1. Introduction
Sarcoptic mange, known as scabies in humans, is a highly contagious skin disease caused by
Sarcoptes scabiei, a mite that burrows into the top layer of the skin (epidermis). These mites are astigmatids that belong to the Sarcoptinae family. They actively penetrate the outermost layer of the skin, known as the stratum corneum [
1]. The adult mites mate, and the females lay eggs on the skin. Once hatched, larvae create small burrows, referred to as molting pouches, where they undergo molting and develop into nymphs, eventually becoming adults [
2]. These parasites are found worldwide and can infect more than 150 different host species. Interestingly, they exhibit a surprising ability to be transmitted between different hosts, showcasing their epidemiological flexibility [
3]. In animals, the disease can manifest as a mild infection. The common symptoms include itchy papules, redness (erythema), scales, and hair loss (alopecia). In chronic cases, hyperkeratosis (thickening of the skin) and/or the formation of crusts with discharge may occur [
4,
5]. Specific identification of this species depends on the host it infests. For example, in humans, it is
S. scabiei var.
hominis, whereas, in rabbits, it is referred to as
S. scabiei var. cuniculi [
6].
Sarcoptic mange is a highly contagious skin disease that spreads through direct skin-to-skin contact, contact with contaminated objects, and exposure to an infected environment inhabited by severely affected hosts [
7,
8]. Nymphs and female mites have a longer off-host survival period of up to 21 days than larvae and males, indicating the need for environmental application of biocides and repellents [
9,
10].
S. scabiei infection causes significant morbidity and mortality in wild and domestic mammals, leading to potential economic losses. Primary morbidity is associated with secondary bacterial infections caused by
Streptococcus pyogenes and
Staphylococcus aureus [
11]. Rabbits infected with
S. scabiei experience weight loss, reduced productivity, and compromised wool-fiber quality [
12,
13]. Additionally, affected rabbits may develop dermatitis, pyoderma, eczema, and urticaria [
12].
Ticks are of great economic significance as pests in the global livestock industry, affecting cattle and other domestic species [
14]. According to FAO [
15], over 80% of the global cattle population is infected with ticks. Among these pests,
Rhipicephalus (
Boophilus)
microplus is of major concern in tropical and subtropical regions. The impact of
R. microplus infestation includes reduced milk production and weight gain, increased mortality rates, hide damage, disease morbidity, control costs, and transmission of tick-borne pathogens, such as
Babesia bigemina,
B. bovis, and
Anaplasma marginale. The global financial losses caused by tick infestation are estimated to be approximately USD 14,000–18,000 million. Moreover, the livestock industry in India and Pakistan spends approximately USD 498.7 million annually on tick and TBD (tick-borne disease) control [
16].
Currently, scabies and
R. microplus are primarily managed by chemical acaricides. These acaricides consist of active components categorized as macrocyclic lactones, organophosphates, formamidines, synthetic pyrethroids, phenyl pyrazoles, and growth inhibitors [
17]. Unfortunately, excessive, and sometimes inappropriate, use of these acaricides against ticks has led to the emergence of resistant tick populations [
18,
19]. Developing new synthetic compounds is a costly and time-consuming endeavor, underscoring the need for plant-based alternatives to effectively control tick and mite infestation [
20,
21,
22].
Green tea, scientifically known as
Camellia sinensis, holds a prominent position among beverages worldwide and has deep cultural significance in China and Japan. The numerous health benefits of
C. sinensis are primarily associated with its polyphenol content. These polyphenols consist mainly of catechins and derivatives such as (−)-epigallocatechin-3-gallate (EGCG), (−)-epicatechin, (−)-epigallocatechin, (−)-epicatechin gallate, and (−)-gallocatechin gallate [
23]. Studies have shown that
C. sinensis exhibits anticancer, antitrypanosomal, and anti-
Plasmodium properties [
24,
25]. Despite extensive research on the pharmacological aspects of
C. sinensis, no studies have been conducted to explore its acaricidal activity.
Glutathione transferase (GST), classified under the EC 2.5.1.18 enzyme superfamily, plays a pivotal role in cellular detoxification processes. These enzymes facilitate the conjugation of reduced glutathione (GSH) with a wide range of endogenous and exogenous electrophilic compounds. By doing so, they protect cells from oxidative damage, contributing to overall cellular health.
In ticks, GST enzymes are believed to support tick survival by neutralizing toxins and aiding in the detoxification of various substances. This detoxification capacity is particularly relevant as overexpression of GST has been associated with drug resistance [
26,
27]. Several studies have observed an upregulation in the transcription of GST genes and an increase in GST enzyme activity in ticks when exposed to both endogenous and exogenous compounds [
28,
29].
Given these critical biological functions, GSTs emerge as promising targets for the development of novel acaricidal chemotherapeutic drugs. By selectively inhibiting GST activity, it is possible to disrupt detoxification mechanisms in ticks and mites, potentially rendering them more susceptible to acaricides. This approach holds promise in addressing challenges associated with mite and tick infestations and drug resistance, ultimately contributing to more effective mite and tick control strategies.
The pivotal role of GST enzymes in detoxification processes and their association with drug resistance make them attractive candidates for targeted acaricidal drug development, offering new avenues for parasitic Acari control and management.
The current study aimed to determine the in vitro efficacy of
C. sinensis plant extract against ticks and mites. Natural products are a mix of different phytochemicals. Testing the relative contribution of individual compounds is difficult and inefficient given complex mixtures. We selected the most abundant compounds (
Table 1) for the in silico evaluation of inhibitory interactions with tick and mite proteins. This provides a foundation for future bioassays to evaluate the insecticidal role of individual compounds.
2. Materials and Methods
2.1. Plant Extract Preparation
C. sinensis aerial materials were collected from the Jalala region in Mardan, Khyber Pakhtunkhwa (coordinates: 34.3345° N, 71.9075° E). The collected plant materials were inspected for any physical damage and then rinsed in running tap water to remove surface debris. The leaves were then submitted to the herbarium of the Department of Botany, Abdul Wali Khan University Mardan (AWKUM) and were issued the accession number Awkum.Bot.917. They were then kept in a shady place at room temperature, away from sunlight, for air drying.
After 15 d of air drying, the plant material was ground to form a coarse powder using a plant grinder (YUEYUEHONG Model: HC-3000A Zhejiang, China). Powdered material (100 g) of each plant was then soaked in 1000 mL of 80% ethanol and agitated for 48 h using an orbital shaker (labForce Model 1165U07, Thomas Scientific, Swedesboro, NJ, USA) at 300RPM. The agitated solution was then reduced to a concentrated solution using a rotary evaporator (BUCHI Rotavapor Model: R-300 Flawil, Switzerland) at 40 °C under vacuum to remove ethanol. The solution was further concentrated in a water bath (Model WTB15, Memmert GmbH & Co. KG, Schwabach, Germany) at 45 °C until a very high concentration of the extract less than 10% of the original volume remained. The concentrated extract was used as a stock solution at different concentrations.
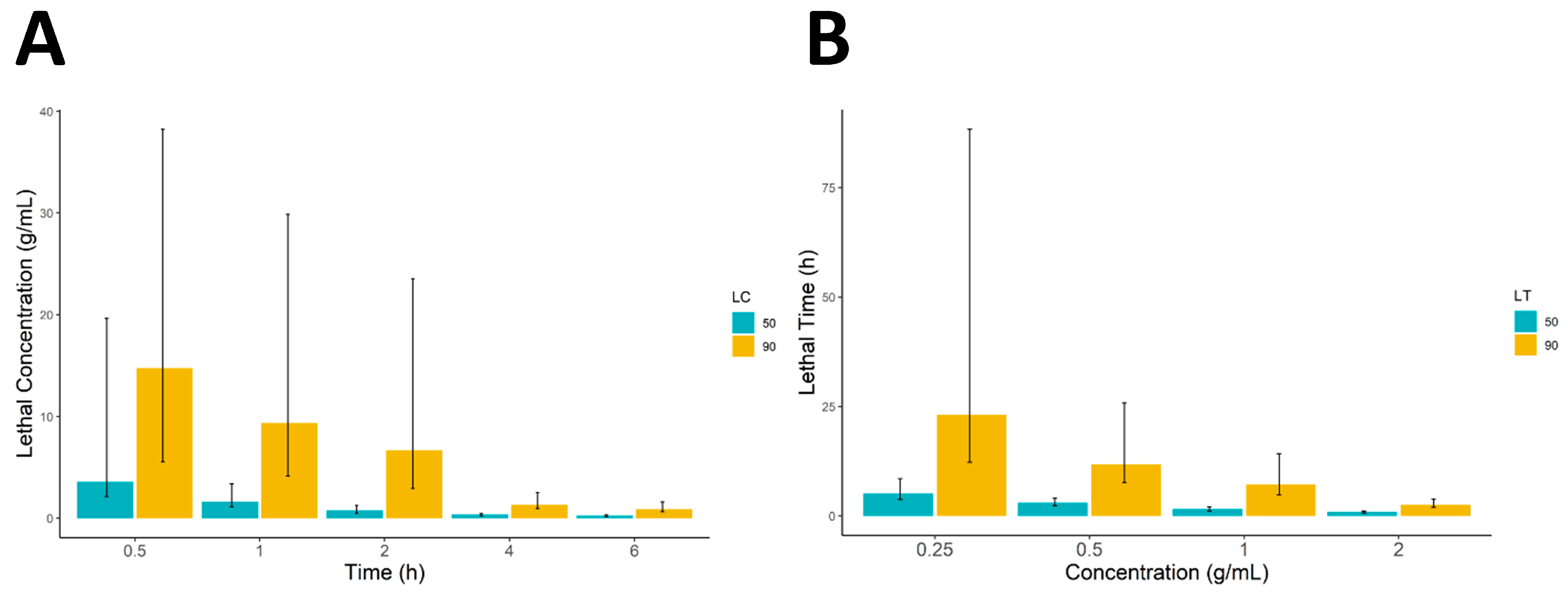
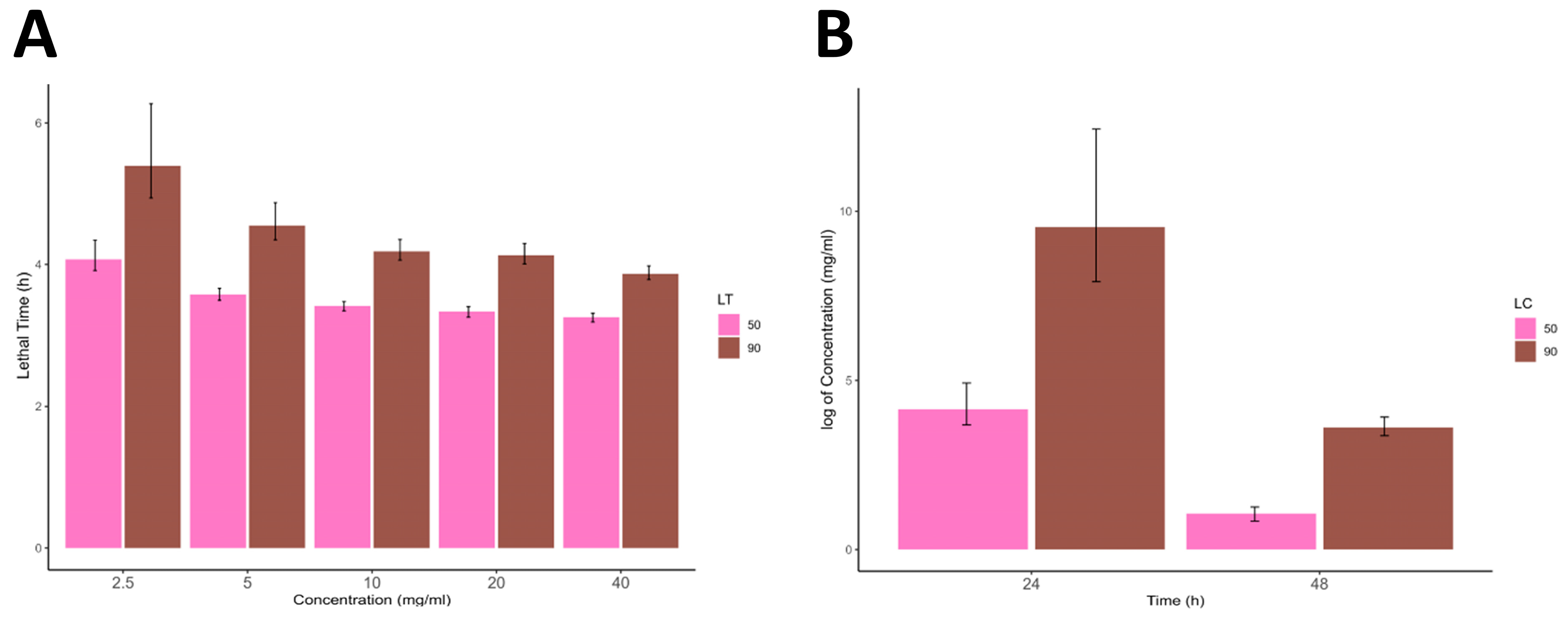
Different concentrations of 0.25, 0.5, 1, and 2 g/mL were prepared according to the procedure described by [
13] for the acaricidal contact bioassay against mites and 2.5, 5, 10, 20, and 40 mg/mL concentrations for the acaricidal bioassay against ticks [
30].
2.2. Mite Collection and Identification
Mites were collected from rabbits kept on rabbit farms at the Abdul Wali Khan University Mardan (AWKUM). Hay was provided as bedding to the rabbits on the farm, which was changed daily. The rabbits were first diagnosed for any signs of mange, and upon confirmation, skin scraps were taken using the procedure described by [
13], and the rabbits were treated immediately. The skin scraps were then made into slides and observed under a microscope for
S. scabiei.
2.3. Tick Collection and Incubation
Engorged adult female ticks were collected from the ground and cattle bodies in different locations near AWKUM in Mardan, Khyber Pakhtunkhwa, Pakistan. Standard tick identification keys were utilized to morphologically identify these ticks as
R. (
B.)
microplus under a stereo zoom microscope [
31]. The engorged ticks were then transported to the parasitology lab, AWKUM, and incubated to lay the eggs. The eggs were then incubated to hatch into larvae for larval assays in an incubator (BIOBASE Model: BJPX-H50IV Shandong, China). Adult ticks and larvae were then used in AIT and LPT.
2.4. Contacts Bioassay for Mites
Scabies-infested rabbits were used for isolating mites in the current study. Skin scraps were taken from the rabbit adhering to the established animal-care guidelines [
32]. The infested skin was briefly cleaned and then scraped into a micro-Petri plate using a sterile surgical blade until the skin appeared red. The Petri plates containing skin scraps were incubated at 37 °C for 30 min to allow mites to emerge from the skin scraps. Ten mites were introduced into a Petri plate using a fine needle, and then 0.5 mL of samples of the plant extract were directly added onto the mites in the Petri plate. The procedure was performed in triplicate for each extract concentration.
2.5. Adult Immersion Test (AIT)
Completely engorged adult female ticks were used in the present study. The adult ticks were first rinsed in distilled water to remove any debris from their skin, and their weights were recorded. Ten (10) ticks were then dipped for 2–3 min at a concentration of the extract and then incubated for two days. This procedure was repeated for each extract concentration. Three replicates were performed for the study on separate days using a fresh new concentration of the extract for each replicate. Data were recorded for the total weight of eggs laid and tick mortality. The calculation of the percentage inhibition of oviposition (% IO) was performed using the following formula:
The egg-laying index is calculated by dividing the mean weight of eggs laid by the mean weight of engorged females.
2.6. Larval Packet Test (LPT)
Newly hatched larvae were used in this experiment. Whatman no. 1 filter paper was immersed in a 0.6 mL extract concentration and then dried in an incubator at 37 °C. The filter paper was then made into a pouch by bending it and taping its sides with adhesive tape, with the upper side left open. Then, 100 larvae were carefully placed in the pouch, making a packet of larvae. The packet was then incubated at 28 ± 1 °C in a bio-oxygen demand incubator (BOD incubator) (BIOBASE Model: BJPX-B100 Shandong, China). Larvae were inspected for mortality at 24 and 48 h. Larvae that did not respond to light or had no appendage movement when teased with a needle were considered dead. This assay was also performed in triplicates.
2.7. Selection of Phytochemicals
Following a thorough examination of the current literature using Google Scholar (
https://scholar.google.com/, accessed on 25 June 2023, Keywords; “
Camellia sinensis” AND “phytochemistry” OR phytochemicals AND “Pakistan” OR “India”) and PubMed (
https://pubmed.ncbi.nlm.nih.gov/advanced/, accessed on 25 June 2023, Keyword; (Camellia sinensis [Title/Abstract]) AND (Pakistan [Title/Abstract]) AND (Phytochemistry) OR (phytochemicals) OR (GC-MS)), the
C. sinensis phytochemicals were chosen. Articles that studied the phytochemical content of
C. sinensis from Pakistan, India, or other related climates, utilizing GC-MS, TLC, and HPLC analytical methodologies were specifically chosen [
33,
34,
35,
36]. Ten phytochemicals were chosen from extracts of
C. sinensis (L) because of their ability to inhibit the
R. microplus and
S. scabiei glutathione transferase (GST) protein. These phytochemicals, which possess anti-inflammatory, antiseptic, and astringent properties, were identified through an extensive review of the existing literature. The corresponding structures of the ligands targeting GST proteins were acquired from the PubChem-NCBI database in the structure data format (SDF). Subsequently, these structures were converted into the protein databank (PDB) format using PyMOL for further analysis.
2.8. Preparation of Protein by Homology Modeling
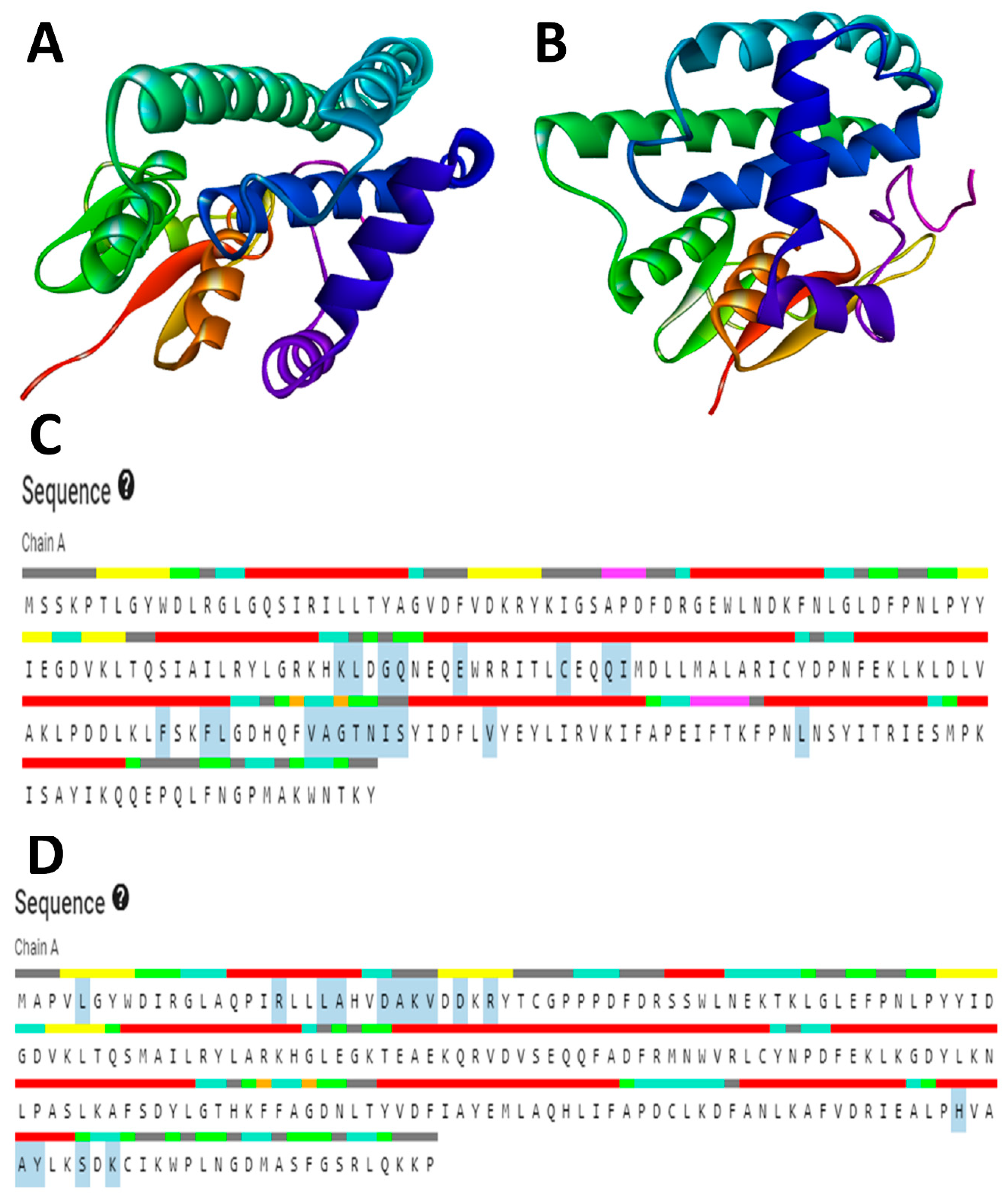
The RCSB’s (Research Collaboratory for Structural Bioinformatics) protein database (PDB) does not contain the available three-dimensional (3D) structures of the drug targets selected for this study. Therefore, homology modeling was used to obtain the 3D structures. The primary structures of
S. scabiei glutathione transferase (Uniprot accession no: Q8I9R9) and
R. microplus glutathione transferase (Uniprot accession no: O97117) were obtained in FASTA format from the UniProt Knowledgebase (UniProt KB database). To predict the 3D structure of SsGST and RmGST proteins, the SWISS-MODEL protein modeling server was employed [
37]. This server generates homology models by performing a target-template sequence alignment using the BLASTp and HHBlits programs and searching through template structures in the Protein Data Bank (PDB) [
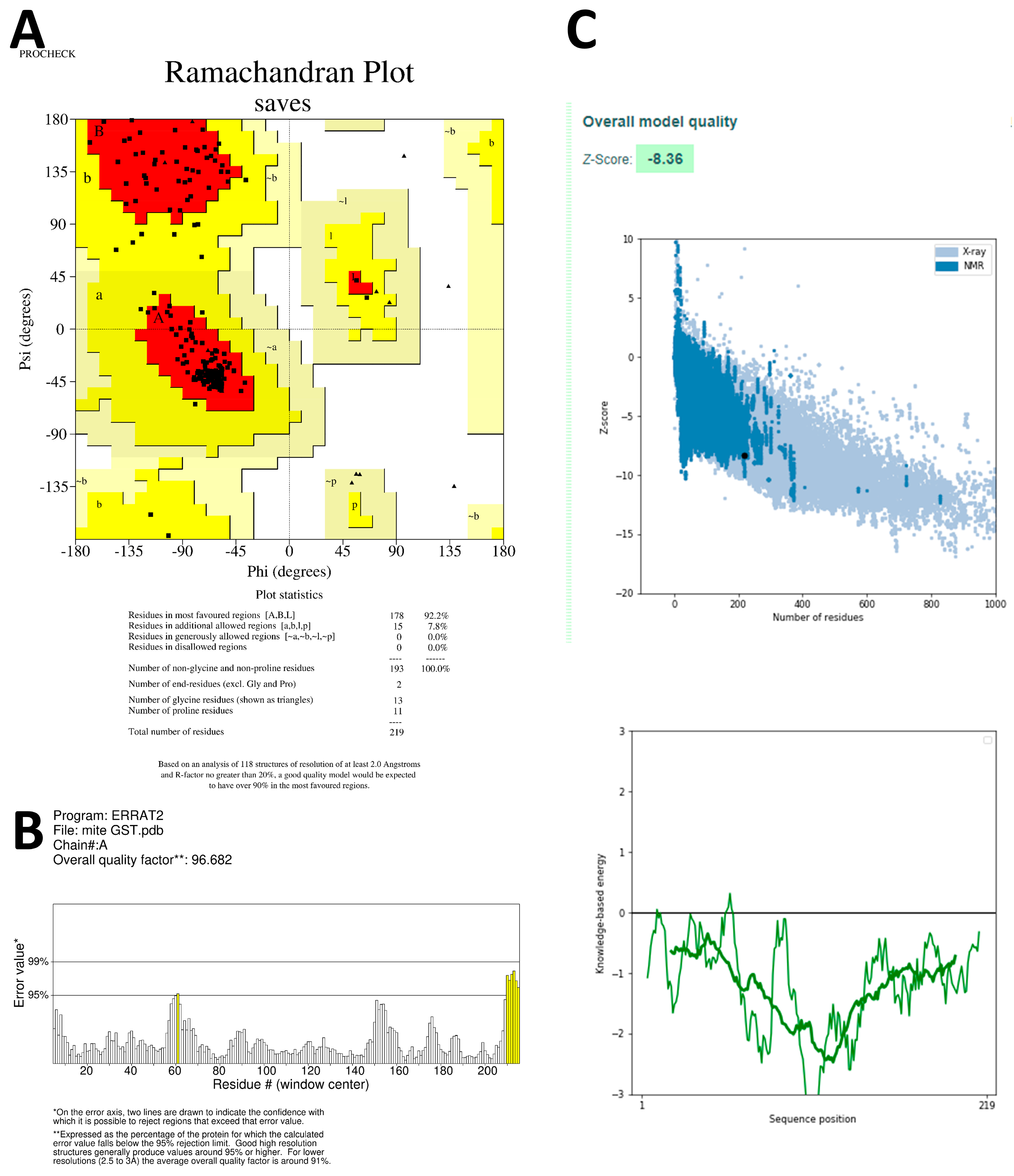
38] and SWISS-MODEL Template Library (SMTL) repositories. The top-ranked alignments of the templates are compared using the global model quality estimate (GMQE) and quaternary structure quality estimate (QSQE) to generate sets of descriptive three-dimensional structures, sequence dissimilarity, and quaternary protein structure information. The QMEAN values were utilized to predict 3D protein structures, taking into account modeling errors and quality estimation. These predicted protein structures are then assessed for stereochemical quality using the SAVES v6.0 server (
https://saves.mbi.ucla.edu/, accessed on 27 June 2023). Additionally, the Ramachandran plot, which plots Ψ versus Φ conformational angles of the 3D macromolecule, is used to measure the torsion angles of Cα (ideal) -N-Cβ (obs). To predict the active site amino acids, the CastP calculation server (
http://sts.bioe.uic.edu/castp/calculation.html, accessed on 28 June 2023) is employed, which calculates the delineating surface area and surface volume of the 3D protein structure [
39].
2.9. Preparation of Modeled Proteins and Ligands for Docking
To facilitate docking, homology-modelled SsGST and RmGST proteins were prepared. Gasteiger charges and polar hydrogens were added, and nonpolar and polar hydrogen atoms were merged by introducing partial charges using AutoDock Vina 4.2 [
40]. To obtain structural information about bioactive compounds isolated from the leaves, fruit, and bark of
C. sinensis, the relevant literature by [
41] was reviewed. The 3D structures of these compounds were retrieved from the PubChem website (
https://pubchem.ncbi.nlm.nih.gov/, accessed on 30 June 2023). Prior to docking, rotatable bonds were determined and nonpolar hydrogens were combined with polar hydrogen atoms for both phytochemical structures and conventional drugs for comparison purposes.
The docking process was conducted using AutoDock Vina software (version 1.1.2). Grid dimensions of 40 × 40 × 40 Å (grid size) with a grid-point spacing of 1.000 Å were employed for both proteins. The X, Y, and Z coordinates (grid centers) varied based on the specific receptor. The exhaustiveness parameter was set to a default value of eight (8) for all docking runs. The binding energy/affinity between the ligand and protein was calculated using the search algorithm within the AutoDock Vina software package. Following the completion of the docking runs, multiple binding modes representing different conformations of the ligands were obtained, along with their respective binding energy/affinity values. The most stable conformation, characterized by the lowest binding energy/affinity, was selected as the pose and utilized for postdocking analysis using BIOVIA Discovery Studio 2021.
2.10. Molecular Dynamics Simulation Analysis
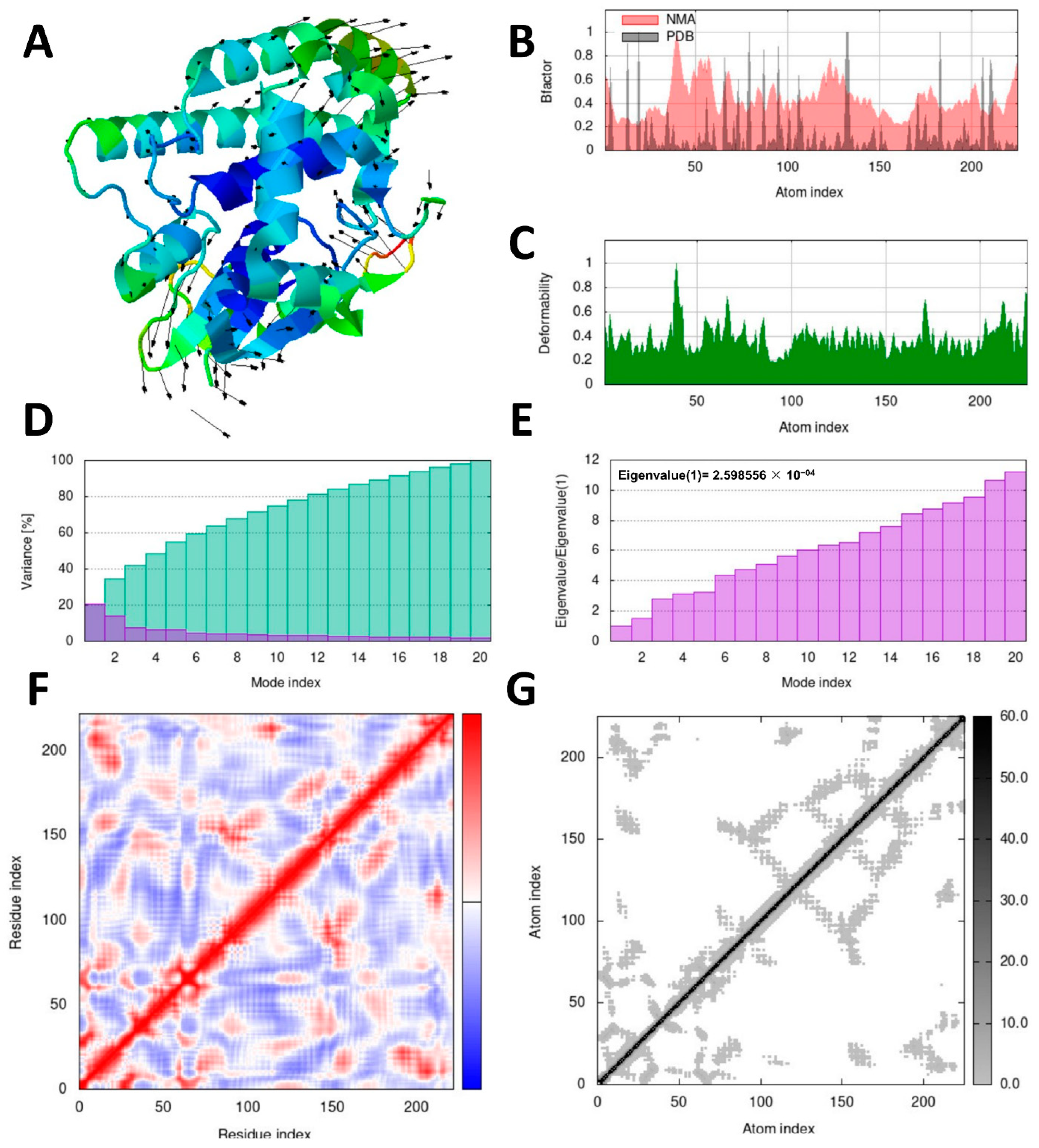
Investigating the dynamic motion of atoms is crucial to understanding the stability and functionality of protein complexes. Molecular dynamics simulation plays a significant role in this regard [
42]. To perform dynamic simulations of the docking complex, we utilized the iMODS server, which is specifically designed for this purpose [
43]. The iMOD server (iMODS) (
http://imods.chaco nlab.org, accessed on 3 July 2023) was employed for conducting molecular dynamics (MD) simulations of the protein–ligand complexes. These simulations allowed us to assess the stability and molecular motion of docked complexes.
Utilizing the iMOD server (iMODS), we performed molecular dynamics simulations to analyze the structural dynamics of the docking complexes and determine molecular motion. Various parameters, such as deformability, B-factor, eigenvalues, variance, covariance map, and elastic network, were employed to evaluate the stability of the two protein–ligand complexes. The input files used for the simulations were docked PDB files that were uploaded to the iMODS server with default parameter settings.
2.11. Statistical Analysis
All statistical analyses were performed using R (version 4.3) running in RStudio (version 2023.06.1). The data were first arranged in Microsoft Excel (v. 2302) and imported into the R working environment for further statistical analysis. Descriptive statistics of the data were calculated and are presented as mean ± standard deviation. The significance difference between the different concentrations was calculated using a one-way analysis of variance (ANOVA) followed by the Tukey honestly significance difference (HSD) test. Furthermore, 50% and 90% lethal concentrations and lethal times (LC and LT) were calculated in RStudio using the ecotox package and all the data were graphically presented using the ggplot2 and ggpubr R packages.
4. Discussion
The use of plant extracts for pest and disease control has gained attention because of their natural degradation properties [
44]. Despite their advantages, biopesticides represent a small portion of the pesticide market [
45]. However, the biopesticide sector has experienced significant growth in recent years, with an annual growth rate predicted to surpass that of chemical pesticides [
45,
46].
This study focused on investigating the acaricidal efficacy of
C. sinensis extract as a potential source for developing herbal acaricides and identifying bioactive compounds against ticks and mites. Ticks and mites were treated with different concentrations of the ethanol extracts. Previous studies have explored the acaricidal properties of various herbs against mites and ticks [
13,
30,
47,
48].
C. sinensis has been reported to possess antioxidant [
49], antibacterial [
50], anti-inflammatory, and antihistaminic properties [
51], as well as insecticidal properties [
52], whereas no acaricidal potential of the plant has been documented. The plant extract has resulted in greater mortality in mites and comparable mortality in ticks as compared to the positive control permethrin and deltamethrin. The decrease in the chemical acaricides toxicity can be due to the acaricidal resistance by these mites and ticks. In a study by Gu, et al. [
53], the
Ailanthus altissima bark extract has resulted in similar results with the extract having more significant acaricidal potential against
S. scabiei and
Psoroptes scabiei compared to the available chemical acaricide fenvalerate. The findings of this study are consistent with previous research of Seddiek, et al. [
54]. Moreover,
Dodonaea angustifolia,
Eucalyptus globulus,
Millettia ferruginea, and
Euphorbia abyssinica plant extracts have been found to have acaricidal potential against these mites [
52].
Molecular docking studies have been widely utilized to predict ligand–target interactions and gain insights into the biological activity of natural products. These studies also provide clues regarding the mechanisms of action and binding modes within the active sites of enzymes [
55]. In this study, ten representative compounds from
C. sinensis were selected for docking analyses against two target proteins:
S. scabiei glutathione transferase (UniProt accession no: Q8I9R9) and
R. microplus glutathione transferases (UniProt accession no: O97117).
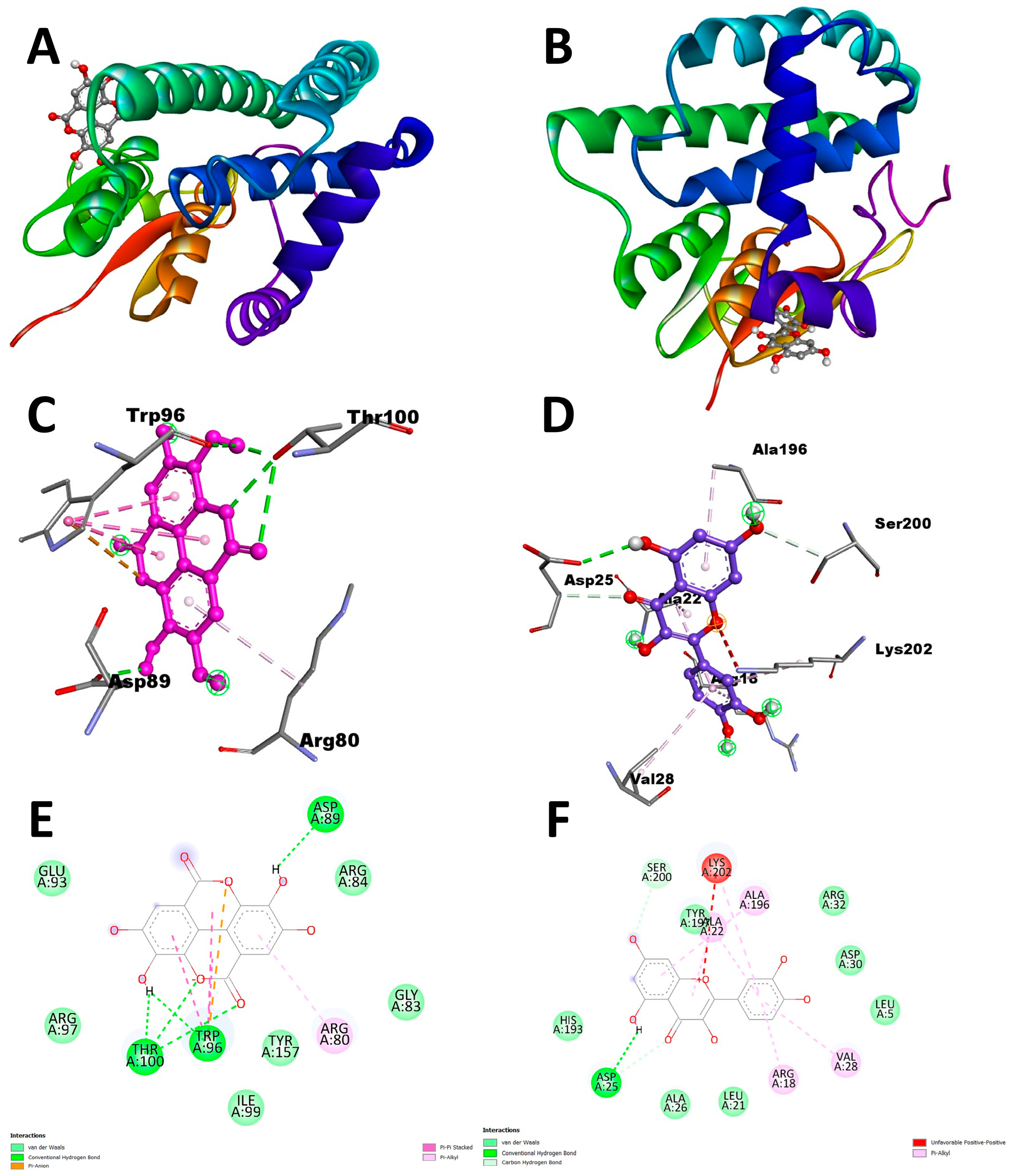
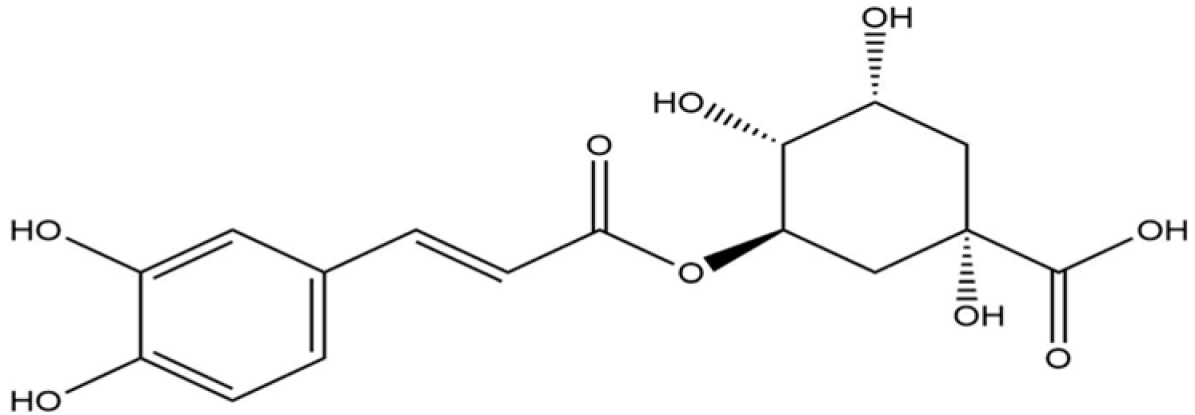
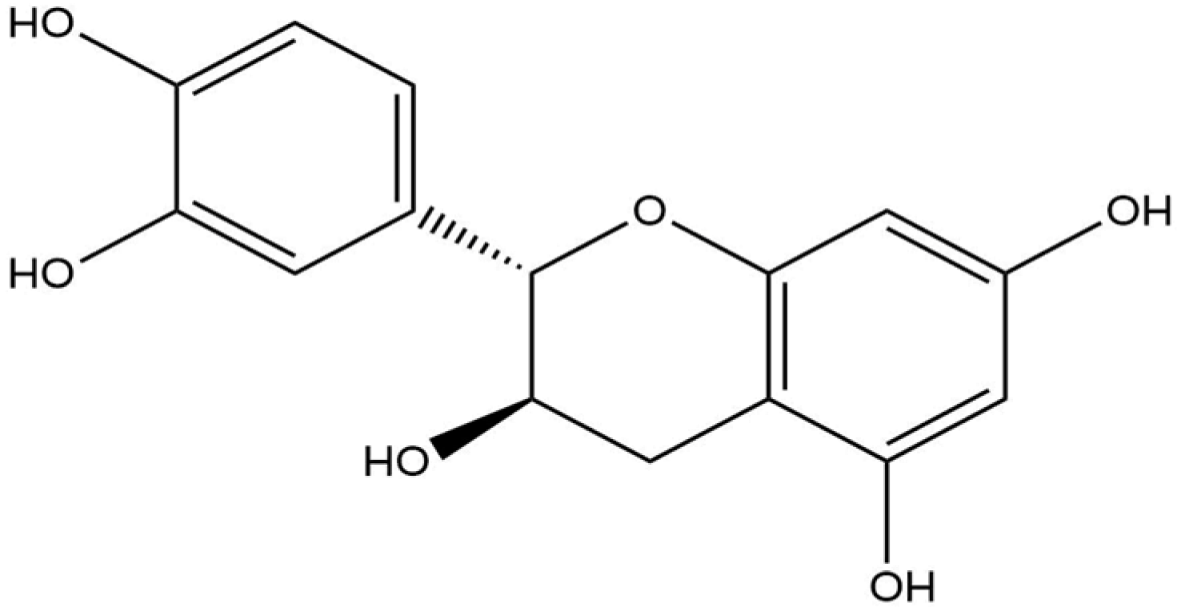
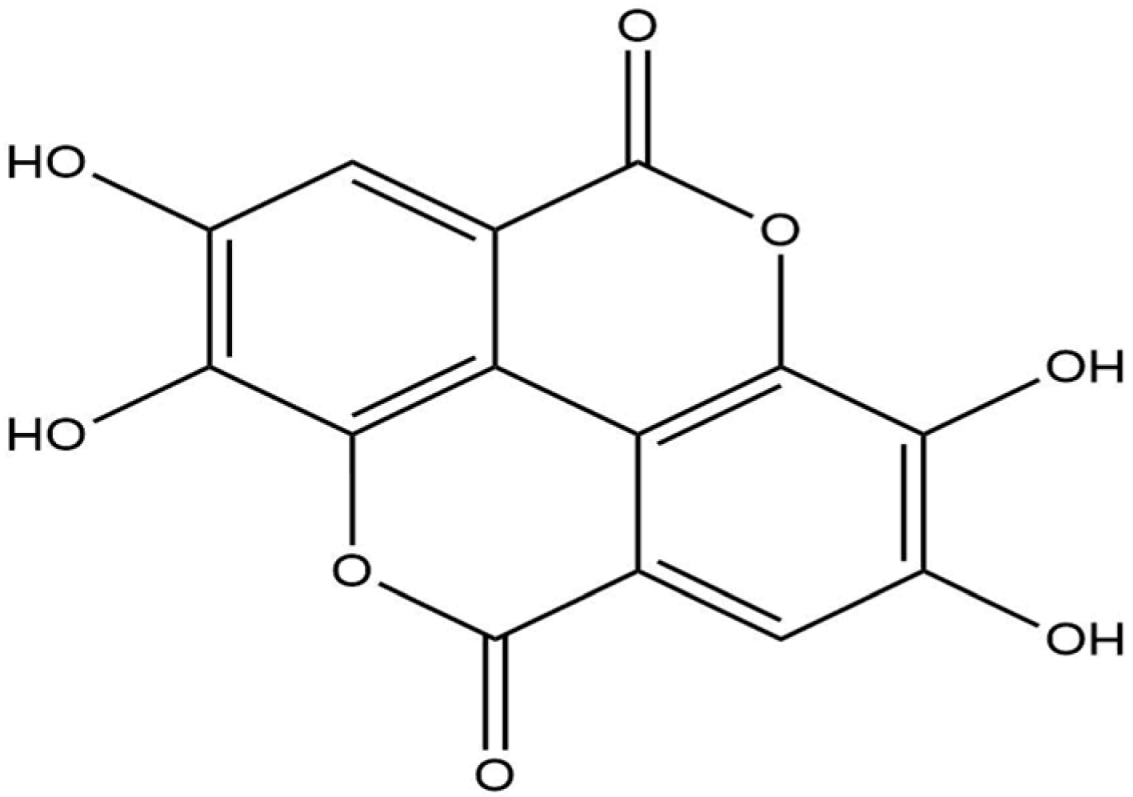
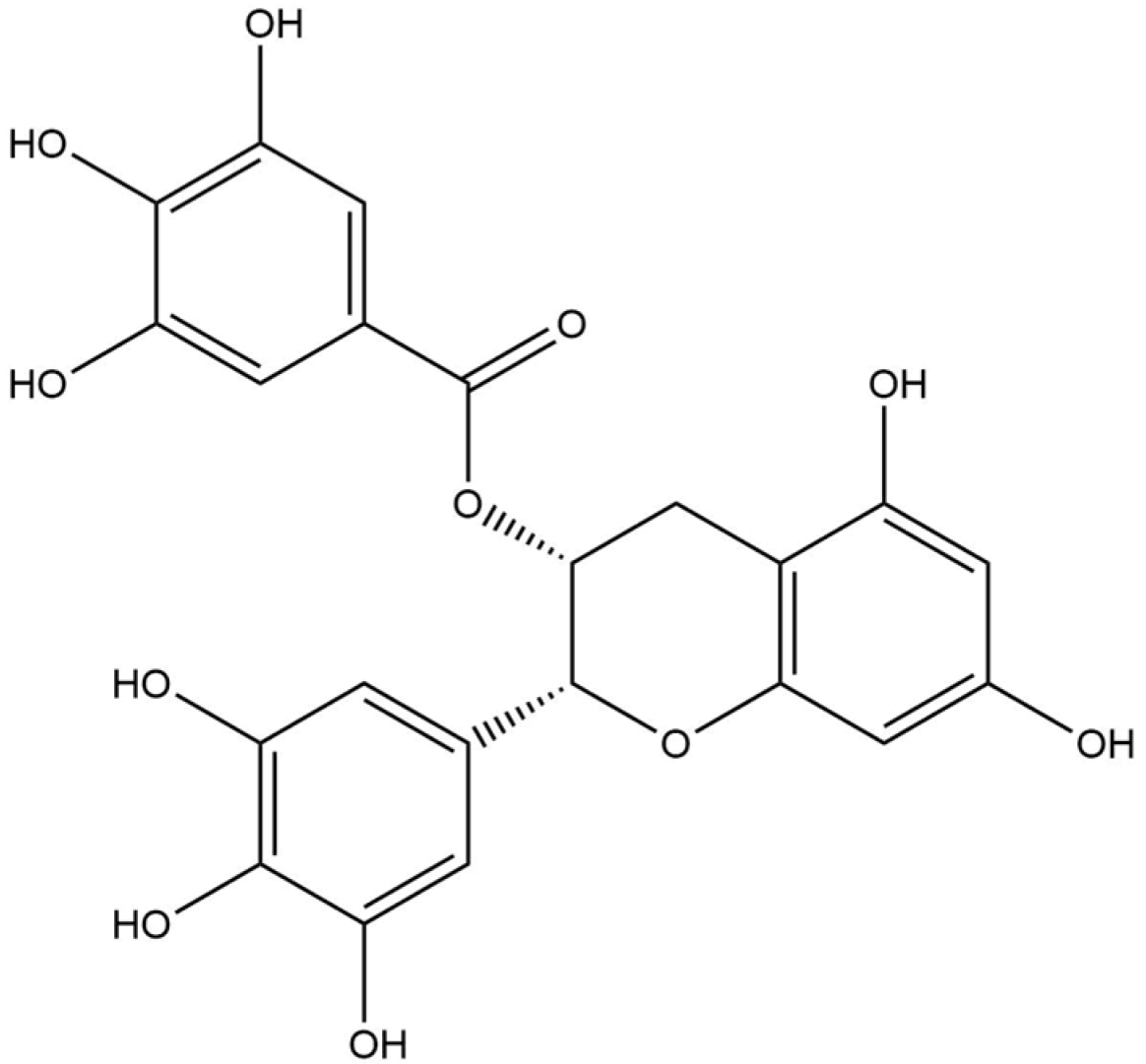
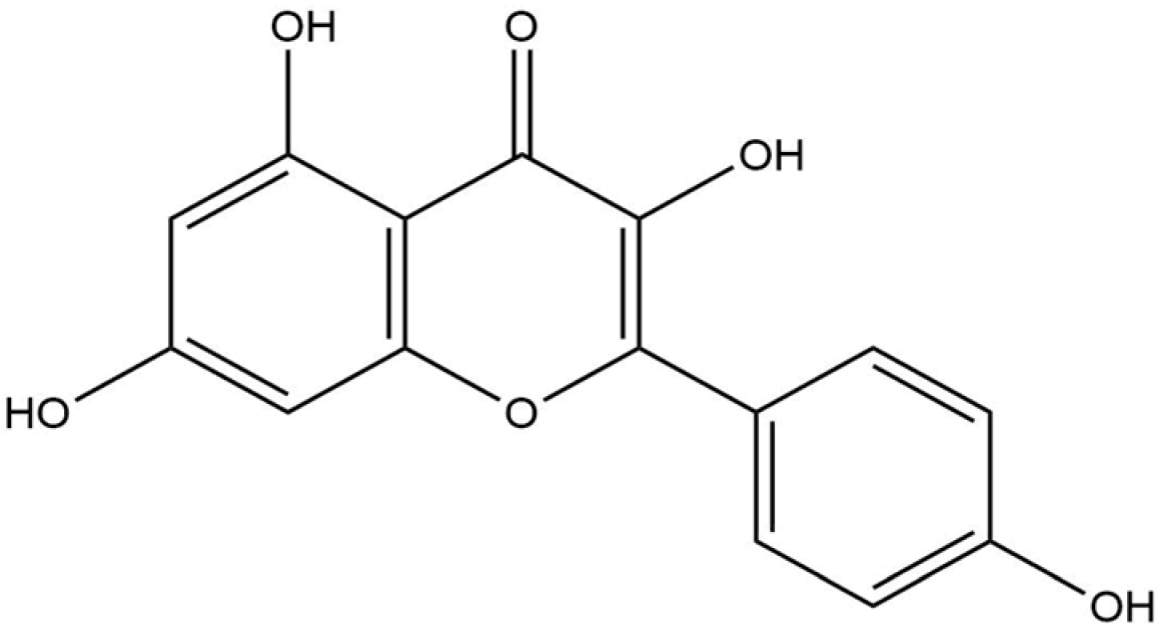
Docking analysis with S scabiei glutathione transferase revealed that, among the ten compounds, ellagic acid exhibited strong interactions with several amino acid residues through hydrogen bonds (Thr-100, Trp-96, Asp-89) and hydrophobic interactions (Trp-96 and Arg-80), with a docking score of −7.3 kcal/mol. Epicatechingallate, epigallocatechingallate, epicatechin, quercetin, caffeoylquinicacid, kaempferol, catechin, gallicacid, and theanine also displayed docking scores, suggesting their potential involvement in the antimite activity of C. sinensis through interactions with the target protein.
In the antitick docking study, the ten compounds were docked with
R. microplus glutathione transferases, and they exhibited docking scores ranging from −5.2 to −8.82 kcal/mol. Quercetin displayed the highest score against RmGST protein, followed by kaempferol, catechin, epigallocatechin gallate, epicatechin, caffeoylquinic acid, epicatechin gallate, ellagic acid, gallic acid, and theanine. Molecular dynamics simulations confirmed the low deformability of the docked proteins, supporting the validity of the in silico-predicted acaricide. Ellagic acid and quercetin have been reported to have antibacterial, antiviral, antimalarial, antiparasitic, antioxidant, and anti-inflammatory properties in previous studies [
56,
57,
58,
59]. The GST from
R. microplus has also been reported to be inhibited by several other compounds such as anonaine from
Annona crassiflora [
60], norapoatropine, and 7-Hydroxyhyoscyamine from
Datura innoxia [
48].
Overall, this study provides computational evidence for the potential inhibition of SsGST and RmGST proteins. Further research should focus on evaluating the clinical efficacy of these compounds, which could contribute to the development of novel resources for managing the Acari species.