Parkia speciosa Hassk. Empty Pod Extract Prevents Cardiomyocyte Hypertrophy by Inhibiting MAPK and Calcineurin-NFATC3 Signaling Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Collection and Sequential Extraction of Empty Pods
2.2. Cell Culture
2.3. Cell Viability Measurement
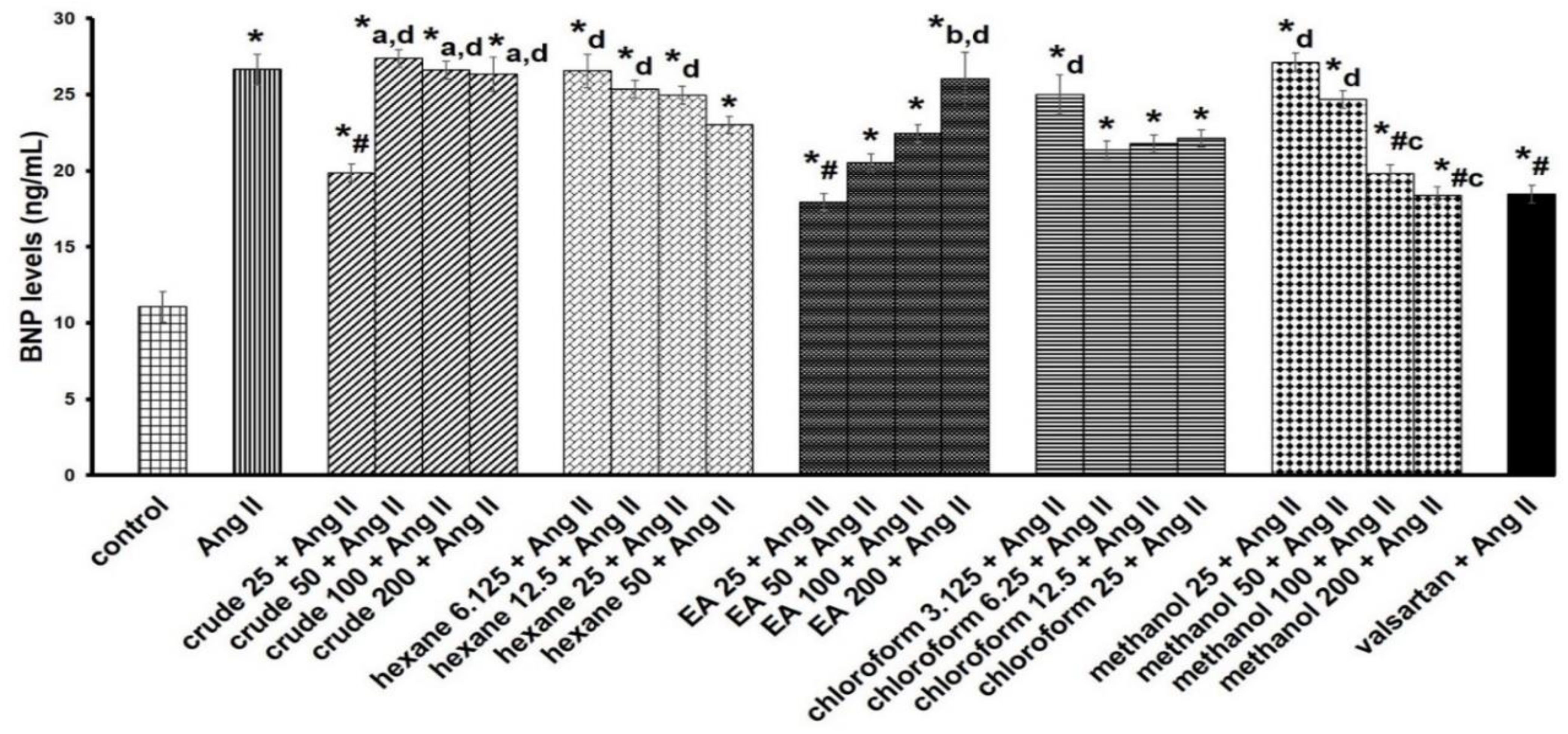
2.4. Antihypertrophic Screening of Extract Fractions
2.5. Chromatography Analysis
2.6. Experimental Groups
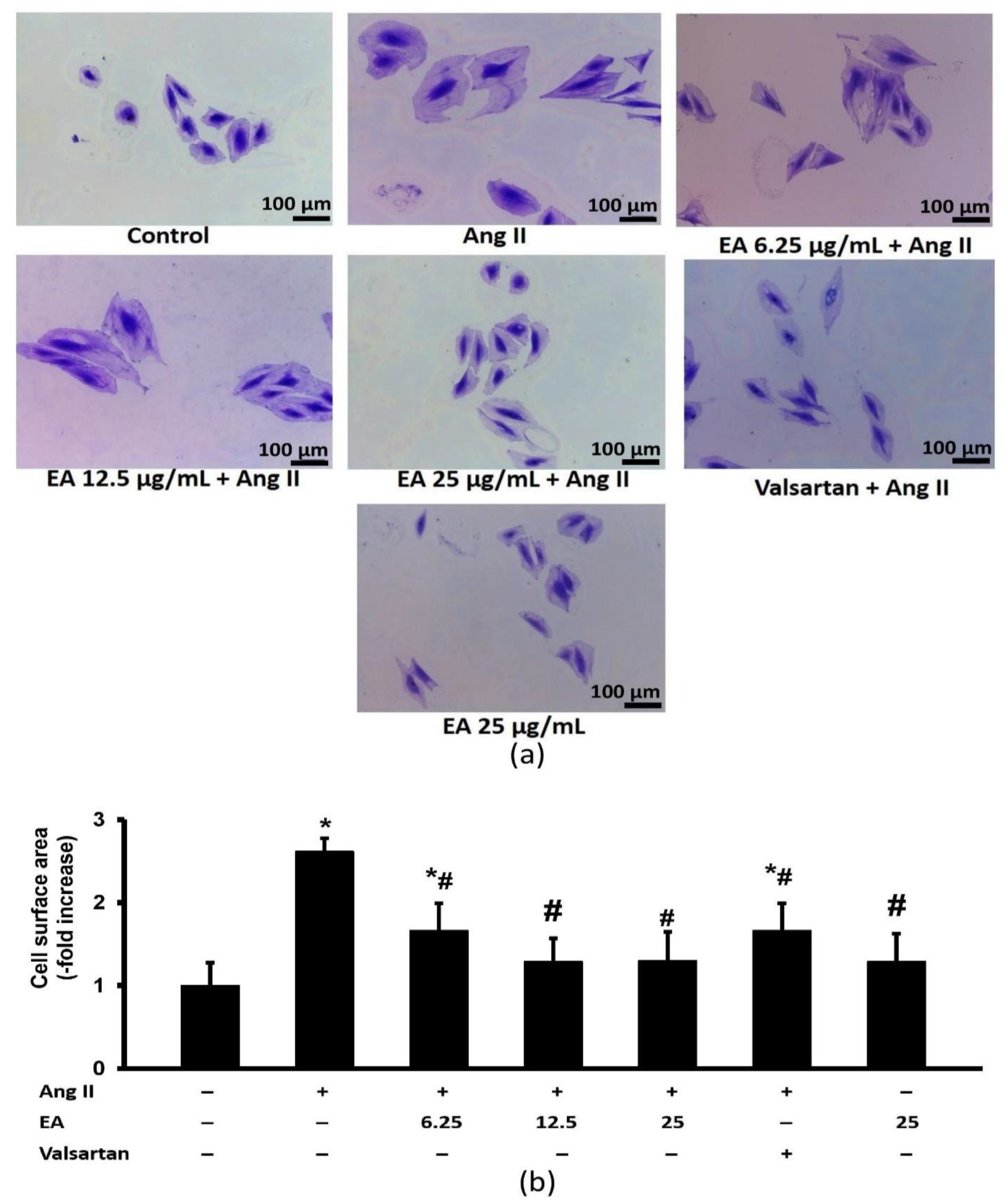
2.7. Cell Size Measurement
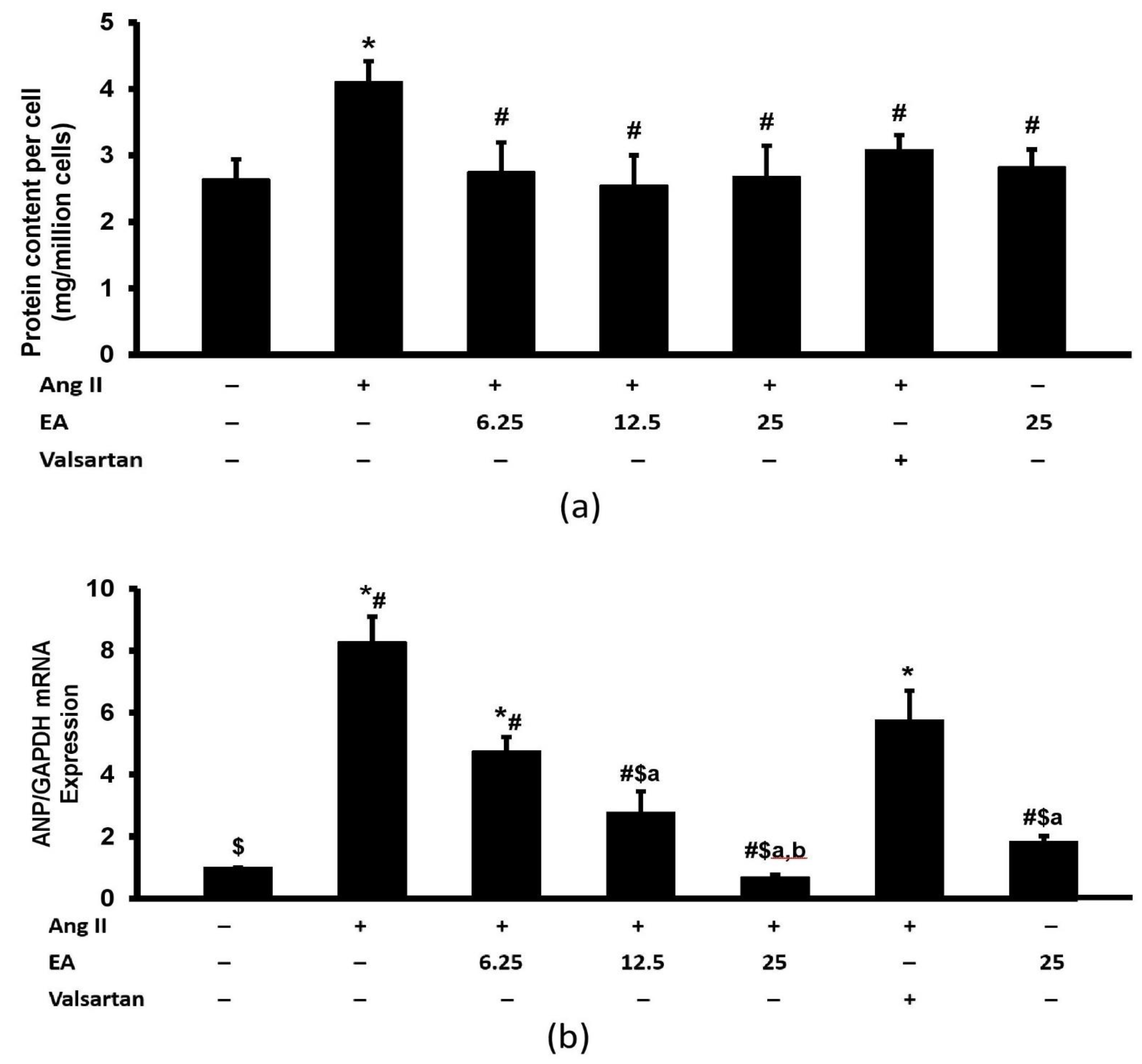
2.8. Protein Content Assay
2.9. ROS Assay
2.10. NADPH Oxidase 4 (Nox4) Immunofluorescence Assay
2.11. ANP and NFATC3 Gene Expression Assay
2.12. Protein Expression
2.13. Statistical Analysis
3. Results
3.1. Cell Cytotoxicity and Antihypertrophic Screening of Fractions
3.2. UHPLC-QTOF-MS/MS Identification
3.3. Ethyl Acetate Fraction Inhibited Ang-II Induced Cardiomyocyte Hypertrophy
3.4. Ethyl Acetate Fraction Inhibited Ang II-Induced Increased Total Protein Content and ANP Expression
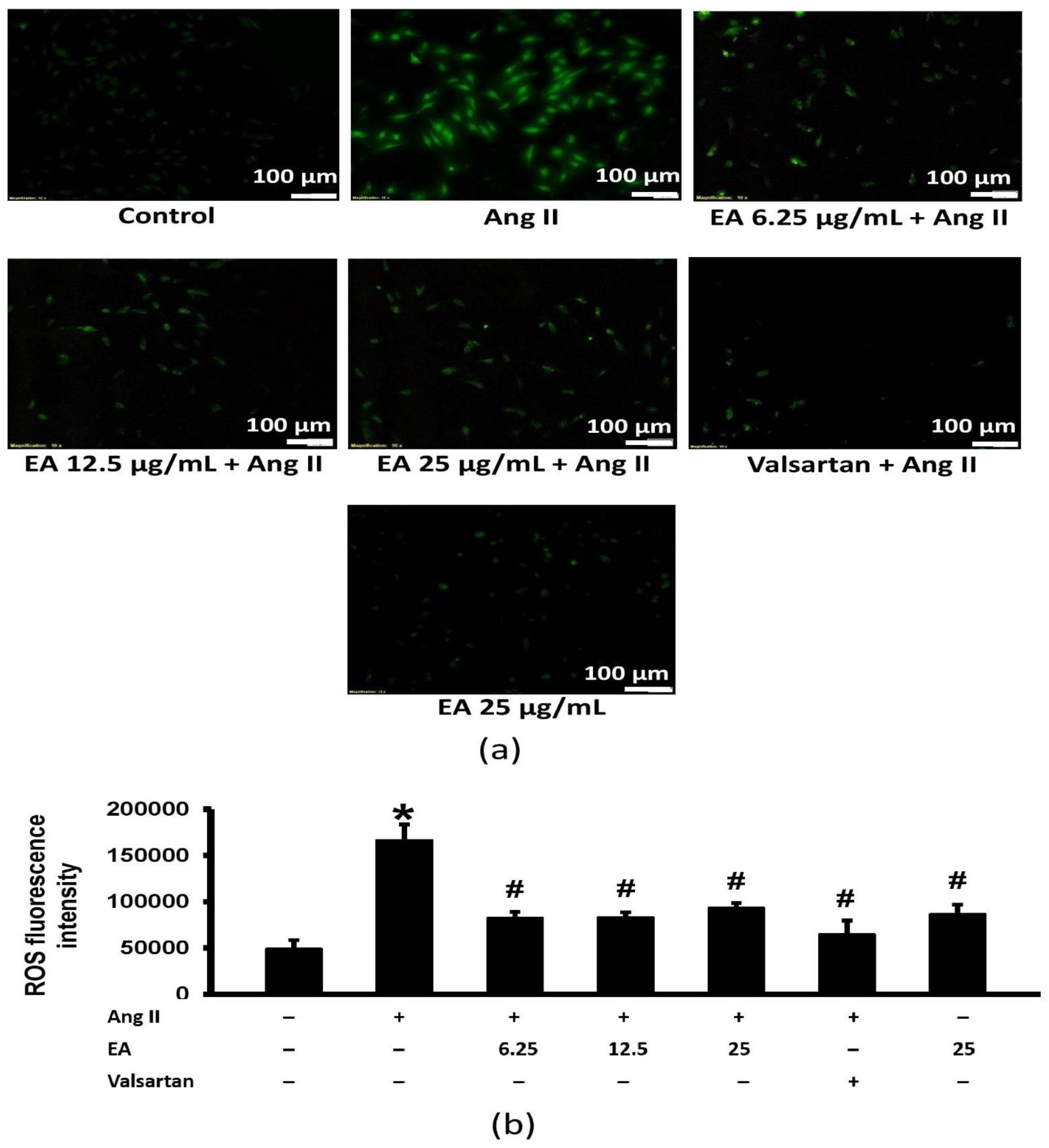
3.5. Ethyl Acetate Fraction Suppressed Ang II-Induced Intracellular ROS Generation
3.6. Ethyl Acetate Fraction Suppressed Ang II-Induced Nox4 Expression
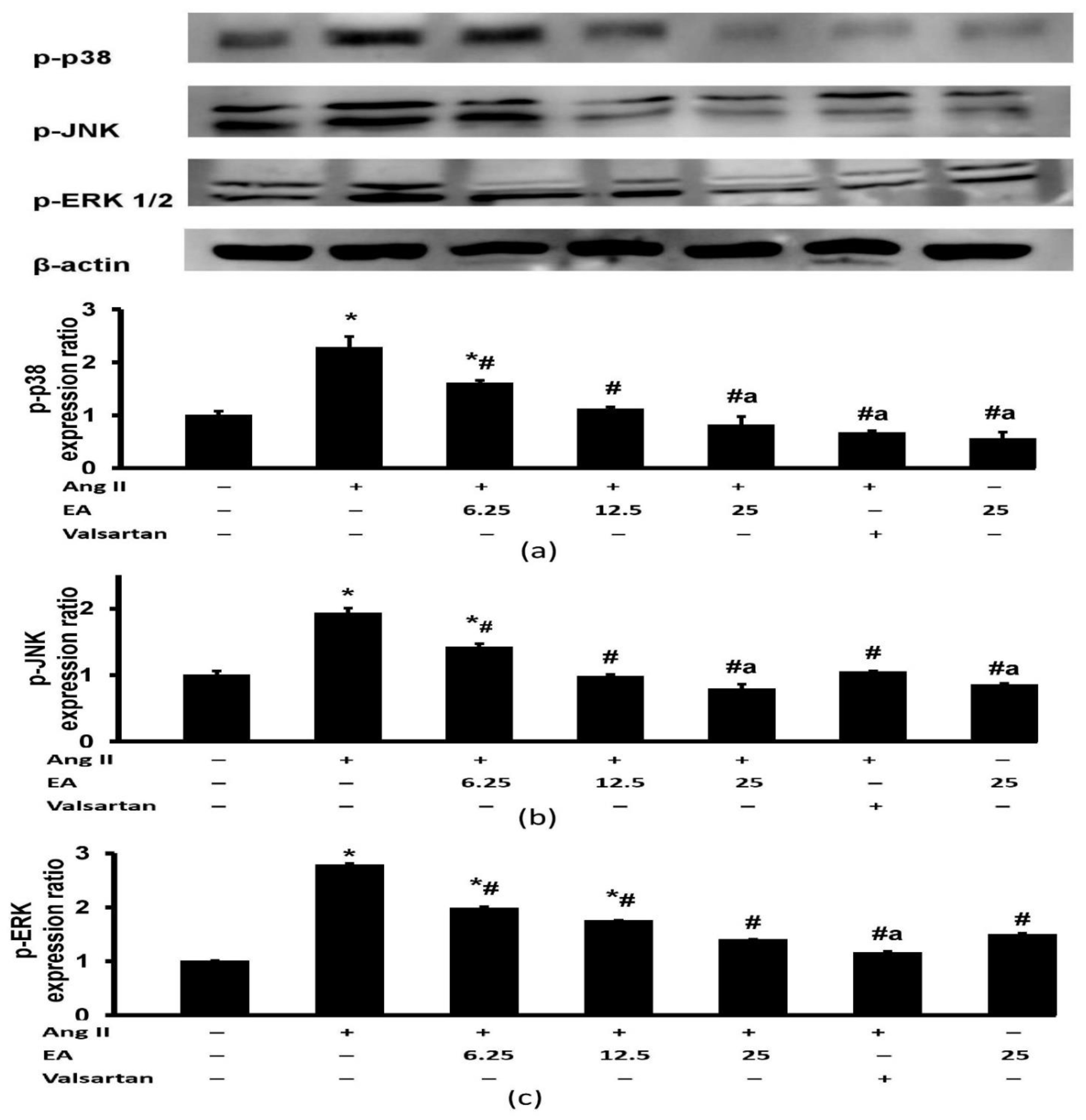
3.7. Effect of Ethyl Acetate Fraction on Ang II-Induced MAPK Signaling Pathway Activation
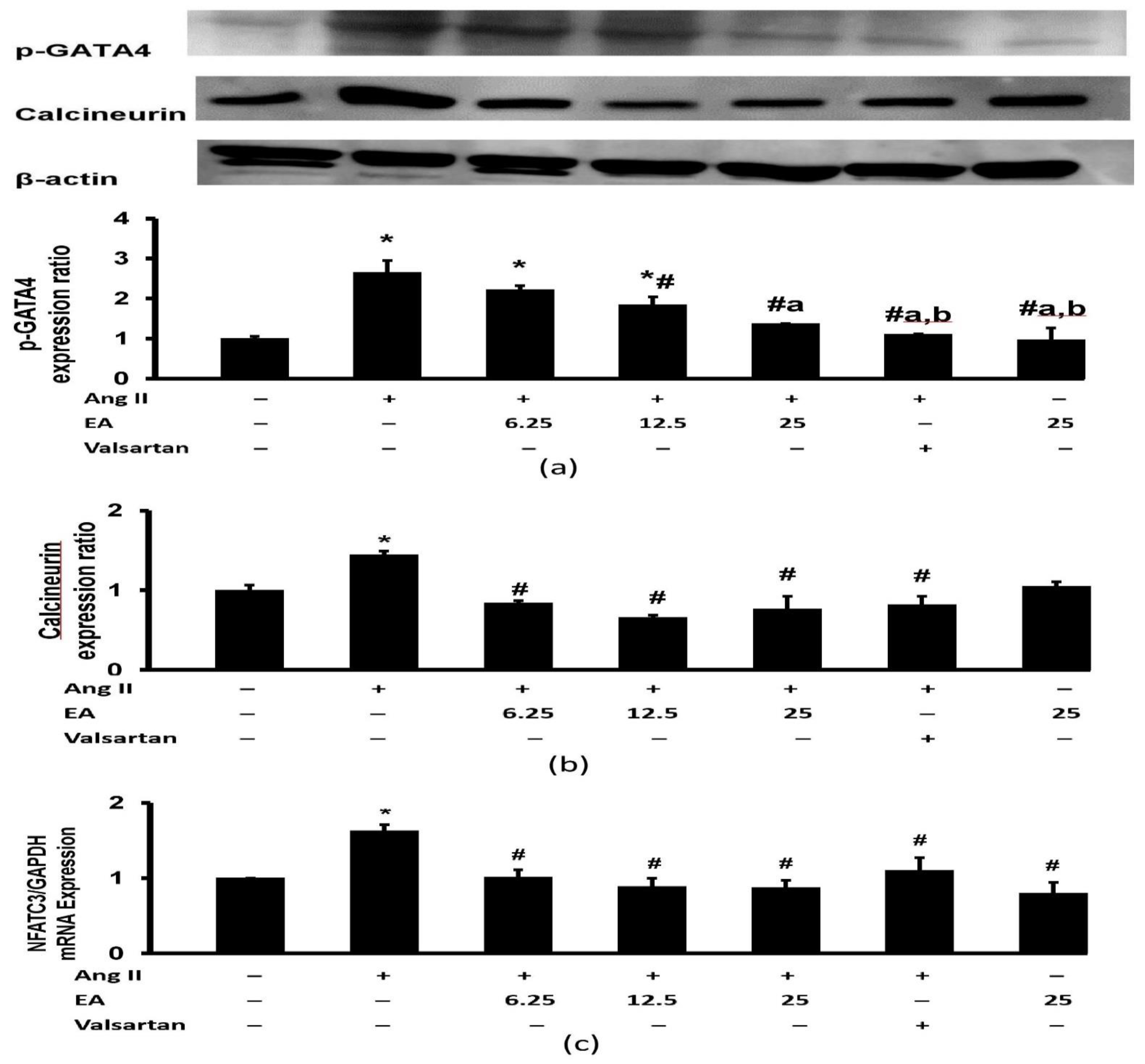
3.8. Effect of Ethyl Acetate Fraction on Ang II-Induced Calcineurin Related Pathway Activation
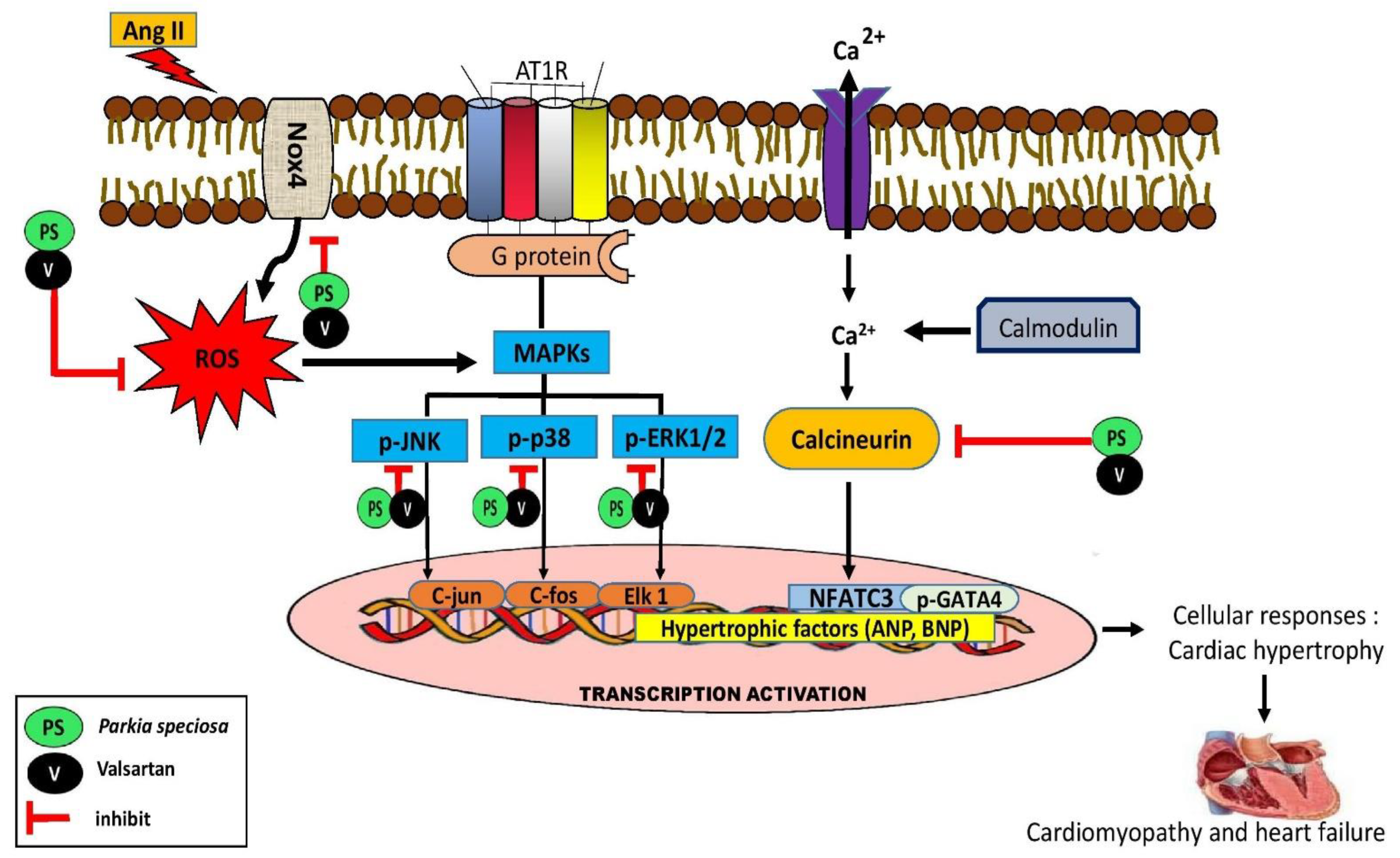
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mascolo, A.; di Mauro, G.; Cappetta, D.; De Angelis, A.; Torella, D.; Urbanek, K.; Berrino, L.; Nicoletti, G.F.; Capuano, A.; Rossi, F. Current and future therapeutic perspective in chronic heart failure. Pharmacol. Res. 2022, 175, 106035. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.Y.; Leong, X.F.; Masbah, N.; Adam, S.K.; Kamisah, Y.; Jaarin, K. Heated vegetable oils and cardiovascular disease risk factors. Vasc. Pharmacol. 2014, 61, 1–9, reprinted in Vascul. Pharmacol. 2014, 62, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Siti, H.N.; Jalil, J.; Asmadi, A.Y.; Kamisah, Y. Roles of rutin in cardiac remodeling. J. Funct. Foods 2020, 64, 103606. [Google Scholar] [CrossRef]
- Szekeres, F.L.M.; Walum, E.; Wikström, P.; Arner, A. A small molecule inhibitor of Nox2 and Nox4 improves contractile function after ischemia-reperfusion in the mouse heart. Sci. Rep. 2021, 11, 11970. [Google Scholar] [CrossRef]
- Lee, C.Y.; Park, H.K.; Lee, B.S.; Jeong, S.; Hyun, S.A.; Choi, J.W.; Kim, S.W.; Lee, S.; Lim, S.; Hwang, K.C. Novel therapeutic effects of pterosin B on Ang II-induced cardiomyocyte hypertrophy. Molecules 2020, 25, 5279. [Google Scholar] [CrossRef]
- Ba, L.; Gao, J.; Chen, Y.; Qi, H.; Dong, C.; Pan, H.; Zhang, Q.; Shi, P.; Song, C.; Guan, X.; et al. Allicin attenuates pathological cardiac hypertrophy by inhibiting autophagy via activation of P3K/AKT/mTOR and MAPK/ERK/mTOR signaling pathways. Phytomedicine 2019, 58, 152765. [Google Scholar] [CrossRef]
- Dong, B.; Liu, C.; Xue, R.; Wang, Y.; Sun, Y.; Liang, Z.; Fan, W.; Jiang, J.; Zhao, J.; Su, Q.; et al. Fisetin inhibits cardiac hypertrophy by suppressing oxidative stress. J. Nutr. Biochem. 2018, 62, 221–229. [Google Scholar] [CrossRef]
- Liao, H.H.; Zhang, N.; Meng, Y.Y.; Feng, H.; Yang, J.J.; Li, W.J.; Chen, S.; Wu, H.M.; Deng, W.; Tang, Q.Z. Myricetin alleviates pathological cardiac hypertrophy via TRAF6/TAK1/MAPK and Nrf2 signaling pathway. Oxidative Med. Cell. Longev. 2019, 2019, 6304058. [Google Scholar] [CrossRef]
- Yin, Z.; Wang, X.; Zhang, L.; Zhou, H.; Wei, L.; Dong, X. Aspirin attenuates angiotensin II-induced cardiomyocyte hypertrophy by inhibiting the Ca2+/calcineurin-NFAT signaling pathway. Cardiovasc. Ther. 2016, 34, 21–29. [Google Scholar] [CrossRef]
- Lin, K.H.; Shibu, M.A.; Peramaiyan, R.; Chen, Y.F.; Shen, C.Y.; Hsieh, Y.L.; Chen, R.J.; Viswanadha, V.P.; Kuo, W.W.; Huang, C.Y. Bioactive flavone fisetin attenuates hypertension associated cardiac hypertrophy in H9c2 Cells and in spontaneously hypertension rats. J. Funct. Foods 2019, 52, 212–218. [Google Scholar] [CrossRef]
- Luo, Y.; Jiang, N.; May, H.I.; Luo, X.; Ferdous, A.; Schiattarella, G.G.; Chen, G.; Li, Q.; Li, C.; Rothermel, B.A.; et al. Cooperative binding of ETS2 and NFAT links Erk1/2 and calcineurin signaling in the pathogenesis of cardiac hypertrophy. Circulation 2021, 144, 34–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Pandey, S.; Sivalingam, K.; Shibu, M.A.; Kuo, W.W.; Yu, L.; Viswanadha, V.P.; Lin, Y.C.; Liao, S.C.; Huang, C.Y. Leech extract: A candidate cardioprotective against hypertension-induced cardiac hypertrophy and fibrosis. J. Ethnopharmacol. 2021, 264, 113346. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, N.H.; Jalil, J.; Zainalabidin, S.; Saleh, M.S.M.; Asmadi, A.Y.; Kamisah, Y. Molecular mechanisms of sacubitril/valsartan in cardiac remodeling. Front. Pharmacol. 2022, 13, 892460. [Google Scholar] [CrossRef]
- Siti, H.N.; Jalil, J.; Asmadi, A.Y.; Kamisah, Y. Parkia speciosa Hassk. empty pod extract alleviates angiotensin II-induced cardiomyocyte hypertrophy in H9c2 cells by modulating the Ang II/ROS/NO axis and MAPK pathway. Front. Pharmacol. 2021, 12, 741623. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Zhang, B.; Wu, C.; Wu, H.; Wu, J.; Wu, S.; Zhang, J.; Yang, X.; Yang, L.; Hu, Z.; et al. Plantago asiatica L. seeds extract protects against cardiomyocyte injury in isoproterenol-induced cardiac hypertrophy by inhibiting excessive autophagy and apoptosis in mice. Phytomedicine 2021, 91, 153681. [Google Scholar] [CrossRef]
- Ding, B.; Niu, W.; Wang, S.; Zhang, F.; Wang, H.; Chen, X.; Chen, S.; Ma, S.; Kang, W.; Wang, M.; et al. Centella asiatica (L.) Urb. attenuates cardiac hypertrophy and improves heart function through multi-level mechanisms revealed by systems pharmacology. J. Ethnopharmacol. 2022, 291, 115106. [Google Scholar] [CrossRef]
- Saleh, M.S.M.; Jalil, J.; Zainalabidin, S.; Asmadi, A.Y.; Mustafa, N.H.; Kamisah, Y. Genus Parkia: Phytochemical, medicinal uses, and pharmacological properties. Int. J. Mol. Sci. 2021, 22, 618. [Google Scholar] [CrossRef]
- Gui, J.S.; Mustafa, N.H.; Jalil, J.; Jubri, Z.; Kamisah, Y. Modulation of Nox4 and MAPK signaling pathways by Parkia speciosa empty pods in H9c2 cardiomyocytes exposed to H2O2. Indian J. Pharm. Sci. 2019, 81, 1029–1035. [Google Scholar] [CrossRef]
- Gui, J.S.; Jalil, J.; Jubri, Z.; Kamisah, Y. Parkia speciosa empty pod extract exerts anti-inflammatory properties by modulating NFκB and MAPK pathways in cardiomyocytes exposed to tumor necrosis factor-α. Cytotechnology 2019, 71, 79–89. [Google Scholar] [CrossRef]
- Mustafa, N.H.; Ugusman, A.; Jalil, J.; Kamisah, Y. Anti-inflammatory property of Parkia speciosa empty pod extract in human umbilical vein endothelial cells. J. Appl. Pharm. Sci. 2018, 8, 152–158. [Google Scholar]
- Kamisah, Y.; Zuhair, J.S.F.; Juliana, A.H.; Jaarin, K. Parkia speciosa empty pod prevents hypertension and cardiac damage in rats given N(G)-nitro-L-arginine methyl ester. Biomed. Pharmacother. 2017, 96, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhang, W.; Yang, L.; Fan, H.; Olatunji, O.J. Stink bean (Parkia speciosa) empty pod: A potent natural antidiabetic agent for the prevention of pancreatic and hepatorenal dysfunction in high fat diet/streptozotocin-induced type 2 diabetes in rats. Arch. Physiol. Biochem. 2021, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.S.M.; Jalil, J.; Mustafa, N.H.; Ramli, F.F.; Asmadi, A.Y.; Kamisah, Y. UPLC-MS-based metabolomics profiling for α-glucosidase inhibiting property of Parkia speciosa pods. Life 2021, 11, 78. [Google Scholar] [CrossRef]
- Ko, H.J.; Ang, L.H.; Ng, L.T. Antioxidant activities and polyphenolic constituents of bitter bean Parkia speciosa. Int. J. Food Prop. 2014, 17, 1977–1986. [Google Scholar] [CrossRef]
- Singh, K.; Gupta, A.; Sarkar, A.; Gupta, I.; Rana, S.; Sarkar, S.; Khan, S. Arginyltransferase knockdown attenuates cardiac hypertrophy and fibrosis through TAK1-JNK1/2 Pathway. Sci Rep. 2020, 10, 598. [Google Scholar] [CrossRef] [PubMed]
- Al-Mazroua, H.A.; Al-Rasheed, N.M.; Korashy, H.M. Downregulation of the cardiotrophin-1 gene expression by valsartan and spironolactone in hypertrophied heart rats in vivo and rat cardiomyocyte H9c2 cell line in vitro: A novel mechanism of cardioprotection. J. Cardiovasc. Pharmacol. 2013, 61, 337–344. [Google Scholar] [CrossRef]
- Riaz, S.; Abdulrahman, N.; Uddin, S.; Jabeen, A.; Gadeau, A.P.; Fliegel, L.; Mraiche, F. Anti-hypertrophic effect of Na+/H+ exchanger-1 inhibition is mediated by reduced cathepsin B. Eur. J. Pharmacol. 2020, 888, 173420. [Google Scholar] [CrossRef]
- Wang, H.; Jessup, J.A.; Lin, M.S.; Chagas, C.; Lindsey, S.H.; Groban, L. Activation of GPR30 attenuates diastolic dysfunction and left ventricle remodelling in oophorectomized mRen2. Lewis rats. Cardiovasc. Res. 2012, 94, 96–104. [Google Scholar] [CrossRef]
- Ramli, F.F.; Hashim, S.A.S.; Raman, B.; Mahmod, M.; Kamisah, Y. Role of trientine in hypertrophic cardiomyopathy: A review of mechanistic aspects. Pharmaceuticals 2022, 15, 1145. [Google Scholar] [CrossRef]
- Xu, X.; Wang, J.; Wang, X. Silencing of circHIPK3 inhibits pressure overload-induced cardiac hypertrophy and dysfunction by sponging miR-185-3p. Drug Des. Devel. Ther. 2020, 14, 5699–5710. [Google Scholar] [CrossRef]
- Nagai, R.; Low, R.B.; Stirewalt, W.S.; Alpert, N.R.; Litten, R.Z. Efficiency and capacity of protein synthesis are increased in pressure overload cardiac hypertrophy. Am. J. Physiol. 1988, 255, H325–H328. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Ping, P.; Wang, F.; Luo, L. Synthesis, secretion, function, metabolism and application of natriuretic peptides in heart failure. J. Biol. Eng. 2018, 12, 2. [Google Scholar] [CrossRef]
- Forte, M.; Madonna, M.; Schiavon, S.; Valenti, V.; Versaci, F.; Zoccai, G.B.; Frati, G.; Sciarretta, S. Cardiovascular pleiotropic effects of natriuretic peptides. Int. J. Mol. Sci. 2019, 20, 3874. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, T.C.; Knobel, J.; Burkert-Rettenmaier, S.; Li, X.; Meyer, I.S.; Jungmann, A.; Sicklinger, F.; Backs, J.; Lasitschka, F.; Müller, O.J.; et al. Secretome analysis of cardiomyocytes identifies PCSK6 (proprotein convertase subtilisin/kexin type 6) as a novel player in cardiac remodeling after myocardial infarction. Circulation 2020, 141, 1628–1644. [Google Scholar] [CrossRef] [PubMed]
- Siti, H.N.; Jalil, J.; Asmadi, A.Y.; Kamisah, Y. Rutin modulates MAPK pathway differently from quercetin in angiotensin II-induced H9c2 cardiomyocyte hypertrophy. Int. J. Mol. Sci. 2021, 22, 5063. [Google Scholar] [CrossRef] [PubMed]
- Togliatto, G.; Lombardo, G.; Brizzi, M.F. The future challenge of reactive oxygen species (ROS) in hypertension: From bench to bed side. Int. J. Mol. Sci. 2017, 18, 1988. [Google Scholar] [CrossRef]
- Zeng, S.Y.; Yang, L.; Lu, H.Q.; Yan, Q.J.; Gao, L.; Qin, X.P. Rutaecarpine prevents hypertensive cardiac hypertrophy involving the inhibition of Nox4-ROS-ADAM17 pathway. J. Cell. Mol. Med. 2019, 23, 4196–4207. [Google Scholar] [CrossRef]
- Chiang, C.J.; Chao, Y.P.; Ali, A.; Day, C.H.; Ho, T.J.; Wang, P.N.; Lin, S.C.; Padma, V.V.; Kuo, W.W.; Huang, C.Y. Probiotic Escherichia coli Nissle inhibits IL-6 and MAPK-mediated cardiac hypertrophy during STZ-induced diabetes in rats. Benef. Microbes 2021, 12, 283–293. [Google Scholar] [CrossRef]
- Yoon, J.J.; Son, C.O.; Kim, H.Y.; Han, B.H.; Lee, Y.J.; Lee, H.S.; Kang, D.G. Betulinic Acid protects Dox-triggered cardiomyocyte hypertrophy response through the GATA-4/Calcineurin/NFAT pathway. Molecules 2020, 26, 53. [Google Scholar] [CrossRef]
- Yang, N.; Zou, C.; Luo, W.; Xu, D.; Wang, M.; Wang, Y.; Wu, G.; Shan, P.; Liang, G. Sclareol attenuates angiotensin II-induced cardiac remodeling and inflammation via inhibiting MAPK signaling. Phytother. Res. 2022. [Google Scholar] [CrossRef]
- Chen, L.; Song, M.; Yao, C. Calcineurin in development and disease. Genes Dis. 2021, 9, 915–927. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, L.; Hamo, C.E.; Butler, J. Heart Failure Guidelines on Pharmacotherapy. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2017; Volume 243, pp. 109–129. [Google Scholar]
- Jin, L.; Piao, Z.H.; Sun, S.; Liu, B.; Kim, G.R.; Seok, Y.M.; Lin, M.Q.; Ryu, Y.; Choi, S.Y.; Kee, H.J.; et al. Gallic acid reduces blood pressure and attenuates oxidative stress and cardiac hypertrophy in spontaneously hypertensive rats. Sci. Rep. 2017, 7, 15607. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Yu, S.S.; He, Y.; Bi, X.Y.; Gao, S.; Yan, T.D.; Zheng, G.D.; Chen, T.T.; Ye, J.T.; Liu, P.Q. EGCG inhibits pressure overload-induced cardiac hypertrophy via the PSMB5/Nmnat2/SIRT6-dependent signalling pathways. Acta Physiol. 2021, 231, e13602. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, L.; Guo, S.; Liu, Y.; Zhao, X.; Li, R.; Yan, X.; Li, Y.; Wang, S.; Niu, X.; et al. Kaempferol alleviates angiotensin II-induced cardiac dysfunction and interstitial fibrosis in mice. Cell. Physiol. Biochem. 2017, 43, 2253–2263. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, J.; Wang, J.; Zhang, D. Luteolin suppresses lipopolysaccharide-induced cardiomyocyte hypertrophy and autophagy In Vitro. Mol. Med. Rep. 2019, 19, 1551–1560. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, Y.; Yu, M.; Shang, Y.; Chang, Y.; Zhao, H.; Kang, Y.; Zhao, L.; Xu, L.; Zhao, X.; et al. Discovery of herbacetin as a novel SGK1 inhibitor to alleviate myocardial hypertrophy. Adv. Sci. 2022, 9, e2101485. [Google Scholar] [CrossRef] [PubMed]

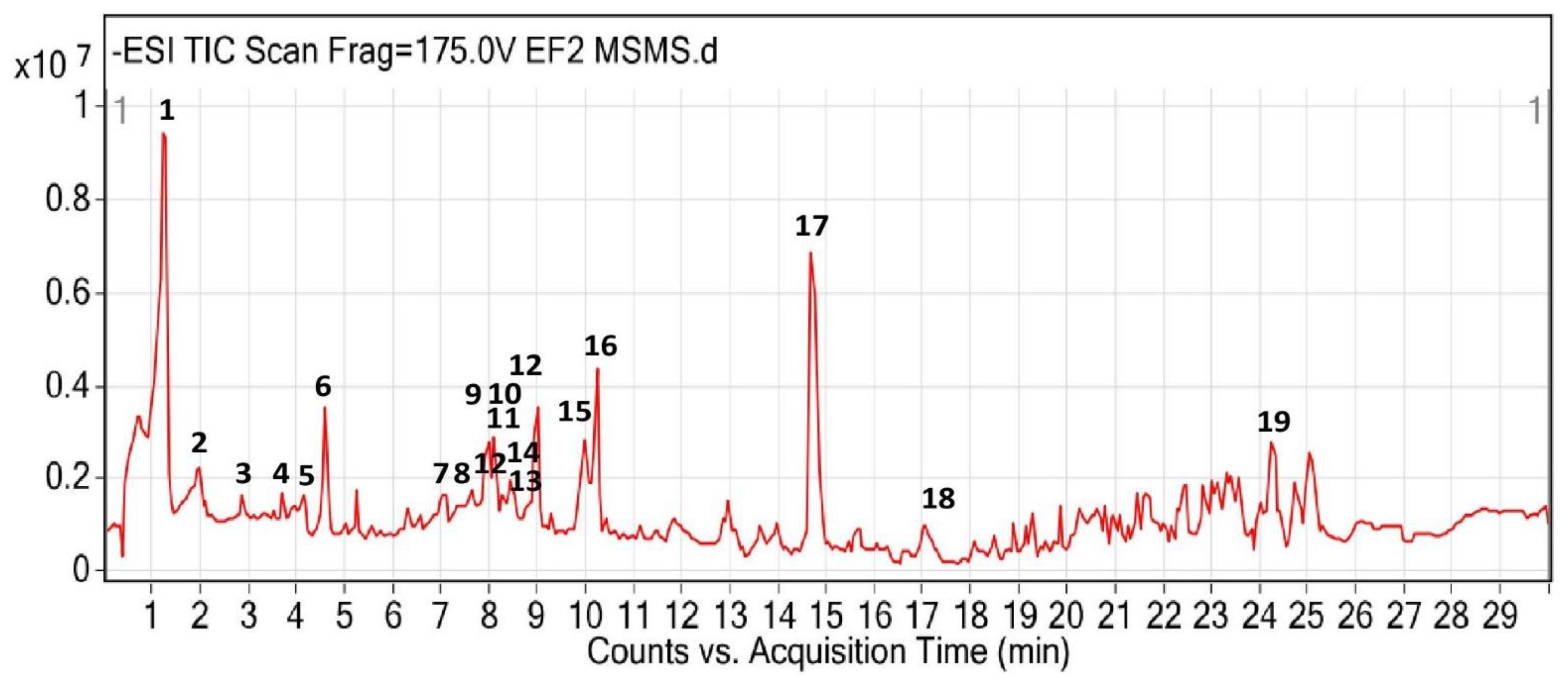

| No | RT (min) | [M-H]− (m/z) | m/z | Formula | MS/MS | Tentative Identification | Class of Compound |
|---|---|---|---|---|---|---|---|
| 1 | 1.1 | 169.0135 | 170.0208 | C7 H6 O5 | 125.0278 | Gallic acid | Phenolic |
| 2 | 2.108 | 218.1041 | 219.1114 | C9 H17 N O5 | 146.0824 | Pantothenic acid | Amino acid |
| 3 | 2.879 | 761.1266 | 762.1431 | C37 H30 O18 | 423.0714, 591.1116 | 2′-2′-Bisepigallocatechin monogallate | Flavonoid |
| 4 | 3.709 | 913.1353 | 914.1547 | C44 H34 O22 | 177.0194, 285.0393, 423.0708, 591.1129, 743.1223 | Theasinensin A | Flavonoid |
| 5 | 4.132 | 342.1058 | 343.1041 | C14 H18 O7 | 223.061 | Picein | Phenolic |
| 6 | 4.585 | 914.1601 | 915.1514 | C42 H34 O21 | 169.0148, 457.0770, 703.0976 | Thonningianin A | Tannin |
| 7 | 7.096 | 463.086 | 464.0932 | C43 H32 O20 | 300.026 | 3,5,7,2′,6′-Pentahydroxyflavone 2′-glucoside | Flavonoid glycoside |
| 8 | 7.579 | 319.0465 | 320.0538 | C15 H12 O8 | 167.0354 | Amaronol A | Flavanoid |
| 9 | 7.9777 | 463.0893 | 464.0963 | C21 H20 O12 | 151.0032, 300.0278, 343.0461 | Quercetin 3′-glucoside | Flavonoid glycoside |
| 10 | 8.023 | 831.1714 | 832.2088 | C38 H40 O21 | 300.0268, 463.0878 | Quercetin 3-(6‴-sinapylglucosyl)(1->2)-galactoside | Flavonoid glycoside |
| 11 | 8.155 | 463.0887 | 464.0958 | C21 H20 O12 | 300.0276 | Spiraeoside | Flavonoid glycoside |
| 12 | 8.29 8.866 | 447.0935 | 448.1008 | C21 H20 O11 | 301.0344 301.0346 | Kaempferol 3-alpha-D-galactoside | Flavonoid glycoside |
| 13 | 8.341 | 433.0779 | 434.0851 | C20 H18 O11 | 151.0031, 300.0276 | 6-Hydroxyluteolin 7-apioside | Flavonoid glycoside |
| 14 | 8.794 | 317.0305 | 318.0378 | C15 H10 O8 | 178.9982 | Gossypetin | Flavonoid |
| 15 | 9.933 | 301.0365 | 302.0437 | C15 H10 O7 | 121.0295, 151.0033, 178.9984, 229.0497 | Herbacetin | Flavonoid |
| 16 | 10.365 | 301.0359 | 302.0431 | C15 H10 O7 | 151.0032 | Bracteatin | Flavonoid |
| 17 | 14.735 | 732.2692 | 733.3104 | C40 H47 N O12 | 160.0144 | 3′-N-Debenzoyl-2′-deoxytaxol | Terpenoid |
| 18 | 17.39 | 297.1499 | 298.1569 | C19 H22 O3 | 183.0101 | Glepidotin C | Flavonoid |
| 19 | 24.398 | 531.4415 | 532.4486 | C34 H60 O4 | 346.9493, 487.4492 | Mayolene-16 | Fatty acid |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustafa, N.H.; Jalil, J.; Saleh, M.S.M.; Zainalabidin, S.; Asmadi, A.Y.; Kamisah, Y. Parkia speciosa Hassk. Empty Pod Extract Prevents Cardiomyocyte Hypertrophy by Inhibiting MAPK and Calcineurin-NFATC3 Signaling Pathways. Life 2023, 13, 43. https://doi.org/10.3390/life13010043
Mustafa NH, Jalil J, Saleh MSM, Zainalabidin S, Asmadi AY, Kamisah Y. Parkia speciosa Hassk. Empty Pod Extract Prevents Cardiomyocyte Hypertrophy by Inhibiting MAPK and Calcineurin-NFATC3 Signaling Pathways. Life. 2023; 13(1):43. https://doi.org/10.3390/life13010043
Chicago/Turabian StyleMustafa, Nor Hidayah, Juriyati Jalil, Mohammed S. M. Saleh, Satirah Zainalabidin, Ahmad Yusof Asmadi, and Yusof Kamisah. 2023. "Parkia speciosa Hassk. Empty Pod Extract Prevents Cardiomyocyte Hypertrophy by Inhibiting MAPK and Calcineurin-NFATC3 Signaling Pathways" Life 13, no. 1: 43. https://doi.org/10.3390/life13010043
APA StyleMustafa, N. H., Jalil, J., Saleh, M. S. M., Zainalabidin, S., Asmadi, A. Y., & Kamisah, Y. (2023). Parkia speciosa Hassk. Empty Pod Extract Prevents Cardiomyocyte Hypertrophy by Inhibiting MAPK and Calcineurin-NFATC3 Signaling Pathways. Life, 13(1), 43. https://doi.org/10.3390/life13010043









