2010, 70, 781–793. [Google Scholar] [CrossRef]
- Liu, C.; Sun, X.; Su, L.; Cai, J.; Zhang, L.; Guo, L. Assessment of phytoplankton community structure and water quality in the Hongmen Reservoir. Water Qual. Res. J. 2021, 56, 19–30. [Google Scholar] [CrossRef]
- Nwonumara, G.N.; Elebe, F.A.; Nwibo, O.D. The physico-chemical variables and phytoplankton of Ufiobodo and Ebonyi Reservoirs, Ebonyi State, Nigeria. Zoologist 2023, 21, 41–48. [Google Scholar] [CrossRef]
- Yena, T.T.H.; Tranga, L.T.; Danga, P.D.; Thaia, T.T.; Tua, N.V.; Lamb, N.N.; Luua, P.T. Seasonal variation of phytoplankton functional groups in Tuyen lam reservoir, Central Highlands, Vietnam. Dalat Univ. J. Sci. 2022, 13, 25–35. [Google Scholar] [CrossRef]
- Siver, P.A. Synurophyte algae. In Freshwater Algae of North America: Ecology and Classification, 2nd ed.; Academic Press: Boston, MA, USA, 2015; pp. 607–651. [Google Scholar]
- Kristiansen, J.; Škaloud, P. Chrysophyta. Handbook of the Protists, 2nd ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–38. [Google Scholar]
- Balonov, I.M. Zolotistyje vodorosli vodojmov Vologodskoj oblasti. AN SSSR Inst. Biol. Vnutrennich Vod. Trudy 1980, 42, 1–26. [Google Scholar]
- Balonov, I.M.; Kuzmina, A.E. Chrysophyta. In Hydrochemical and Hydrobiological Studies in on the Khantay Reservoir; Votinzev, K.K., Ed.; Nauka: Novosibirsk, Russia, 1986; p. 119. [Google Scholar]
- Bessudova, A.Y.; Likhoshway, Y.V. Silica chrysophytes (Chrysophyceae) of the Boguchany Reservoir. Mod. Sci. Actual Probl. Theory Pract. Ser. Nat. Tech. Sci. Gen. Biol. 2017, 11, 4–11. [Google Scholar]
- Gusev, E.S.; Guseva, E.E.; Gabyshev, V.A. Taxonomic composition of silica-scaled chrysophytes in rivers and lakes of Yakutia and Magadanskaya oblast (Russia). Nova Hedwig. Beih. 2018, 147, 105–117. [Google Scholar]
- Barreto, S.; Kristiansen, J.; Acs, E. Silica-scaled chrysophytes during spring in the Kis-Balaton Reservoir, Hungary. Acta Bot. Croat. 2000, 59, 337–349. [Google Scholar]
- Gusev, E.; Martynenko, N.; Kapustin, D.; Doan-Nhu, H.; Nguyen-Ngoc, L. Diversity of silica-scaled chrysophytes of two tropical islands: Phu Quoc and Con Son (Viet Nam). Life 2022, 12, 1611. [Google Scholar] [CrossRef]
- Bessudova, A.; Firsova, A.; Bukin, Y.; Kopyrina, L.; Zakharova, Y.; Likhoshway, Y. Under-ice development of silica-scaled chrysophytes with different trophic mode in two ultraoligotrophic lakes of Yakutia. Diversity 2023, 15, 326. [Google Scholar] [CrossRef]
- Vorobyova, S.S. Phytoplankton of the Angara River; Nauka: Novosibirsk, Russia, 1995; p. 126. (In Russian) [Google Scholar]
- Popovskaya, G.I.; Firsova, A.D.; Bessudova, A.; Sakirko, M.V.; Suturin, A.N.; Likhoshway, Y.V. Phytoplankton of the Irkutsk Reservoir as an indicator of water quality. Oceanol. Hydrobiol. Stud. 2012, 41, 29–38. [Google Scholar] [CrossRef]
- Toman, M.J. Physico-chemical characteristics and seasonal changes of phytoplankton communities in a river reservoir. Lakes Reserv. Res. Man. 1996, 2, 71–76. [Google Scholar] [CrossRef]
- Korneva, L.G. Phytoplankton of Reservoirs of the Volga Basin; Kostroma Printing House: Kostroma, Russia, 2015; p. 284. [Google Scholar]
- Shchur, L.A. Phytoplankton as an indicator of the state of the ecosystem of the cooling pond at Berezovskaya GRES-1 (Krasnoyarsk Territory). Russ. Water Resour. 2009, 5, 597–605. [Google Scholar]
- Talling, J.F.; Prowse, G.A. Selective recruitment and resurgence of tropical river phytoplankton: Evidence from the Nile system of lakes, rivers, reservoirs and ponds. Hydrobiologia 2010, 637, 187–195. [Google Scholar] [CrossRef]
- Sharma, B.K.; Sharma, S. Phytoplankton diversity of a de-mineralized subtropical reservoir of Meghalaya state, northeast India. Aquat. Res. 2021, 4, 233–249. [Google Scholar] [CrossRef]
- Grachev, M.A. On Recent State of Ecological System of Lake Baikal; SB RAS: Novosibirsk, Russia, 2002; 153p. [Google Scholar]
- Matz, V.D. The structure and development of the Baikal rift depression. Earth-Sci. Rev. 1993, 34, 81–118. [Google Scholar] [CrossRef]
- Shimaraev, M.N.; Domysheva, V.M.; Gorbunova, L.A. On oxygen dynamics during spring water renewal. DAN 1996, 347, 814–817. [Google Scholar]
- Galazy, G.I. (Ed.) Atlas of Baikal; Federal Agency for Geodesy and Cartography: Moscow, Russia, 1993; 160p. [Google Scholar]
- Verbolov, V.I. Currents and water exchange in Lake Baikal. Russ. Water Resour. 1996, 23, 381–391. [Google Scholar]
- Znamensky, V.A.; Yanter, N.N. (Eds.) Hydrometeorological Regime of Lakes and Reservoirs; Gidrometeoizdat: Leningrad, Russia, 1980; 140p. [Google Scholar]
- ISO 7890-3: 1988; Water Duality—Determination of Nitrate—Part 3: Spectrometric Method Using Sulfosalicylic Acid. International Organization for Standardization (ISO): Geneva, Switzerland, 2004.
- Wetzel, R.; Likens, G. Limnological Analysis, 3rd ed.; Springer: New York, NY, USA, 2000; pp. 57–112. [Google Scholar]
- ISO 6878: 2004; Water Quality—Determination of Phosphorus—Ammonium Molybdate Spectrometric Method. International Organization for Standardization (ISO): Geneva, Switzerland, 2004.
- Kristiansen, J. Dispersal and biogeography of silica-scaled chrysophytes. Protist Divers. Geogr. Distrib. 2008, 17, 419–426. [Google Scholar] [CrossRef]
- Voloshko, L.N. Golden Algae of Waters of North Russia; Komarov Botanical Institute of the Russian Academy of Sciences: St Petersburg, Russia, 2017; p. 378. [Google Scholar]
- Legendre, P.; Gallagher, E.D. Ecologically meaningful transformations for ordination of species data. Oecologia 2001, 129, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Taiyun, W.; Simko, V. R Package ‘Corrplot’: Visualization of a Correlation Matrix (Version 0.92). 2021. Available online: https://github.com/taiyun/corrplot (accessed on 29 September 2023).
- Harrell, F.E., Jr. Hmisc: Harrell Miscellaneous. R Package Version 4.7-1. 2022. Available online: https://CRAN.R-project.org/package=Hmisc (accessed on 29 September 2023).
- Bessudova, A.; Gabyshev, V.; Firsova, A.; Gabysheva, O.; Bukin, Y.; Likhoshway, Y. Diversity variation of silica-scaled chrysophytes related to differences in physicochemical variables in estuaries of rivers in an Arctic watershed. Sustainability 2021, 13, 13768. [Google Scholar] [CrossRef]
- Asmund, B.; Kristiansen, J. The Genus Mallomonas (Chrysophyceae). A taxonomic survey based on the ultrastructure of silica scales and bristles. Opera Bot. 1986, 85, 1–128. [Google Scholar]
- Kristiansen, J.; Preisig, H.R. Chrysophyta and Haptophyta Algae, 2nd part: Synurophyceae. In Süsswasserflora von Mitteleuropa (Freshwater Flora of Central Europe); Büdel, B., Gärtner, G., Krienitz, L., Preisig, H.R., Schagerl, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1–252. [Google Scholar]
- Bessudova, A.Y.; Domysheva, V.M.; Firsova, A.D.; Likhoshway, Y.V. Silica-scaled chrysophytes of Lake Baikal. Acta Biol. Sib. 2017, 3, 47–56. [Google Scholar] [CrossRef][Green Version]
- Siver, P.A. The Distribution of Chrysophytes along Environmental Gradients: Their Use as Biological Indicators. In Chrysophyte Algae: Ecology, Phylogeny and Development; Sandgren, C.D., Smol, J.P., Kristiansen, J., Eds.; Cambridge University Press: Cambridge, UK, 1995; pp. 232–268. [Google Scholar]
- Firsova, A.; Galachyants, Y.; Bessudova, A.; Titova, L.; Sakirko, M.; Marchenkov, A.; Hilkhanova, D.; Nalimova, M.; Buzevich, V.; Mikhailov, I.; et al. Environmental factors affecting distribution and diversity of phytoplankton in the Irkutsk Reservoir ecosystem in June 2023. Diversity 2023, 15, 1070. [Google Scholar] [CrossRef]
- Grachev, M.A.; Domysheva, V.M.; Khodzher, T.V.; Korovyakova, N.V.; Golobokova, L.P.; Vereshagina, A.L.; Granin, N.G.; Gnatovsky, R.Y.; Kostornova, T.Y. Deep water of Lake Baikal as a standard of fresh water. Chem. Sustain. Dev. 2004, 12, 417–429. [Google Scholar]
- Khodzher, T.V.; Domysheva, V.M. Hydrochemistry of Lake Baikal. In Chemical Balance of the Lake; Rusinek, O.T., Takhteev, V.V., Gladkochub, D.P., Khodzher, T.V., Budnev, N.M., Eds.; Nauka: Novosibirsk, Russia, 2012; pp. 206–228. [Google Scholar]
- Khodzher, T.V.; Domysheva, V.M.; Sorokovikova, L.M.; Sakirko, M.V.; Tomberg, I.V. Current chemical composition of Lake Baikal water. Inland Waters 2017, 7, 250–258. [Google Scholar] [CrossRef]
- Mikhailov, I.S.; Galachyants, Y.P.; Bukin, Y.S.; Petrova, D.P.; Bashenkhaeva, M.V.; Sakirko, M.V.; Blinov, V.V.; Titova, L.A.; Zakharova, Y.R.; Likhoshway, Y.V. Seasonal succession and coherence among bacteria and microeukaryotes in Lake Baikal. Microb. Ecol. 2022, 84, 404–422. [Google Scholar] [CrossRef] [PubMed]
- Kozhova, O.M. Phytoplankton of Irkutsk Reservoir; Science: Moskva, Russia, 1964; pp. 41–114. [Google Scholar]
- Siver, P.A.; Lott, A.M. Fossil species of Mallomonas from an Eocene maar lake with recessed dome structures: Early attempts at securing bristles to the cell covering? Nova Hedwig. 2012, 95, 517–529. [Google Scholar] [CrossRef]
- Smol, J.P. Application of chrysophytes to problems in paleoecology. In Chrysophyte Algae: Ecology, Phylogeny and Development; Sandgren, C.D., Smol, J.P., Kristiansen, J., Eds.; Cambridge University Press: Cambridge, UK, 1995; pp. 303–329. [Google Scholar]
- Dürrschmidt, M. Studies on the Chrysophyceae from Southern Chilean inland waters by means of scanning and transmission microscopy, II. Arch. Hydrobiol. Suppl. 1982, 63, 121–163. [Google Scholar]
- Siver, P.A. The distribution and variation of Synura species (Chrysophyceae) in Connecticut, USA. Nord. J. Bot. 1987, 7, 107–116. [Google Scholar] [CrossRef]
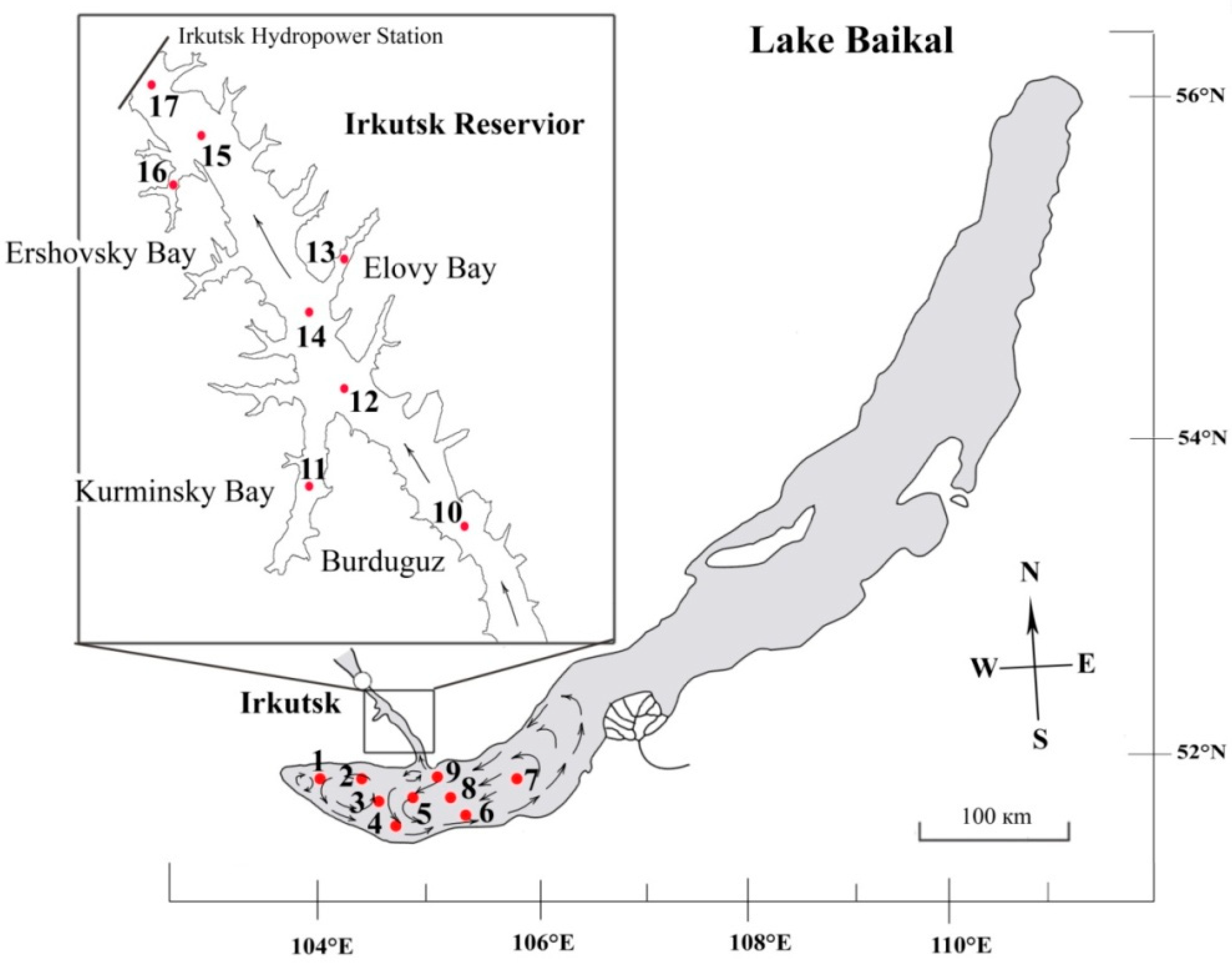
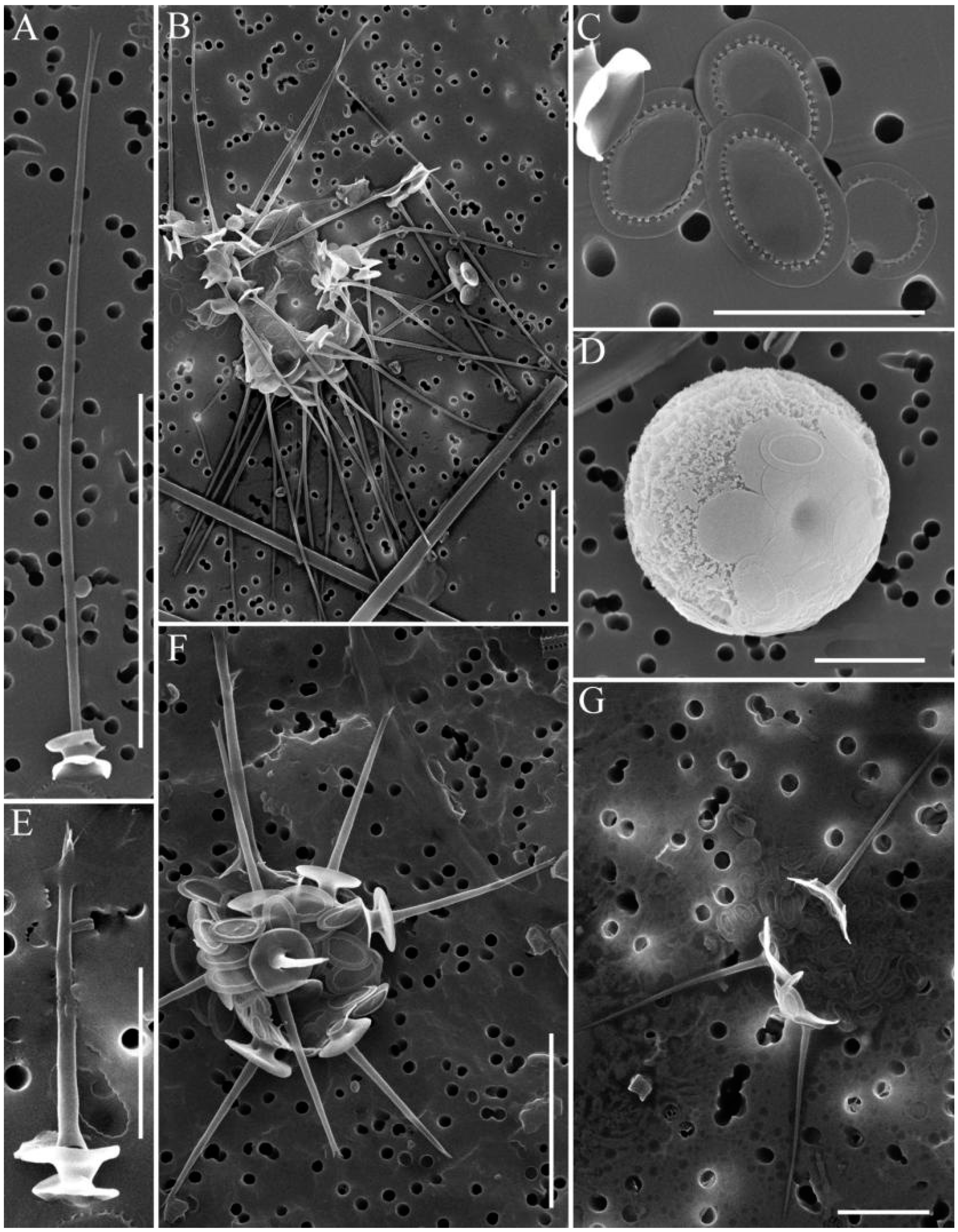

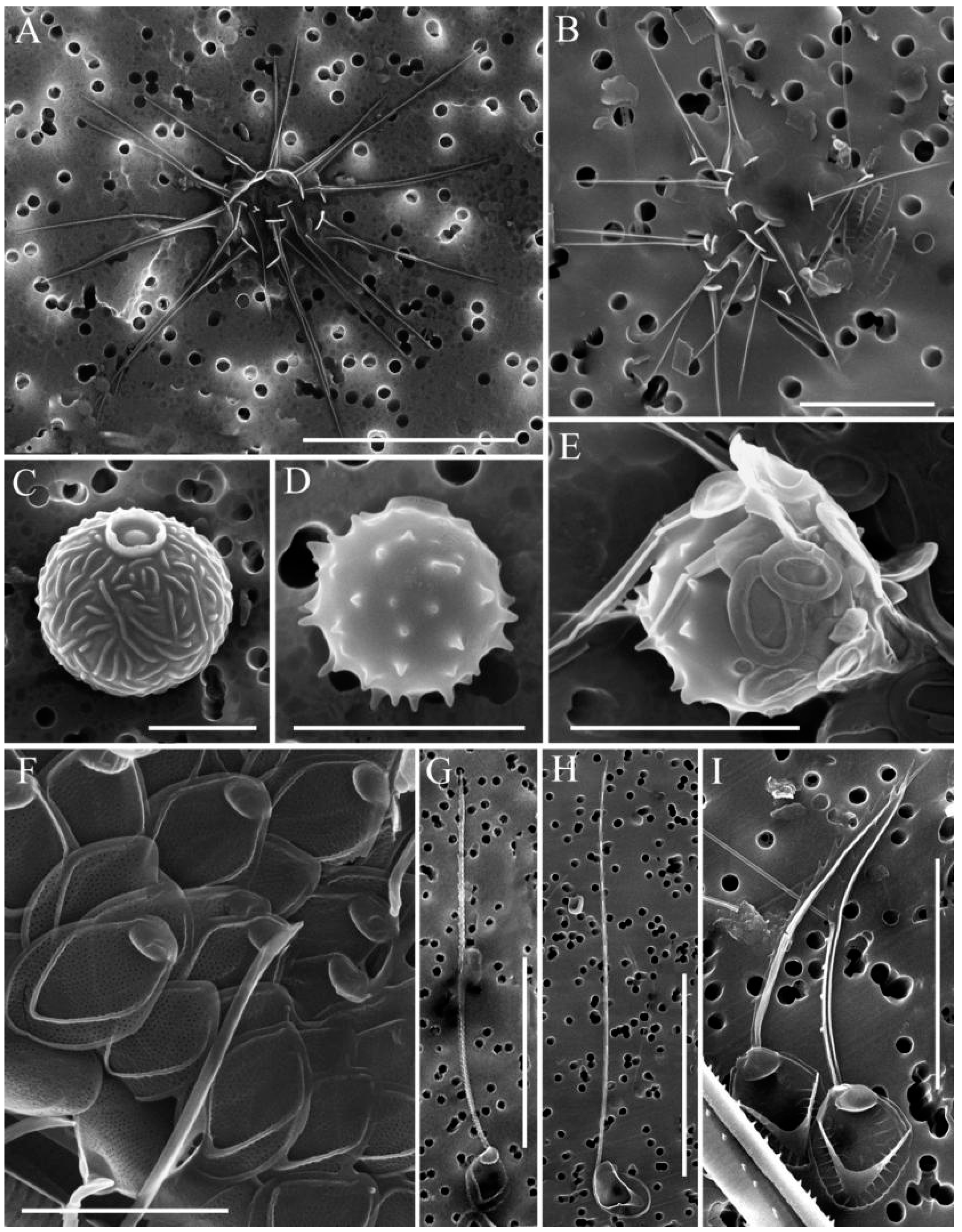

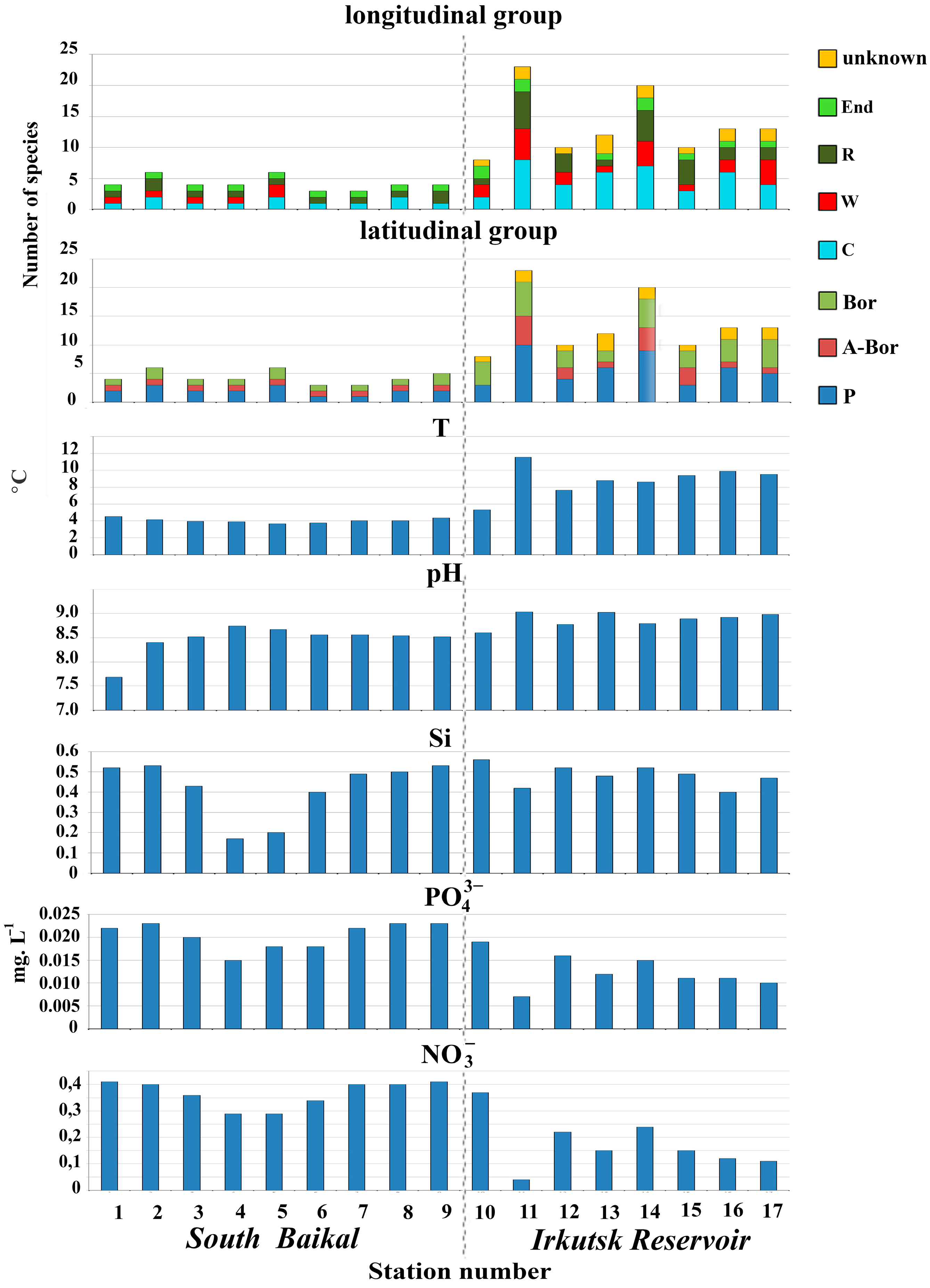

| Station Number | Station Name | Coordinates N/E | Water T, °C | pH | Si, mg·L−1 | PO43−, mg·L−1 | NO3− mg·L−1 |
|---|---|---|---|---|---|---|---|
| 1. | 12 km from Kultuk | 51°40.578/103°52.309 | 4.5 | 7.18 | 0.52 | 0.022 | 0.41 |
| 2. | 3 km from Marituy | 51°45.546/104°13.222 | 4.1 | 7.90 | 0.53 | 0.023 | 0.40 |
| 3. | Marituy–Solzan | 51°38.710/104°13.715 | 3.9 | 8.02 | 0.43 | 0.020 | 0.36 |
| 4. | 3 km from Solzan | 51°31.428/104°14.417 | 3.9 | 8.24 | 0.17 | 0.015 | 0.29 |
| 5. | cape Tolsty–Snezhnaya River | 51°36.402/104°44.147 | 3.6 | 8.17 | 0.20 | 0.018 | 0.29 |
| 6. | 3 km from Tankhoi | 51°35.440/105°06.968 | 3.7 | 8.06 | 0.40 | 0.018 | 0.34 |
| 7. | cape Kadilny–Mishikha | 51°46.731/105°22.528 | 4.0 | 8.06 | 0.49 | 0.022 | 0.40 |
| 8. | Listvyanka–Tankhoi | 51°42.262/105°00.720 | 4.0 | 8.04 | 0.50 | 0.023 | 0.40 |
| 9. | 3 km from Listvyanka | 51°49.033/104°54.616 | 4.3 | 8.02 | 0.53 | 0.023 | 0.41 |
| 10. | IrkR_Burduguz | 52°04.105/104°59.451 | 5.3 | 8.10 | 0.56 | 0.019 | 0.37 |
| 11. | IrkR_Kurma Bay | 52°06.845/104°45.926 | 11.5 | 8.57 | 0.42 | 0.007 | 0.04 |
| 12. | IrkR_center against Kurma Bay | 52°10.874/104°47.935 | 7.6 | 8.27 | 0.52 | 0.016 | 0.22 |
| 13. | IrkR_Elovy Bay | 52°09.906/104°25.172 | 8.8 | 8.52 | 0.48 | 0.012 | 0.15 |
| 14. | IrkR_center against Elovy Bay | 52°14.548/104°45.243 | 8.6 | 8.29 | 0.52 | 0.015 | 0.24 |
| 15. | IrkR_center against Ershovsky Bay | 52°21.511/104°37.550 | 9.4 | 8.39 | 0.49 | 0.011 | 0.15 |
| 16. | IrkR_Ershovsky Bay | 52°20.851/104°34.439 | 9.9 | 8.42 | 0.40 | 0.011 | 0.12 |
| 17. | IrkR_head water | 52°23.478/104°33.722 | 9.5 | 8.48 | 0.47 | 0.010 | 0.11 |
| Species | Latitudinal Group | Longitudinal Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Chrysosphaerella baicalensis Popovskaya | End | Bor | +++ | +++ | +++ | +++ | +++ | +++ | +++ | ++ | ++ | ++ | + | + | + | + | + | + | |
| 2. | C. brevispina Korshikov | R | Bor | ++ | ++ | ++ | ++ | + | ++ | + | + | + | + | |||||||
| 3. | C. coronacircumspina Wujek & Kristiansen | C | P | + | + | |||||||||||||||
| 4. | Paraphysomonas bandaiensis Takahashi | R | A-Bor | + | ||||||||||||||||
| 5. | Paraphysomonas sp. 1 | unkn | unkn | + | ||||||||||||||||
| 6. | Paraphysomonas sp. 2 | unkn | unkn | + | ||||||||||||||||
| 7. | Spiniferomonas abrupta Nielsen | W | Bor | ++ | ++ | ++ | + | |||||||||||||
| 8. | S. bourrellyi Takahashi | C | P | + | ++ | ++ | ++ | + | ||||||||||||
| 9. | S. cornuta Balonov | R | A-Bor | + | ||||||||||||||||
| 10. | S. silverensis Nicholls | R | A-Bor | + | + | ++ | + | |||||||||||||
| 11. | S. triangularis Siver | R | A-Bor | + | + | |||||||||||||||
| 12. | S. trioralis Takahashi | C | P | + | + | + | ++ | ++ | ++ | ++ | ++ | + | ++ | |||||||
| 13. | S. trioralis f. cuspidata Balonov | R | A-Bor | ++ | ++ | ++ | +++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | + | ++ | ++ | |
| 14. | Mallomonas acaroides Perty | C | P | +++ | ++ | +++ | ++ | |||||||||||||
| 15. | M. alpina Pascher & Ruttner | C | P | +++ | ++ | +++ | +++ | +++ | ++ | ++ | ++ | ++ | + | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ |
| 16. | M. crassisquama (Asmund) Fott | C | P | +++ | ++ | ++ | ++ | ++ | ++ | ++ | ||||||||||
| 17. | M. elongata Reverdin | W | P | + | ||||||||||||||||
| 18. | M. grachevii Bessudova | End | Bor | + | + | + | ||||||||||||||
| 19. | M. punctifera Korshikov | C | P | + | + | + | ||||||||||||||
| 20. | M. striata var. getseniae Voloshko | R | A-Bor | + | ||||||||||||||||
| 21. | M. striata Asmund | C | P | + | + | |||||||||||||||
| 22. | M. tonsurata Teiling | C | P | + | ||||||||||||||||
| 23. | M. trummensis Cronberg | R | A-Bor | + | ||||||||||||||||
| 24. | M. vannigera Asmund | W | P | + | + | + | + | + | + | + | + | + | ||||||||
| 25. | Mallomonas sp. | unkn | unkn | + | + | |||||||||||||||
| 26. | Synura echinulata Korshikov | C | P | + | ||||||||||||||||
| 27. | S. cf. glabra (Korshikov) Škaloud & Kynclová | W | Bor | ++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | |||||||||
| 28. | S. punctulosa Balonov | W | Bor | + | + | + | ||||||||||||||
| 29. | S. spinosa f. longispina Petersen & Hansen | W | P | + | ||||||||||||||||
| 30. | Synura sp. 1 | unkn | unkn | ++ | ++ | |||||||||||||||
| 31. | Synura sp. 2 | unkn | unkn | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | |||||||||
| Total | 4 | 6 | 4 | 4 | 6 | 3 | 3 | 4 | 4 | 8 | 23 | 10 | 12 | 20 | 10 | 13 | 13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bessudova, A.; Galachyants, Y.; Firsova, A.; Hilkhanova, D.; Nalimova, M.; Marchenkov, A.; Mikhailov, I.; Sakirko, M.; Likhoshway, Y. Changes in Diversity of Silica-Scaled Chrysophytes during Lake–River–Reservoir Transition (Baikal–Angara–Irkutsk Reservoir). Life 2023, 13, 2052. https://doi.org/10.3390/life13102052
Bessudova A, Galachyants Y, Firsova A, Hilkhanova D, Nalimova M, Marchenkov A, Mikhailov I, Sakirko M, Likhoshway Y. Changes in Diversity of Silica-Scaled Chrysophytes during Lake–River–Reservoir Transition (Baikal–Angara–Irkutsk Reservoir). Life. 2023; 13(10):2052. https://doi.org/10.3390/life13102052
Chicago/Turabian StyleBessudova, Anna, Yuri Galachyants, Alena Firsova, Diana Hilkhanova, Maria Nalimova, Artyom Marchenkov, Ivan Mikhailov, Maria Sakirko, and Yelena Likhoshway. 2023. "Changes in Diversity of Silica-Scaled Chrysophytes during Lake–River–Reservoir Transition (Baikal–Angara–Irkutsk Reservoir)" Life 13, no. 10: 2052. https://doi.org/10.3390/life13102052
APA StyleBessudova, A., Galachyants, Y., Firsova, A., Hilkhanova, D., Nalimova, M., Marchenkov, A., Mikhailov, I., Sakirko, M., & Likhoshway, Y. (2023). Changes in Diversity of Silica-Scaled Chrysophytes during Lake–River–Reservoir Transition (Baikal–Angara–Irkutsk Reservoir). Life, 13(10), 2052. https://doi.org/10.3390/life13102052







