ECMO Retrieval Program: What Have We Learned So Far
Abstract
1. Introduction
2. Materials and Methods
2.1. ECMO-Center Protocol
2.2. Data Collection
2.3. Outcome Analysis
2.4. Ethics
2.5. Statistical Methods
3. Results
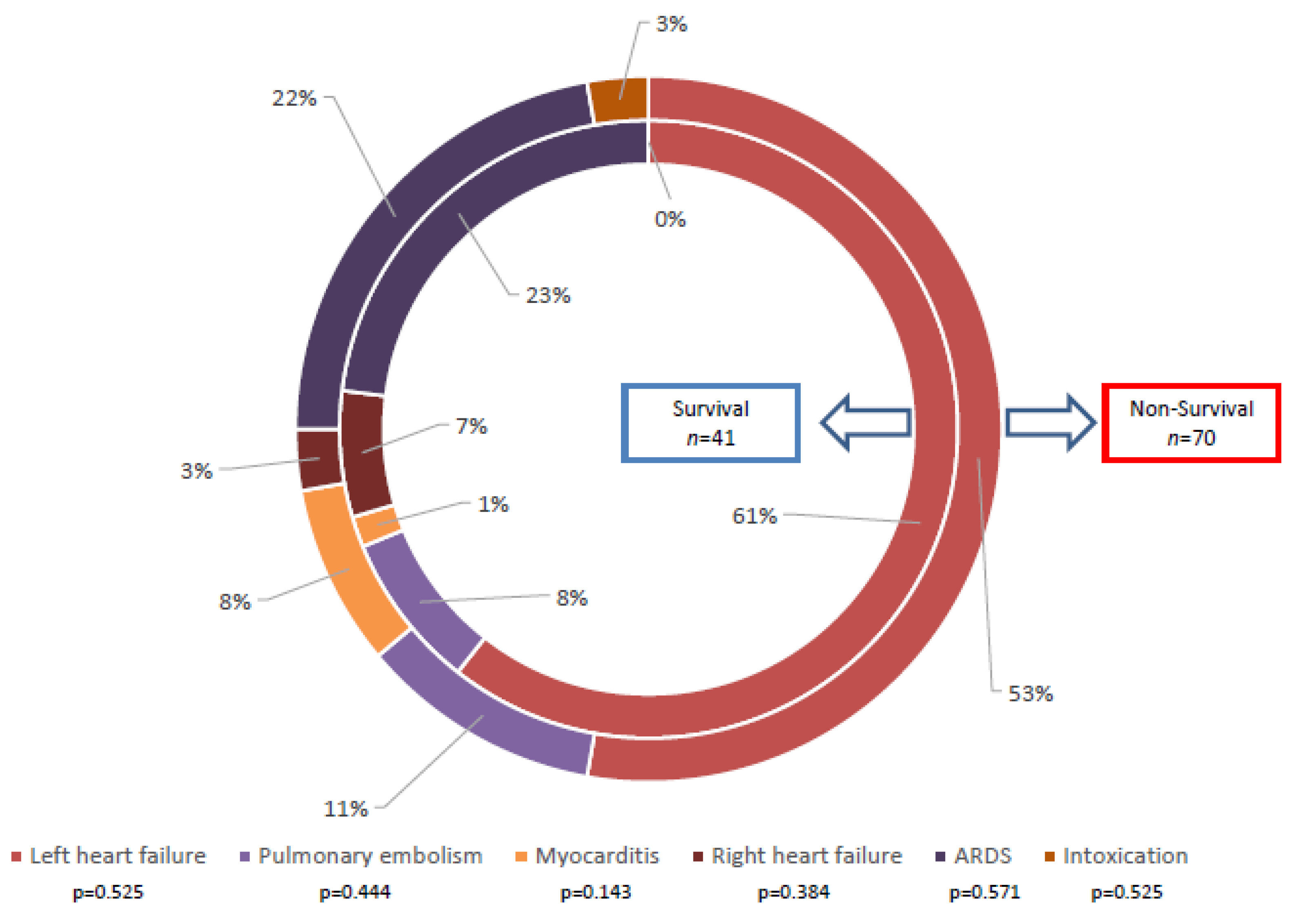
3.1. Indication of ECMO Therapy
3.2. Demographic and Clinical Characteristics
3.3. Clinical Characteristics of Implantation
3.4. Laboratory Parameters at Admission and 48 h after ECMO Implantation
3.5. Complications after ECMO Implantation
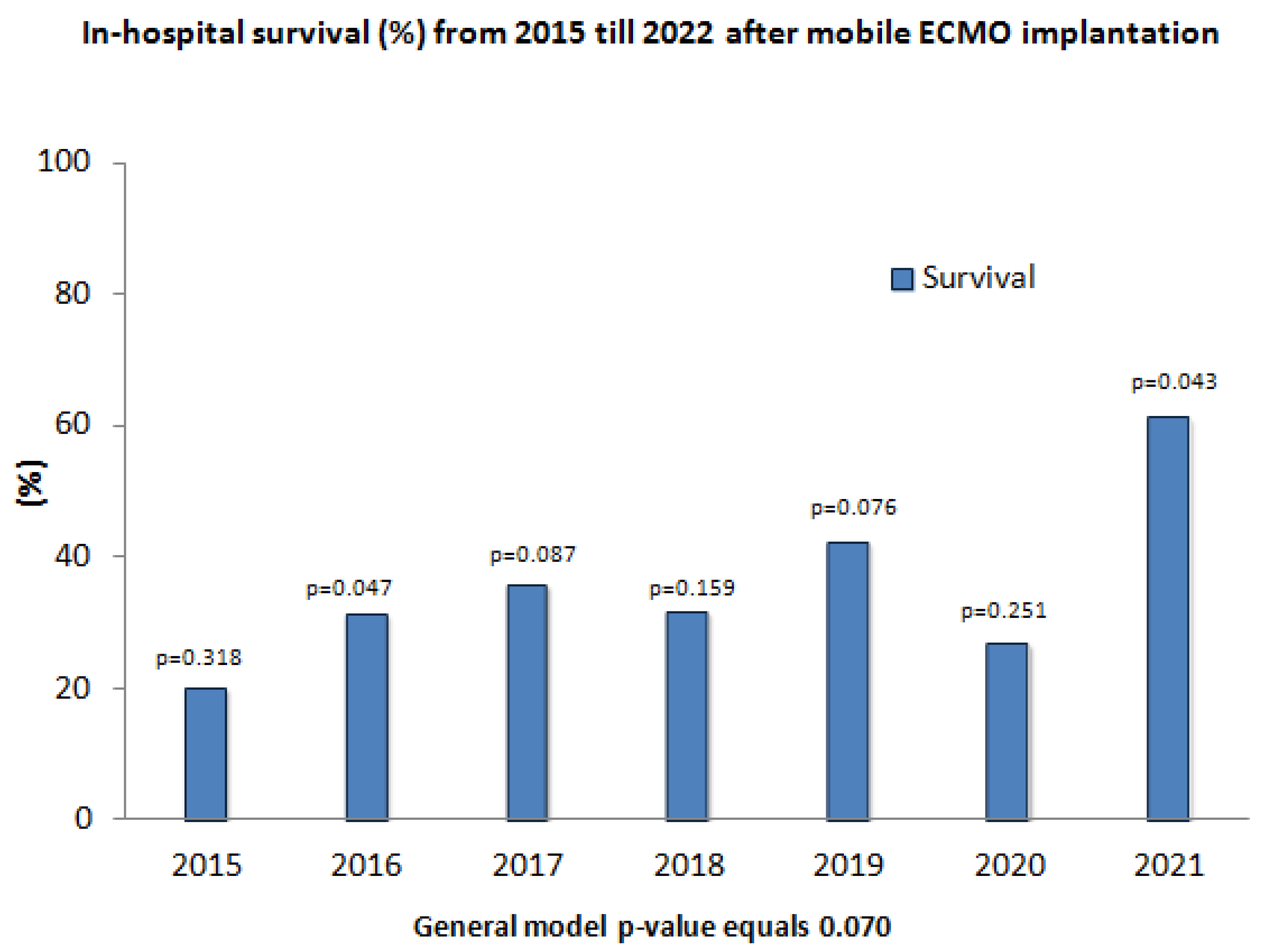
3.6. Survival Rates for Each Year from 2015 Till 2021
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lunz, D.; Calabrò, L.; Belliato, M.; Contri, E.; Broman, L.M.; Scandroglio, A.M.; Patricio, D.; Malfertheiner, M.; Creteur, J.; Philipp, A.; et al. Extracorporeal membrane oxygenation for refractory cardiac arrest: A retrospective multicenter study. Intensive Care Med. 2020, 46, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Ostadal, P.; Rokyta, R.; Kruger, A.; Vondrakova, D.; Janotka, M.; Smíd, O.; Smalcova, J.; Hromadka, M.; Linhart, A.; Bělohlávek, J. Extra corporeal membrane oxygenation in the therapy of cardiogenic shock (ECMO-CS): Rationale and design of the multicenter randomized trial. Eur. J. Heart Fail. 2017, 19 (Suppl. 2), 124–127. [Google Scholar] [CrossRef] [PubMed]
- Carroll, B.J.; Shah, R.V.; Murthy, V.; McCullough, S.A.; Reza, N.; Thomas, S.S.; Song, T.H.; Newton-Cheh, C.H.; Camuso, J.M.; MacGillivray, T.; et al. Clinical Features and outcomes in adults with cardiogenic shock supported by extracorporeal membrane oxygenation. Am. J. Cardiol. 2015, 116, 1624–1630. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, R.; Shekar, K.; MacLaren, G.; Schmidt, M.; Pellegrino, V.; Meyns, B.; Haft, J.; Vercaemst, L.; Pappalardo, F.; Bermudez, C.; et al. ELSO Interim Guidelines for Venoarterial Extracorporeal Membrane Oxygenation in Adult Cardiac Patients. Asaio J. 2021, 67, 827–844. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, M.; Schwartz, J.; Gupta, N.; Im, J.; Leff, J.; Jakobleff, W.A.; Leyvi, G. Patient Demographics and Extracorporeal Membranous Oxygenation (ECMO)-Related Complications Associated With Survival to Discharge or 30-Day Survival in Adult Patients Receiving Venoarterial (VA) and Venovenous (VV) ECMO in a Quaternary Care Urban Center. J. Cardiothorac. Vasc. Anesth. 2019, 33, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Yukawa, T.; Kashiura, M.; Sugiyama, K.; Tanabe, T.; Hamabe, Y. Neurological outcomes and duration from cardiac arrest to the initiation of extracorporeal membrane oxygenation in patients with out-of-hospital cardiac arrest: A retrospective study. Scand. J. Trauma Resusc. Emerg. Med. 2017, 25, 95. [Google Scholar] [CrossRef] [PubMed]
- Schmiady, M.O.; Hofmann, M.; Sromicki, J.; Halbe, M.; van Tilburg, K.; Aser, R.; Mestres, C.A.; Maisano, F.; Ferrari, E. Initiation of an inter-hospital extracorporeal membrane oxygenation transfer programme for critically ill patients with coronavirus disease 2019: Bringing extracorporeal membrane oxygenation support to peripheral hospitals. Interact. Cardiovasc. Thorac. Surg. 2021, 32, 812–816. [Google Scholar] [CrossRef]
- Banjas, N.; Hopf, H.B.; Hanisch, E.; Friedrichson, B.; Fichte, J.; Buia, A. ECMO-treatment in patients with acute lung failure, cardiogenic, and septic shock: Mortality and ECMO-learning curve over a 6-year period. J. Intensive Care 2018, 6, 84. [Google Scholar] [CrossRef]
- Yeo, H.J.; Cho, W.H.; Kim, D. Learning curve for multidisciplinary team setup in veno-venous extracorporeal membrane oxygenation for acute respiratory failure. Perfusion 2019, 34, 30–38. [Google Scholar] [CrossRef]
- Krasivskyi, I.; Ivanov, B.; Vehrenberg, J.; Eghbalzadeh, K.; Gerfer, S.; Gaisendrees, C.; Kuhn, E.; Sabashnikov, A.; Mader, N.; Djordjevic, I.; et al. Sex-Related Differences in Short-Term Outcomes after Mobile VA-ECMO Implantation: Five-Year Experience of an ECMO Retrieval Program. Life 2022, 12, 1746. [Google Scholar] [CrossRef]
- Djordjevic, I.; Gaisendrees, C.; Adler, C.; Eghbalzadeh, K.; Braumann, S.; Ivanov, B.; Merkle, J.; Deppe, A.C.; Kuhn, E.; Stangl, R.; et al. Extracorporeal cardiopulmonary resuscitation for out-of-hospital cardiac arrest: First results and outcomes of a newly established ECPR program in a large population area. Perfusion 2022, 37, 249–256. [Google Scholar] [CrossRef]
- Napp, L.C.; Kuhn, C.; Bauersachs, J. ECMO in cardiac arrest and cardiogenic shock. Herz 2017, 42, 27–44. [Google Scholar] [CrossRef]
- Vakil, D.; Soto, C.; D’Costa, Z.; Volk, L.; Kandasamy, S.; Iyer, D.; Ikegami, H.; Russo, M.J.; Lee, L.Y.; Lemaire, A. Short-term and intermediate outcomes of cardiogenic shock and cardiac arrest patients supported by venoarterial extracorporeal membrane oxygenation. J. Cardiothorac. Surg. 2021, 16, 290. [Google Scholar] [CrossRef]
- Scolari, F.L.; Trott, G.; Schneider, D.; Goldraich, L.A.; Frederico Tonietto, T.; Moura, L.Z.; Bertoldi, E.G.; Rover, M.M.; Wolf, J.M.; Souza, D.; et al. Cardiogenic shock treated with temporary mechanical circulatory support in Brazil: The effect of learning curve. Int. J. Artif. Organs 2022, 45, 292–300. [Google Scholar] [CrossRef]
- Russo, J.J.; Aleksova, N.; Pitcher, I.; Couture, E.; Parlow, S.; Faraz, M.; Visintini, S.; Simard, T.; Di Santo, P.; Mathew, R.; et al. Left Ventricular Unloading during Extracorporeal Membrane Oxygenation in Patients with Cardiogenic Shock. J. Am. Coll. Cardiol. 2019, 73, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Fiorelli, F.; Panoulas, V. Impella as unloading strategy during VA-ECMO: Systematic review and meta-analysis. Rev. Cardiovasc. Med. 2021, 22, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, Z.C.; Trevas, S.; Ferreira, H.; Côrte-Real, H. ECMELLA: Successful rescue cardiopulmonary support in post-coronary artery bypass graft cardiogenic shock with cardiac arrest-case report. Eur. Heart J. Case Rep. 2020, 4, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Cappannoli, L.; Galli, M.; Zito, A.; Restivo, A.; Princi, G.; Laborante, R.; Vergallo, R.; Romagnoli, E.; Leone, A.M.; Aurigemma, C.; et al. Venoarterial extracorporeal membrane oxygenation (VA-ECMO) with vs without left ventricular unloading by Impella: A systematic review and meta-analysis. Eur. Heart J. Qual. Care Clin. Outcomes 2022. [Google Scholar] [CrossRef]
- Schrage, B.; Becher, P.M.; Bernhardt, A.; Bezerra, H.; Blankenberg, S.; Brunner, S.; Colson, P.; Cudemus Deseda, G.; Dabboura, S.; Eckner, D.; et al. Left Ventricular Unloading Is Associated with Lower Mortality in Patients with Cardiogenic Shock Treated with Venoarterial Extracorporeal Membrane Oxygenation: Results From an International, Multicenter Cohort Study. Circulation 2020, 142, 2095–2106. [Google Scholar] [CrossRef]
- Makhoul, M.; Heuts, S.; Mansouri, A.; Taccone, F.S.; Obeid, A.; Mirko, B.; Broman, L.M.; Malfertheiner, M.V.; Meani, P.; Raffa, G.M.; et al. Understanding the “extracorporeal membrane oxygenation gap” in veno-arterial configuration for adult patients: Timing and causes of death. Artif. Organs 2021, 45, 1155–1167. [Google Scholar] [CrossRef]
- Panuccio, G.; Neri, G.; Macri, L.M.; Salerno, N.; De Rosa, S.; Torella, D. Use of Impella device in cardiogenic shock and its clinical outcomes: A systematic review and meta-analysis. Int. J. Cardiol. Heart Vasc. 2022, 40, 101007. [Google Scholar] [CrossRef]
- Smith, M.; Vukomanovic, A.; Brodie, D.; Thiagarajan, R.; Rycus, P.; Buscher, H. Duration of veno-arterial extracorporeal life support (VA ECMO) and outcome: An analysis of the Extracorporeal Life Support Organization (ELSO) registry. Crit. Care 2017, 21, 45. [Google Scholar] [CrossRef] [PubMed]
- Provaznik, Z.; Philipp, A.; Müller, T.; Kostiantyn, K.; Lunz, D.; Schmid, C.; Floerchinger, B. Outcome after veno-arterial extracorporeal membrane oxygenation in elderly patients: A 14-year single-center experience. Artif. Organs 2022. [Google Scholar] [CrossRef] [PubMed]
- Fleissner, F.; Mogaldea, A.; Martens, A.; Natanov, R.; Rumke, S.; Salman, J.; Kaufeld, T.; Ius, F.; Beckmann, E.; Haverich, A.; et al. ECLS supported transport of ICU patients: Does out-of-house implantation impact survival? J. Cardiothorac. Surg. 2021, 16, 158. [Google Scholar] [CrossRef]
- Yang, J.T.; Kim, H.S.; Kim, K.I.; Ko, H.H.; Lim, J.H.; Lee, H.K.; Ra, Y.J. Outcomes of Urgent Interhospital Transportation for Extracorporeal Membrane Oxygenation Patients. J. Chest Surg. 2022, 55, 452–461. [Google Scholar] [CrossRef]
- Fux, T.; Holm, M.; Corbascio, M.; Lund, L.H.; van der Linden, J. VA-ECMO Support in Nonsurgical Patients with Refractory Cardiogenic Shock: Pre-Implant Outcome Predictors. Artif. Organs 2019, 43, 132–141. [Google Scholar] [CrossRef]
- Zhang, R.; Kofidis, T.; Kamiya, H.; Shrestha, M.; Tessmann, R.; Haverich, A.; Klima, U. Creatine kinase isoenzyme MB relative index as predictor of mortality on extracorporeal membrane oxygenation support for postcardiotomy cardiogenic shock in adult patients. Eur. J. Cardiothorac. Surg. 2006, 30, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, B.G.; Powers, A.J.; Sheikh, A.Y.; Lee, P.H.; Lobato, R.L.; Wong, J.K. Resource use trends in extracorporeal membrane oxygenation in adults: An analysis of the Nationwide Inpatient Sample 1998–2009. J. Thorac. Cardiovasc. Surg. 2014, 148, 416–421. [Google Scholar] [CrossRef]
- Ostermann, M.; Lumlertgul, N. Acute kidney injury in ECMO patients. Crit. Care 2021, 25, 313. [Google Scholar] [CrossRef] [PubMed]
- Tarzia, V.; Bagozzi, L.; Ponzoni, M.; Pradegan, N.; Banchelli, F.; Bortolussi, G.; Bellanti, E.; Bianco, R.; Zanella, F.; Bottio, T.; et al. Prognosticating mortality of primary cardiogenic shock requiring extracorporeal life support: The RESCUE score. Curr. Probl. Cardiol. 2022, 2022, 101554. [Google Scholar] [CrossRef]
- Kohs, T.C.L.; Liu, P.; Raghunathan, V.; Amirsoltani, R.; Oakes, M.; McCarty, O.J.T.; Olson, S.R.; Masha, L.; Zonies, D.; Shatzel, J.J. Severe thrombocytopenia in adults undergoing extracorporeal membrane oxygenation is predictive of thrombosis. Platelets 2022, 33, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Shao, M.; Zhang, C.; Fang, M.; Jin, M.; Han, X.; Liu, N. Serum Total Bilirubin with Hospital Survival in Adults during Extracorporeal Membrane Oxygenation. Front. Med. (Lausanne) 2022, 9, 914557. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.; Cho, S.; Lee, J.H.; Min, J.; Kwon, H.W.; Kwak, J.G.; Kim, W.H. Postcardiotomy Extracorporeal Membrane Oxygenation Support in Patients with Congenital Heart Disease. J. Chest Surg. 2022, 55, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Dobrilovic, N.; March, R.; Yin, K.; Lateef, O.; Alimohamed, M.; Bak, E.; Delibasic, M.; Karlson, K.; Edwards, N.; Raman, J. Liver Dysfunction Associated with In-Hospital Mortality in Adult Extracorporeal Membrane Oxygenation Support. Crit. Care Explor. 2021, 3, e0484. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Park, S. Blood Transfusion Strategies in Patients Undergoing Extracorporeal Membrane Oxygenation. Korean J. Crit. Care Med. 2017, 32, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Shudo, Y.; Cheng, N.; He, H.; Rosenberg, C.; Hiesinger, W.; Hadhazy, E.; Shepard, J.; Krishna, P.; Resnik, J.; Fong, R.; et al. A value-based approach to optimize red blood cell transfusion in patients receiving extracorporeal membrane oxygenation. Perfusion 2022, 2676591221128138. [Google Scholar] [CrossRef]
- Cankar, T.; Krepek, M.; Kosmopoulos, M.; Radsel, P.; Yannopoulos, D.; Noc, M.; Goslar, T. Long-Term Survival and Quality of Life in Non-Surgical Adult Patients Supported with Veno-Arterial Extracorporeal Oxygenation. J. Clin. Med. 2022, 11, 6452. [Google Scholar] [CrossRef] [PubMed]
| Non-Survival (n = 70) | Survival (n = 41) | p-Value | |
|---|---|---|---|
| Age (years), mean ± SD | 55 ± 13 | 49 ± 14 | 0.719 |
| Male, n (%) | 53 (75.7%) | 23 (56.1%) | 0.027 |
| BMI (kg/m2), mean ± SD | 28 ± 7 | 28 ± 6 | 0.773 |
| EuroSCORE II (%), mean ± SD | 6.0 ± 3.6 | 7.0 ± 3.6 | 0.849 |
| Previous MI, n (%) | 16 (25.8%) | 9 (22.0%) | 0.419 |
| Previous stroke, n (%) | 2 (3.2%) | 2 (4.9%) | 0.516 |
| COPD, n (%) | 8 (12.7%) | 3 (7.3%) | 0.298 |
| Smoking, n (%) | 21 (33.3%) | 15 (36.6%) | 0.733 |
| Chronic renal insufficiency, n (%) | 9 (14.3%) | 2 (4.9%) | 0.113 |
| Dialysis, n (%) | 4 (6.3%) | 3 (7.3%) | 0.572 |
| Diabetes, n (%) | 18 (28.6%) | 11 (26.8%) | 0.846 |
| Arterial hypertension, n (%) | 29 (46.0%) | 17 (41.5%) | 0.647 |
| Hyperlipidaemia, n (%) | 15 (23.8%) | 10 (24.4%) | 0.946 |
| SARS-CoV-2, n (%) | 7 (11.1%) | 3 (7.3%) | 0.736 |
| Non-Survival (n = 70) | Survival (n = 41) | p-Value | |
|---|---|---|---|
| ALS before ECMO, n (%) | 39 (56.5%) | 22 (53.7%) | 0.770 |
| Duration ALS, min, mean ± SD | 52 ± 49 | 22 ± 24 | 0.005 |
| Distance to patient (km), mean ± SD | 26 ± 24 | 27 ± 28 | 0.547 |
| Implantation technique, PP, n (%) | 55 (94.8%) | 27 (90.0%) | 0.406 |
| Initial ECMO flow, L/m, mean ± SD | 3.9 ± 0.8 | 3.8 ± 0.9 | 0.638 |
| ECMO duration, hours, mean ± SD | 108 ± 110 | 106 ± 78 | 0.003 |
| IABP, n (%) | 3 (4.6%) | 4 (9.8%) | 0.426 |
| Impella, n (%) | 3 (4.6%) | 7 (17.1%) | 0.043 |
| ECMO weaning, n (%) | 8 (11.9%) | 41 (100%) | <0.001 |
| RBC, n, mean ± SD | 19 ± 16 | 17 ± 20 | 0.552 |
| FFP, n, mean ± SD | 10 ± 12 | 7 ± 12 | 0.405 |
| Platelets, n, mean ± SD | 2 ± 2 | 2 ± 4 | 0.355 |
| Non-Survival (n = 70) | Survival (n = 41) | p-Value | |
|---|---|---|---|
| Hb (g/dL), mean ± SD | 9.1 ± 10.8 | 7.7 ± 1.4 | 0.221 |
| RBC (109/L), mean ± SD | 2.6 ± 0.5 | 2.7 ± 0.5 | 0.961 |
| WBC (109/L), mean ± SD | 21.7 ± 9.9 | 22.0 ± 8.7 | 0.218 |
| Platelets (109/L), mean ± SD | 54.2 ± 48.8 | 98.8 ± 129.6 | 0.065 |
| Bilirubin, mg/dL, mean ± SD | 8.2 ± 11.3 | 4.2 ± 7.2 | 0.002 |
| AST (U/L) max, mean ± SD | 3535 ± 4322 | 2388 ± 4516 | 0.557 |
| ALT (U/L) max, mean ± SD | 1350 ± 1595 | 1252 ± 2212 | 0.148 |
| Creatinine (mg/dL), mean ± SD | 2.2 ± 1.7 | 2.5 ± 4.1 | 0.140 |
| Lactate (mmol/L), mean ± SD | 9.7 ± 6.7 | 6.6 ± 4.9 | 0.098 |
| CK, U/L, mean ± SD | 2921 ± 3730 | 3868 ± 1391 | 0.131 |
| CK-MB, U/L, mean ± SD | 336 ± 362 | 167 ± 220 | 0.012 |
| Non-Survival (n = 70) | Survival (n = 41) | p-Value | |
|---|---|---|---|
| pO2 (mmHg), mean ± SD | 130 ± 65 | 122 ± 45 | 0.085 |
| pCO2 (mmHg), mean ± SD | 39 ± 5 | 37 ± 6 | 0.201 |
| pH, mean ± SD | 7.4 ± 0.07 | 7.4 ± 0.07 | 0.478 |
| FiO2 (%), mean ± SD | 55 ± 26 | 68 ± 51 | 0.052 |
| CK, U/L, mean ± SD | 3377 ± 5736 | 2725 ± 7002 | 0.713 |
| CK-MB, U/L, mean ± SD | 126 ± 119 | 71 ± 92 | 0.020 |
| Creatinine (mg/dL), mean ± SD | 2.7 ± 3.4 | 2.2 ± 1.8 | 0.487 |
| Lactate (mmol/L), mean ± SD | 5.2 ± 5.3 | 2.9 ± 2.4 | 0.007 |
| Bilirubin (mg/dL), mean ± SD | 2.7 ± 3.7 | 2.4 ± 2.5 | 0.376 |
| AST (U/L), mean ± SD | 2395 ± 3388 | 1604 ± 3846 | 0.502 |
| ALT (U/L), mean ± SD | 915 ± 1339 | 917 ± 1844 | 0.207 |
| aPTT (s), mean ± SD | 64 ± 27 | 60 ± 28 | 0.657 |
| Non-Survival (n = 70) | Survival (n = 41) | p-Value | |
|---|---|---|---|
| Stroke, n (%) | 9 (14.3%) | 2 (5.0%) | 0.195 |
| Thromboembolic events, n (%) | 21 (33.3%) | 3 (7.3%) | 0.002 |
| Bleeding, n (%) | 31 (51.7%) | 14 (35.0%) | 0.101 |
| Limb ischemia, n (%) | 12 (19.0%) | 7 (17.1%) | 0.799 |
| Limb ischemia requiring intervention, n (%) | 6 (9.5%) | 5 (12.2%) | 0.749 |
| ARDS, n (%) | 19 (30.6%) | 7 (17.5%) | 0.137 |
| Pneumonia, n (%) | 19 (30.6%) | 14 (35.0%) | 0.646 |
| Pneumothorax, n (%) | 5 (8.1%) | 2 (5.0%) | 0.702 |
| Hepatic failure, n (%) | 28 (44.4%) | 5 (12.2%) | <0.001 |
| Gastrointestinal bleeding, n (%) | 4 (6.5%) | 1 (2.4%) | 0.646 |
| Ventricular rhythm disorders, n (%) | 21 (33.9%) | 16 (39.0%) | 0.594 |
| PPI, n (%) | 1 (1.6%) | 3 (7.3%) | 0.299 |
| Renal failure, n (%) | 45 (71.4%) | 16 (39.0%) | 0.002 |
| Dialysis, n (%) | 31 (50.0%) | 8 (19.5%) | 0.002 |
| Oxygenator failure, n (%) | 2 (3.0%) | 0 (0.0%) | 0.526 |
| Wound infection, n (%) | 2 (3.2%) | 5 (12.2%) | 0.112 |
| SIRS, n (%) | 25 (39.7%) | 8 (20.5%) | 0.044 |
| Septic shock, n (%) | 25 (39.7%) | 9 (22.5%) | 0.071 |
| ICU stay (days), mean ± SD | 7 ± 7 | 17 ± 14 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krasivskyi, I.; Großmann, C.; Dechow, M.; Djordjevic, I.; Ivanov, B.; Gerfer, S.; Bennour, W.; Kuhn, E.; Sabashnikov, A.; Mader, N.; et al. ECMO Retrieval Program: What Have We Learned So Far. Life 2023, 13, 157. https://doi.org/10.3390/life13010157
Krasivskyi I, Großmann C, Dechow M, Djordjevic I, Ivanov B, Gerfer S, Bennour W, Kuhn E, Sabashnikov A, Mader N, et al. ECMO Retrieval Program: What Have We Learned So Far. Life. 2023; 13(1):157. https://doi.org/10.3390/life13010157
Chicago/Turabian StyleKrasivskyi, Ihor, Clara Großmann, Marit Dechow, Ilija Djordjevic, Borko Ivanov, Stephen Gerfer, Walid Bennour, Elmar Kuhn, Anton Sabashnikov, Navid Mader, and et al. 2023. "ECMO Retrieval Program: What Have We Learned So Far" Life 13, no. 1: 157. https://doi.org/10.3390/life13010157
APA StyleKrasivskyi, I., Großmann, C., Dechow, M., Djordjevic, I., Ivanov, B., Gerfer, S., Bennour, W., Kuhn, E., Sabashnikov, A., Mader, N., Eghbalzadeh, K., & Wahlers, T. (2023). ECMO Retrieval Program: What Have We Learned So Far. Life, 13(1), 157. https://doi.org/10.3390/life13010157









