Bioinformatics Analysis and Experimental Verification of Exercise for Aging Mice in Different Brain Regions Based on Transcriptome Sequencing
Abstract
:1. Introduction
2. Results
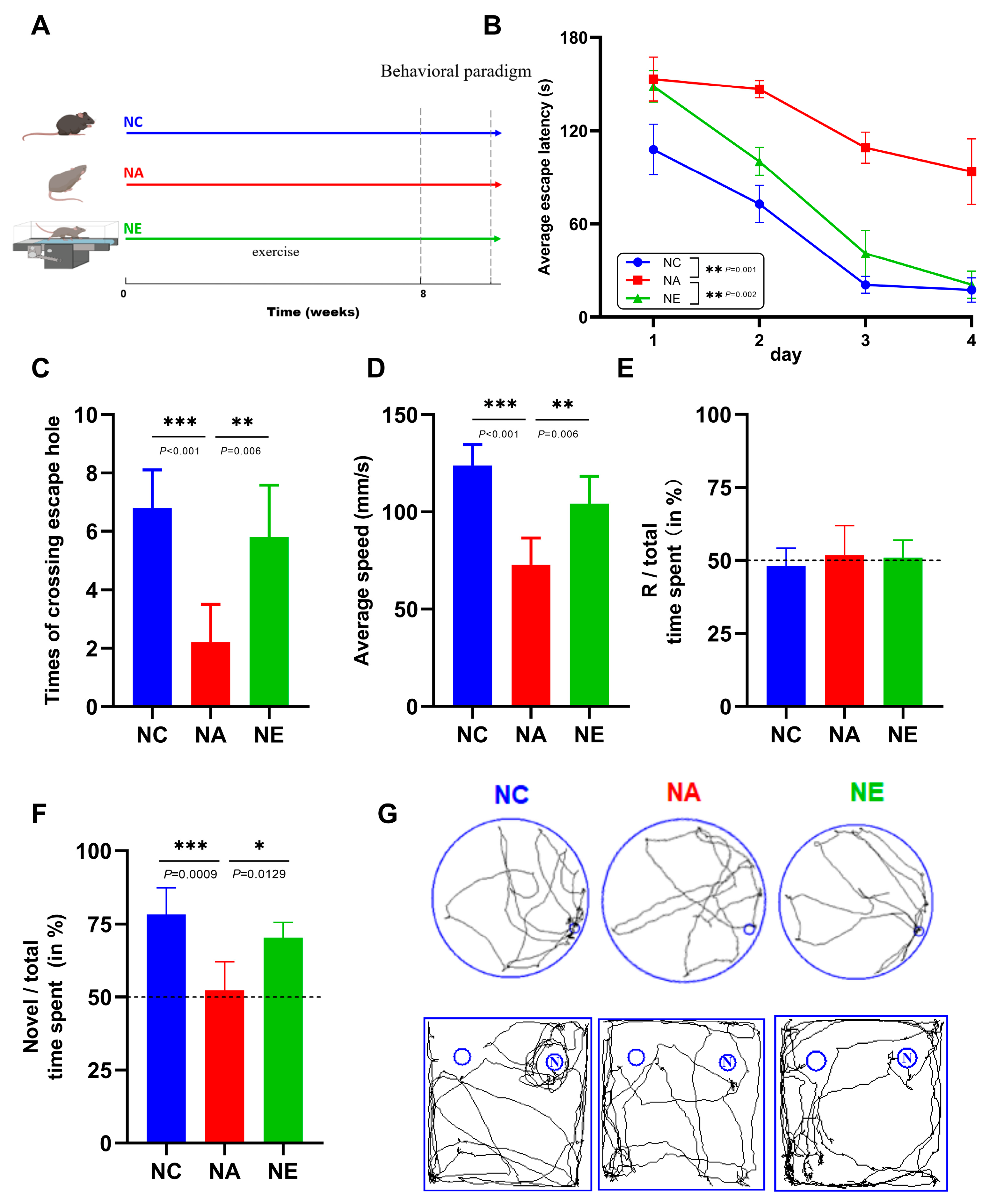
2.1. Aerobic Exercise Ameliorated the Deficits of Cognitive Function in Barnes Maze and Novel Object Recognition (NOR) Test
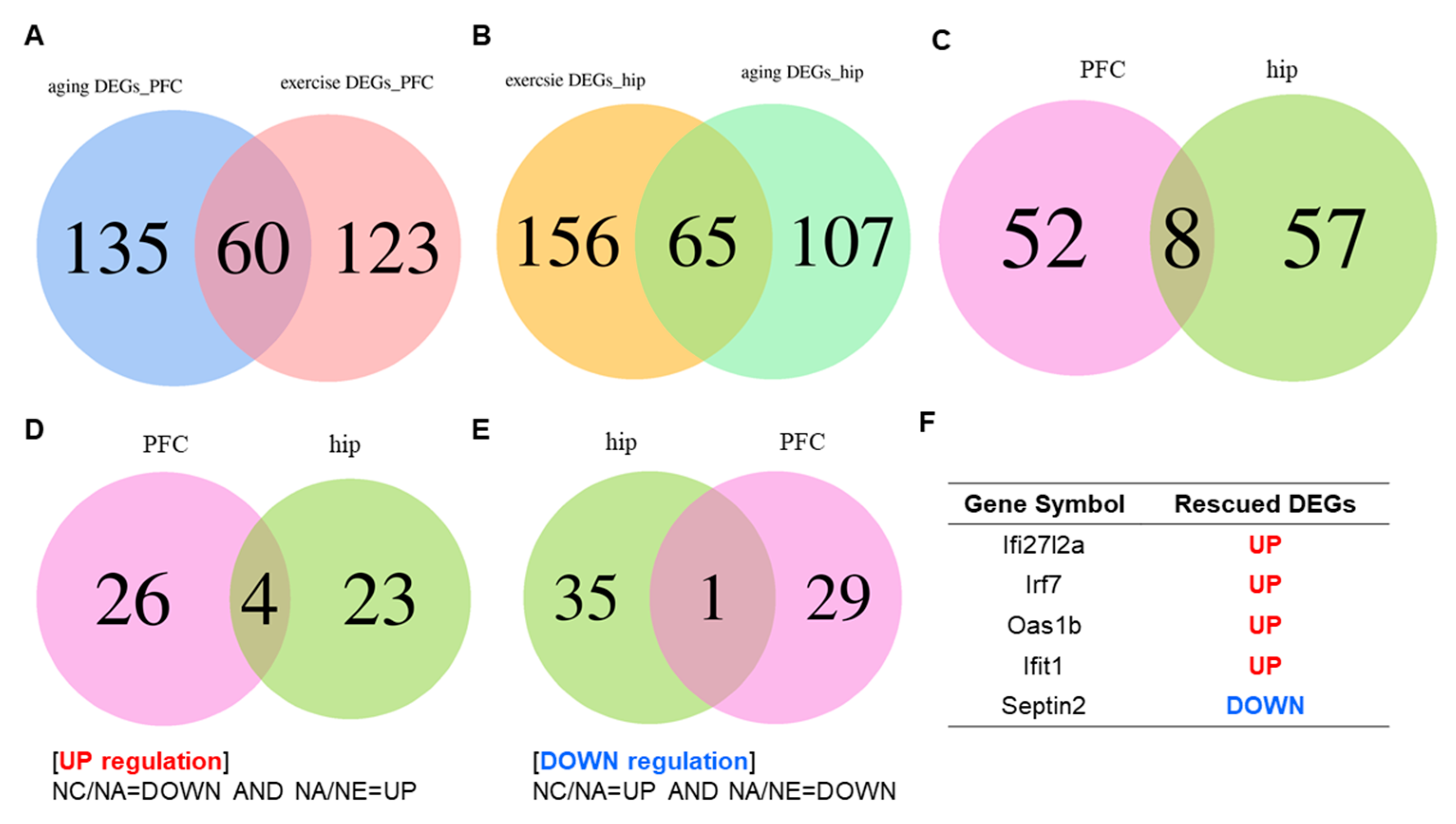
2.2. Conversion of Aging Relevant Gene Expression across Brain Area by Exercise
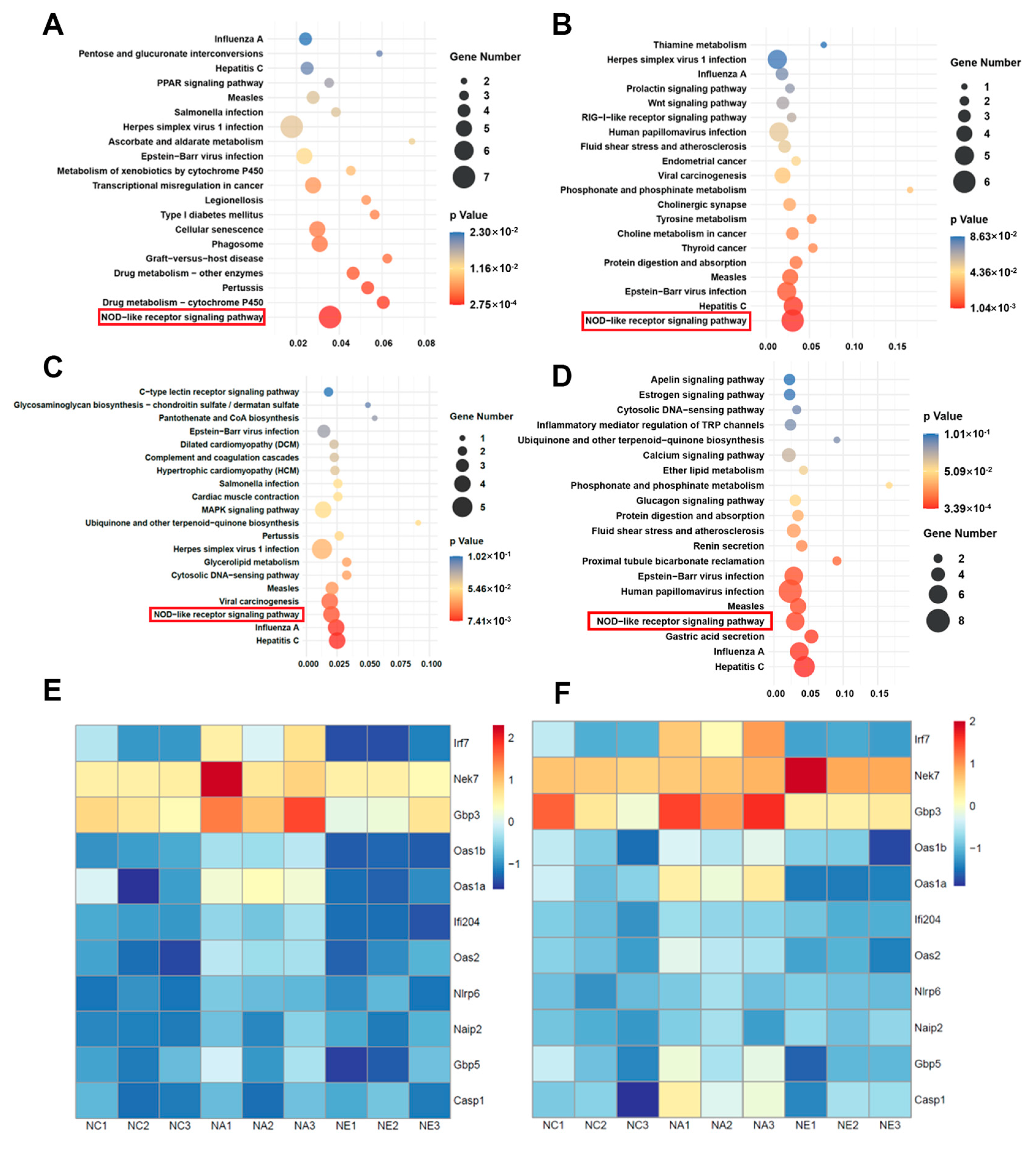
2.3. GO Terms and KEGG Pathway Enrichment Analyses
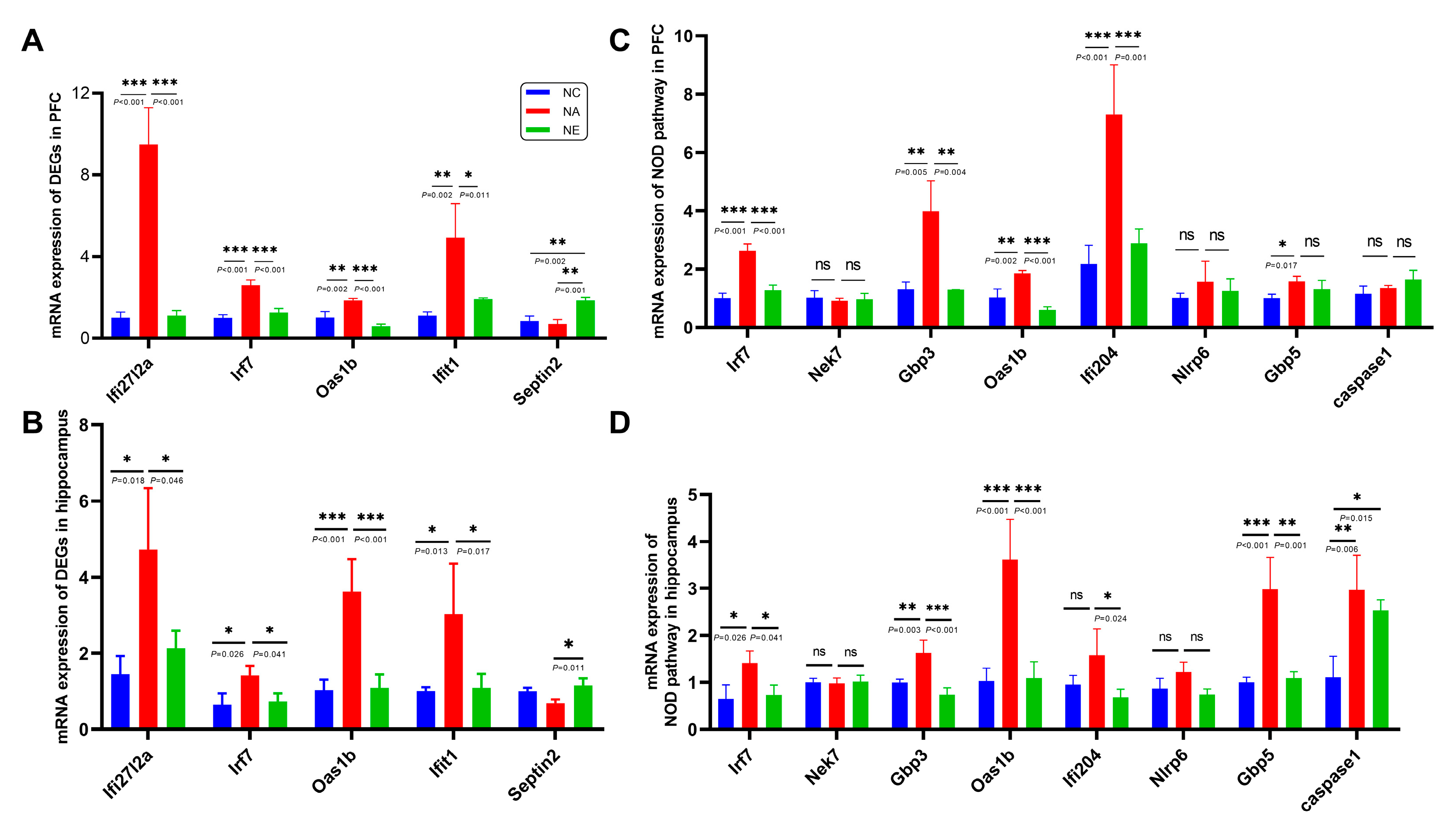
2.4. Identification of Genes Expression by RT-qPCR
3. Discussion
4. Materials & Methods
4.1. Experimental Design and Exercise Protocol
4.2. Behavioral Test
4.2.1. Barnes Maze
4.2.2. Novel Object Recognition (NOR)
4.3. Data Acquisition and Analysis
4.3.1. DEGs
4.3.2. Venn Diagram
4.3.3. Heatmaps
4.3.4. GO and KEGG Analysis
4.4. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR) Assay
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations. World Population Ageing 2019; United Nations: New York, NY, USA, 2020. Available online: https://www.un.org/development/desa/pd/news/world-population-ageing-2019 (accessed on 20 September 2022).
- Partridge, L.; Deelen, J.; Slagboom, P.E. Facing up to the Global Challenges of Ageing. Nature 2018, 561, 45–56. [Google Scholar] [CrossRef] [PubMed]
- RAZ, N.; RODRIGUE, K.M.; HAACKEb, E.M. Brain Aging and Its Modifiers: Insights from in Vivo Neuromorphometry and Susceptibility Weighted Imaging. Ann. N. Y. Acad. Sci. 2009, 1097, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Pamplona, R.; Borras, C.; Jové, M.; Pradas, I.; Ferrer, I.; Viña, J. Redox Lipidomics to Better Understand Brain Aging and Function. Free Radic. Biol. Med. 2019, 144, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Tyndall, A.V.; Clark, C.M.; Anderson, T.J.; Al, E. Protective Effects of Exercise on Cognition and Brain Health in Older Adults. Exerc. Sport Sci. Rev. 2018, 46, 215–223. [Google Scholar] [CrossRef] [PubMed]
- McGurran, H.; Glenn, J.M.; Madero, E.N.; Bott, N.T. Prevention and Treatment of Alzheimer’s Disease: Biological Mechanisms of Exercise. J. Alzheimer’s Dis. 2019, 69, 311–338. [Google Scholar] [CrossRef]
- Cooper, C.; Moon, H.Y.; Van Praag, H. On the Run for Hippocampal Plasticity. Cold Spring Harb. Perspect. Med. 2018, 8, a029736. [Google Scholar] [CrossRef]
- Mattson, M.P.; Arumugam, T.V. Hallmarks of Brain Aging: Adaptive and Pathological Modification by Metabolic States. cell Metab. 2018, 27, 1176–1199. [Google Scholar] [CrossRef]
- Valenzuela, P.L.; Castillo-García, A.; Morales, J.S.; de la Villa, P.; Hampel, H.; Emanuele, E.; Lista, S.; Lucia, A. Exercise Benefits on Alzheimer’s Disease: State-of-the-Science. Ageing Res. Rev. 2020, 62, 101108. [Google Scholar] [CrossRef]
- Mercer, D. Alzheimer ’ s Disease, Neural Plasticity, and Functional Recovery. J. Alzheimer’s Dis. 2021, 82, S37–S50. [Google Scholar] [CrossRef]
- Liu, B.; Kou, J.; Li, F.; Huo, D.; Xu, J.; Zhou, X.; Meng, D.; Ghulam, M.; Artyom, B.; Gao, X.; et al. Lemon Essential Oil Ameliorates Age-Associated Cognitive Dysfunction via Modulating Hippocampal Synaptic Density and Inhibiting Acetylcholinesterase. Aging 2020, 12, 8622–8639. [Google Scholar] [CrossRef]
- Singh, P.; Sivanandam, T.M.; Konar, A.; Thakur, M.K. Role of Nutraceuticals in Cognition during Aging and Related Disorders. Neurochem. Int. 2021, 143, 104928. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Huang, Y.; Zhang, Y.; Huang, H.; Hong, S.; Liu, T. Impacts of Exercise Interventions on Different Diseases and Organ Functions in Mice. J. Sport Health Sci. 2020, 9, 53–73. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Tian, H.; Guo, D.; Tian, Q.; Yao, T.; Kong, X. Impacts of Exercise Intervention on Various Diseases in Rats. J. Sport Health Sci. 2020, 9, 211–227. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, L.; Zhang, S.; Hu, X.; Yang, H.; Xi, L. Timing-Dependent Protection of Swimming Exercise against d-Galactose-Induced Aging-like Impairments in Spatial Learning/Memory in Rats. Brain Sci. 2019, 9, 236. [Google Scholar] [CrossRef]
- Cheng, T.; Huang, X.D.; Hu, X.F.; Wang, S.Q.; Chen, K.; Wei, J.A.; Yan, L.; So, K.F.; Yuan, T.F.; Zhang, L. Physical Exercise Rescues Cocaine-Evoked Synaptic Deficits in Motor Cortex. Mol. Psychiatry 2021, 26, 6187–6197. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, Q.; Gao, J.; Lv, L.; Liu, R.; Wu, Y.; Li, X.; Jin, Y.; Wang, L. Moderate-Intensity Intermittent Training Alters the DNA Methylation Pattern of PDE4D Gene in Hippocampus to Improve the Ability of Spatial Learning and Memory in Aging Rats Reduced by D-Galactose. Brain Sci. 2023, 13, 422. [Google Scholar] [CrossRef]
- Dong, J.; Liu, Y.; Zhan, Z.; Wang, X. MicroRNA-132 Is Associated with the Cognition Improvement Following Voluntary Exercise in SAMP8 Mice. Brain Res. Bull. 2018, 140, 80–87. [Google Scholar] [CrossRef]
- Enge, M.; Arda, H.E.; Mignardi, M.; Beausang, J.; Bottino, R.; Kim, S.K.; Quake, S.R. Single-Cell Analysis of Human Pancreas Reveals Transcriptional Signatures of Aging and Somatic Mutation Patterns. Cell 2017, 171, 321–330. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, Y.; Li, J.; Yu, Y.; Zhang, W.; Song, M.; Liu, Z.; Min, Z.; Hu, H.; Jing, Y.; et al. Single-Cell Transcriptomic Atlas of Primate Ovarian Aging. Cell 2020, 180, 585–600.e19. [Google Scholar] [CrossRef]
- Davie, K.; Janssens, J.; Koldere, D.; De Waegeneer, M.; Pech, U.; Kreft, Ł.; Aibar, S.; Makhzami, S.; Christiaens, V.; Bravo González-Blas, C.; et al. A Single-Cell Transcriptome Atlas of the Aging Drosophila Brain. Cell 2018, 174, 982–998.e20. [Google Scholar] [CrossRef]
- Ma, S.; Sun, S.; Geng, L. Caloric Restriction Reprograms the Single-Cell Transcriptional Landscape of Rattus Norvegicus Aging. Cell 2020, 180, 984–1001.e22. [Google Scholar] [CrossRef] [PubMed]
- Dulken, B.W.; Buckley, M.T.; Navarro Negredo, P.; Saligrama, N.; Cayrol, R.; Leeman, D.S.; George, B.M.; Boutet, S.C.; Hebestreit, K.; Pluvinage, J.V.; et al. Single-Cell Analysis Reveals T Cell Infiltration in Old Neurogenic Niches. Nature 2019, 571, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, A.; De Strooper, B.; Arancibia-Cárcamo, I.L. Cellular Senescence at the Crossroads of Inflammation and Alzheimer’s Disease. Trends Neurosci. 2021, 44, 714–727. [Google Scholar] [CrossRef] [PubMed]
- Mosher, K.I.; Wyss-Coray, T. Microglial Dysfunction in Brain Aging and Alzheimer’s Disease. Biochem. Pharmacol. 2014, 88, 594–604. [Google Scholar] [CrossRef]
- Preston, A.R.; Eichenbaum, H. Interplay of Hippocampus and Prefrontal Cortex in Memory. Curr. Biol. 2013, 23, R764–R773. [Google Scholar] [CrossRef]
- Mu, L.; Cai, J.; Gu, B.; Yu, L.; Li, C.; Liu, Q.-S.; Zhao, L. Treadmill Exercise Prevents Decline in Spatial Learning and Memory in 3 × Tg-AD Mice through Enhancement of Structural Synaptic Plasticity of the Hippocampus and Prefrontal Cortex. Cells 2022, 11, 244. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.S.; Harmon, E.; Gutierrez, M.; Stephenson, J.; Chauhan, A.; Banerjee, A.; Wise, Z.; Doan, A.; Wu, T.; Lee, J.; et al. Single-Cell Analysis Identifies Ifi27l2a as a Novel Gene Regulator of Microglial Inflammation in the Context of Aging and Stroke. Res. Sq. 2023, 15, 2557290. [Google Scholar] [CrossRef]
- Roy, E.R.; Wang, B.; Wan, Y.W.; Chiu, G.; Cole, A.; Yin, Z.; Propson, N.E.; Xu, Y.; Jankowsky, J.L.; Liu, Z.; et al. Type I Interferon Response Drives Neuroinflammation and Synapse Loss in Alzheimer Disease. J. Clin. Investig. 2020, 130, 1912–1930. [Google Scholar] [CrossRef]
- Tamura, T.; Yanai, H.; Savitsky, D.; Taniguchi, T. The IRF Family Transcription Factors in Immunity and Oncogenesis. Annu. Rev. Immunol. 2008, 26, 535–584. [Google Scholar] [CrossRef]
- Romagnoli, M.; Porcellini, E.; Carbone, I.; Veerhuis, R.; Licastro, F. Impaired Innate Immunity Mechanisms in the Brain of Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 1126. [Google Scholar] [CrossRef]
- Xiao, C.; Beitler, J.J.; Higgins, K.A.; Chico, C.E.; Withycombe, J.S.; Zhu, Y.; Zhao, H.; Lin, I.H.; Li, F.; Jeon, S.; et al. Pilot Study of Combined Aerobic and Resistance Exercise on Fatigue for Patients with Head and Neck Cancer: Inflammatory and Epigenetic Changes. Brain. Behav. Immun. 2020, 88, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Rasa, S.M.M.; Annunziata, F.; Krepelova, A.; Nunna, S.; Omrani, O.; Gebert, N.; Adam, L.; Käppel, S.; Höhn, S.; Donati, G.; et al. Inflammaging Is Driven by Upregulation of Innate Immune Receptors and Systemic Interferon Signaling and Is Ameliorated by Dietary Restriction. Cell Rep. 2022, 39, 111017. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Bhattacharya, B.; Puri, R.K.; Maheshwari, R.K. Venezuelan Equine Encephalitis Virus Infection Causes Modulation of Inflammatory and Immune Response Genes in Mouse Brain. BMC Genom. 2008, 9, 289. [Google Scholar] [CrossRef] [PubMed]
- Briskin, E.A.; Casanovas-massana, A.; Ryff, K.R.; Morales-estrada, S.; Castro-arellano, I.; Jr, E.A.W.; Sharp, T.M.; Rivera-garcia, B. Immune Correlates of Protection From West Nile Virus Neuroinvasion and Disease. J. Infect. Dis. 2019, 219, 1162–1171. [Google Scholar] [CrossRef]
- Dhib-Jalbut, S. The Choroid Plexus and the Paradox of Interferons in the Aging Brain. Cytokine 2015, 71, 413–414. [Google Scholar] [CrossRef]
- Gruchała-Niedoszytko, M.; Van Der Vlies, P.; Niedoszytko, P.; Sanjabi, B.; Niedoszytko, M.; Kaczkan, M.; Pieszko, M.; Gierat-Haponiuk, K.; Sliwińska, A.; Szalewska, D.; et al. Differences in Gene Expression Related to the Outcomes of Obesity Treatment, Peak Oxygen Uptake, and Fatty Acid Metabolism Measured in a Cardiopulmonary Exercise Test. Pol. Arch. Intern. Med. 2018, 128, 280–286. [Google Scholar] [CrossRef]
- Ferreira, R.O.; Garcez, P.P. Dissecting the Toxic Effects of Zika Virus Proteins on Neural Progenitor Cells. Neuron 2019, 101, 989–991. [Google Scholar] [CrossRef]
- Mostowy, S.; Cossart, P. Septins: The Fourth Component of the Cytoskeleton. Nat. Rev. Mol. Cell Biol. 2012, 13, 183–194. [Google Scholar] [CrossRef]
- Li, H.; Saucedo-cuevas, L.; Yuan, L.; Shresta, S.; Xu, Z.; Gleeson, J.G.; Li, H.; Saucedo-cuevas, L.; Yuan, L.; Ross, D.; et al. Zika Virus Protease Cleavage of Host Protein Septin- 2 Mediates Mitotic Defects in Neural Progenitors Report Zika Virus Protease Cleavage of Host Protein Septin-2 Mediates Mitotic Defects in Neural Progenitors. Neuron 2019, 101, 1089–1098.e4. [Google Scholar] [CrossRef]
- Liu, P.; Lu, Z.; Liu, L.; Li, R.; Liang, Z.; Shen, M.; Xu, H.; Ren, D.; Ji, M.; Yuan, S.; et al. NOD-like Receptor Signaling in Inflammation-Associated Cancers: From Functions to Targeted Therapies. Phytomedicine 2019, 64, 152925. [Google Scholar] [CrossRef]
- Platnich, J.M.; Muruve, D.A. NOD-like Receptors and Inflammasomes: A Review of Their Canonical and Non-Canonical Signaling Pathways. Arch. Biochem. Biophys. 2019, 670, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.M.; Cookson, M.R.; Van Den Bosch, L.; Zetterberg, H.; Holtzman, D.M.; Dewachter, I. Hallmarks of Neurodegenerative Diseases. Cell 2023, 186, 693–714. [Google Scholar] [CrossRef] [PubMed]
- Bulut, O.; Kilic, G.; Domínguez-Andrés, J. Immune Memory in Aging: A Wide Perspective Covering Microbiota, Brain, Metabolism, and Epigenetics. Clin. Rev. Allergy Immunol. 2022, 63, 499–529. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Han, R.; Cheng, T.; Zheng, Y.; Xiao, J.; So, K. Corticosterone-Mediated Microglia Activation a Ff Ects Dendritic Spine Plasticity and Motor Learning Functions in Minimal Hepatic Encephalopathy. Brain Behav. Immun. 2019, 82, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Molina, P.; Almolda, B.; Giménez-Llort, L.; González, B.; Castellano, B. Chronic IL-10 Overproduction Disrupts Microglia-Neuron Dialogue Similar to Aging, Resulting in Impaired Hippocampal Neurogenesis and Spatial Memory. Brain. Behav. Immun. 2022, 101, 231–245. [Google Scholar] [CrossRef]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System during Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef]
- Meng, N.; Zhou, Y.; Zhang, Q.; Yu, X.; Li, H.; Liu, Y.; Liu, M.; Li, Q. Using Inflammatory Biological Age To Evaluate the Preventing Aging Effect of a Polyphenol-Probiotic-Enhanced Dietary Pattern in Adults Aged 50 Years and Older. J. Agric. Food Chem. 2023, 71, 6314–6325. [Google Scholar] [CrossRef]
- De Miguel, Z.; Khoury, N.; Betley, M.J.; Lehallier, B.; Willoughby, D.; Olsson, N.; Yang, A.C.; Hahn, O.; Lu, N.; Vest, R.T.; et al. Exercise Plasma Boosts Memory and Dampens Brain Inflammation via Clusterin. Nature 2021, 600, 494–499. [Google Scholar] [CrossRef]
- Barrientos, R.M.; Frank, M.G.; Crysdale, N.Y.; Chapman, T.R.; Ahrendsen, J.T.; Day, H.E.W.; Campeau, S.; Watkins, L.R.; Patterson, S.L.; Maier, S.F. Little Exercise, Big Effects: Reversing Aging and Infection-Induced Memory Deficits, and Underlying Processes. J. Neurosci. 2011, 31, 11578–11586. [Google Scholar] [CrossRef]
- Duggan, M.R.; Parikh, V. Microglia and Modifiable Life Factors: Potential Contributions to Cognitive Resilience in Aging. Behav. Brain Res. 2021, 405, 113207. [Google Scholar] [CrossRef]
- Kohman, R.A.; DeYoung, E.K.; Bhattacharya, T.K.; Peterson, L.N.; Rhodes, J.S. Wheel Running Attenuates Microglia Proliferation and Increases Expression of a Proneurogenic Phenotype in the Hippocampus of Aged Mice. Brain. Behav. Immun. 2012, 26, 803–810. [Google Scholar] [CrossRef]
- Poh, L.; Sim, W.L.; Jo, D.G.; Dinh, Q.N.; Drummond, G.R.; Sobey, C.G.; Chen, C.L.H.; Lai, M.K.P.; Fann, D.Y.; Arumugam, T.V. The Role of Inflammasomes in Vascular Cognitive Impairment. Mol. Neurodegener. 2022, 17, 4. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zheng, Y.; Wei, J.A.; Ouyang, H.; Huang, X.; Zhang, F.; Wan Lai, C.S.; Ren, C.; So, K.F.; Zhang, L. Exercise Training Improves Motor Skill Learning via Selective Activation of MTOR. Sci. Adv. 2019, 5, eaaw1888. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-S.; Park, S.; Chun, Y.; Song, W.; Kim, H.-J.; Kim, J. Treadmill Exercise Attenuates Retinal Oxidative Stress in Naturally-Aged Mice: An Immunohistochemical Study. Int. J. Mol. Sci. 2015, 16, 21008–21020. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Tai, C.; Westenbroek, R.E.; Yu, F.H.; Cheah, C.S.; Potter, G.B.; Rubenstein, J.L.; Scheuer, T.; De La Iglesia, H.O.; Catterall, W.A. Autistic-like Behaviour in Scn1a +- Mice and Rescue by Enhanced GABA-Mediated Neurotransmission. Nature 2012, 489, 385–390. [Google Scholar] [CrossRef]
| Gene | Primer (5′ to 3′) |
|---|---|
| caspase1 | F: ACAAGGCACGGGACCTATG R: TCCCAGTCAGTCCTGGAAATG |
| Gbp3 | F: TTCACCAACGGCAAGACCAAGACTC R: GTAGCCCAGCTCAATCTTCTTCCTG |
| Gbp5 | F: CAGACCTATTTGAACGCCAAAGA R: TGCCTTGATTCTATCAGCCTCT |
| Ifi27l2a | F: GCTTGTTGGGAACCCTGTTTG R: GGATGGCATTTGTTGATGTGGAG |
| Ifit1 | F: TTCCGTAGGAAACATCGCGT R: CATGAATGGCCTGTTGTGCC |
| Ifi204 | F: CCAGTCACCAATACTCCACAG R: GAGCACCATCACTGTCAGG |
| Irf7 | F: GAGCGAAGAGAGCGAAGAGG R: GGCCCACAGTAGATCCAAGC |
| Nek7 | F: GCTGTCTGCTATATGAGATGGC R: CCGAATAGTGATCTGACGGGAG |
| Nlrp6 | F: CTCGCTTGCTAGTGACTACAC R: AGTGCAAACAGCGTCTCGTT |
| Oas1b | F: GGCCTCTAAGGGGGTCAAG R: CTGGCAGCACGTCAAACTTC |
| Septin2 | F: GCCCAGCAACAAGCAAAGCACAT R: CCCAACCACCCTAGTTCCTCCG |
| GAPDH | F: GAACGGGAAGCTCACTGG R: GCCTGCTTCACCACCTTCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Y.; Wei, C.; Huang, X.; Zhang, D.; Zhang, L.; Li, X. Bioinformatics Analysis and Experimental Verification of Exercise for Aging Mice in Different Brain Regions Based on Transcriptome Sequencing. Life 2023, 13, 1988. https://doi.org/10.3390/life13101988
Jin Y, Wei C, Huang X, Zhang D, Zhang L, Li X. Bioinformatics Analysis and Experimental Verification of Exercise for Aging Mice in Different Brain Regions Based on Transcriptome Sequencing. Life. 2023; 13(10):1988. https://doi.org/10.3390/life13101988
Chicago/Turabian StyleJin, Yu, Changling Wei, Xiaohan Huang, Deman Zhang, Li Zhang, and Xue Li. 2023. "Bioinformatics Analysis and Experimental Verification of Exercise for Aging Mice in Different Brain Regions Based on Transcriptome Sequencing" Life 13, no. 10: 1988. https://doi.org/10.3390/life13101988
APA StyleJin, Y., Wei, C., Huang, X., Zhang, D., Zhang, L., & Li, X. (2023). Bioinformatics Analysis and Experimental Verification of Exercise for Aging Mice in Different Brain Regions Based on Transcriptome Sequencing. Life, 13(10), 1988. https://doi.org/10.3390/life13101988






