The Ameliorative Effect of Thymoquinone on Vincristine-Induced Peripheral Neuropathy in Mice by Modulating Cellular Oxidative Stress and Cytokine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drugs and Chemicals
2.3. Experimental Protocol
2.4. Behavioral Assessment
2.4.1. Rotarod Test
2.4.2. Hot Plate Test
2.4.3. Cold Plate Test
2.4.4. Formalin Test
2.5. Biochemical Estimation
2.6. Statistical Analysis
3. Results
3.1. Behavioral Assessment
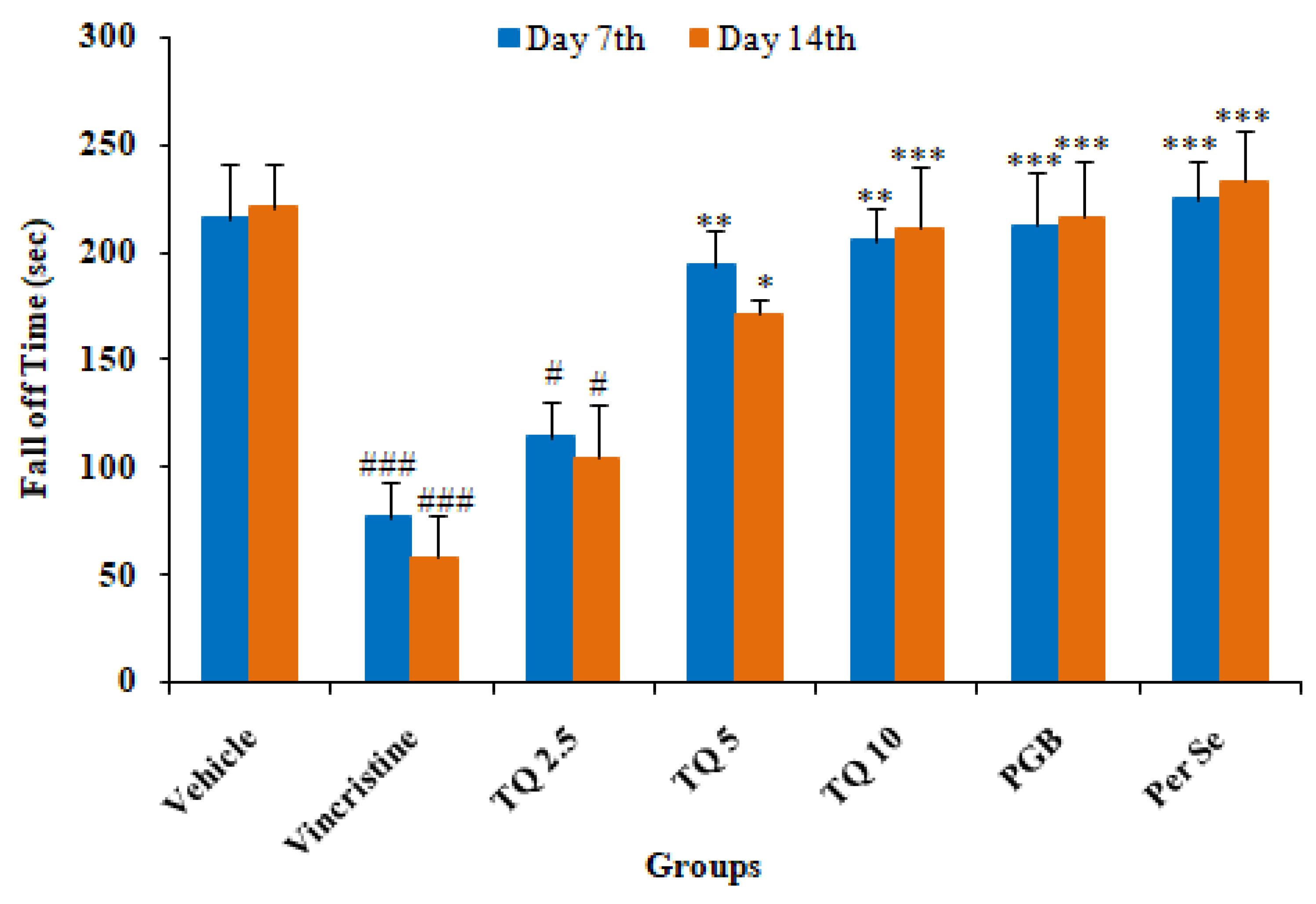
3.1.1. Effect of Thymoquinone on Rotarod Test of Mice
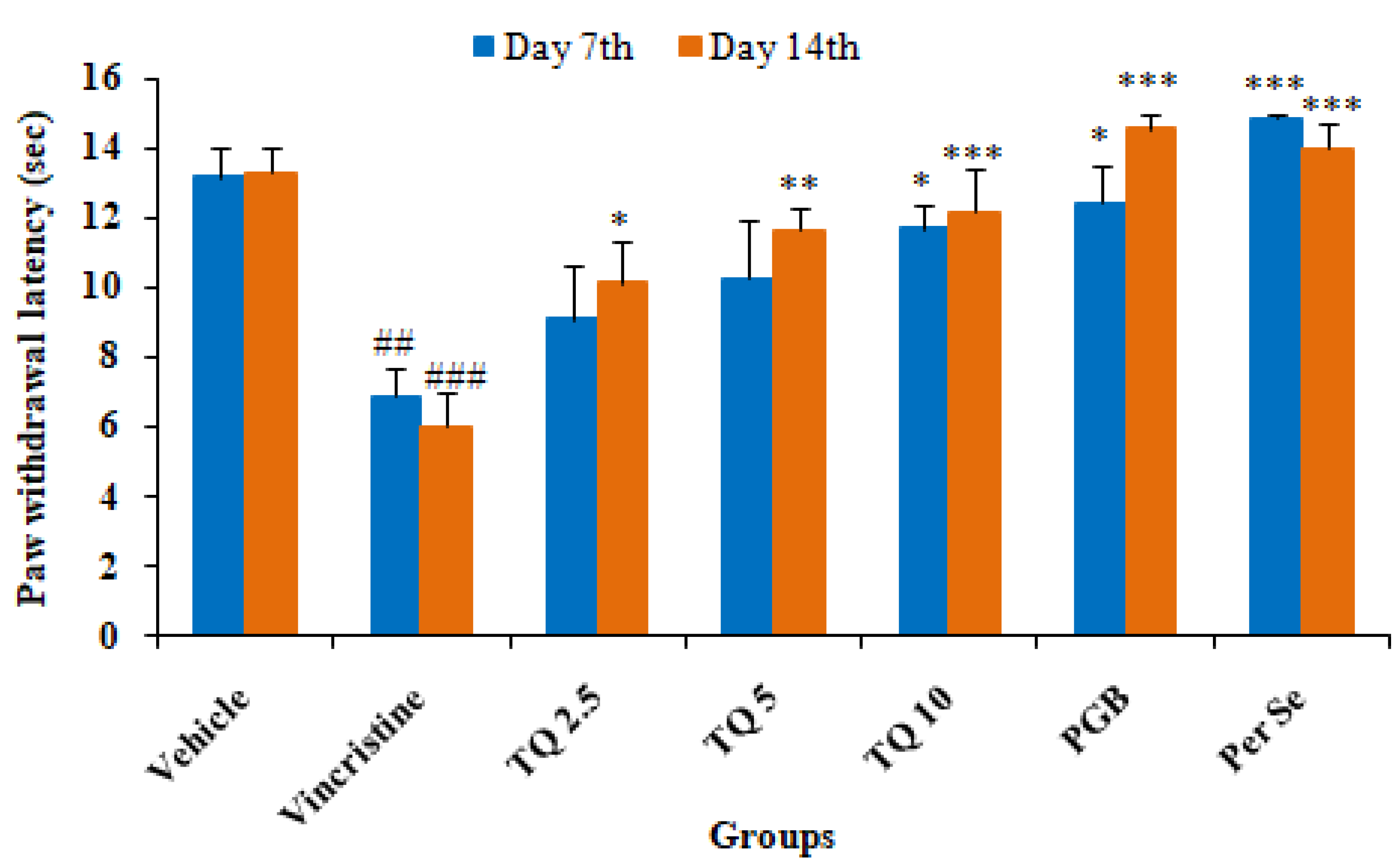
3.1.2. Effect of Thymoquinone on Hot Plate Induced Algesia in Mice
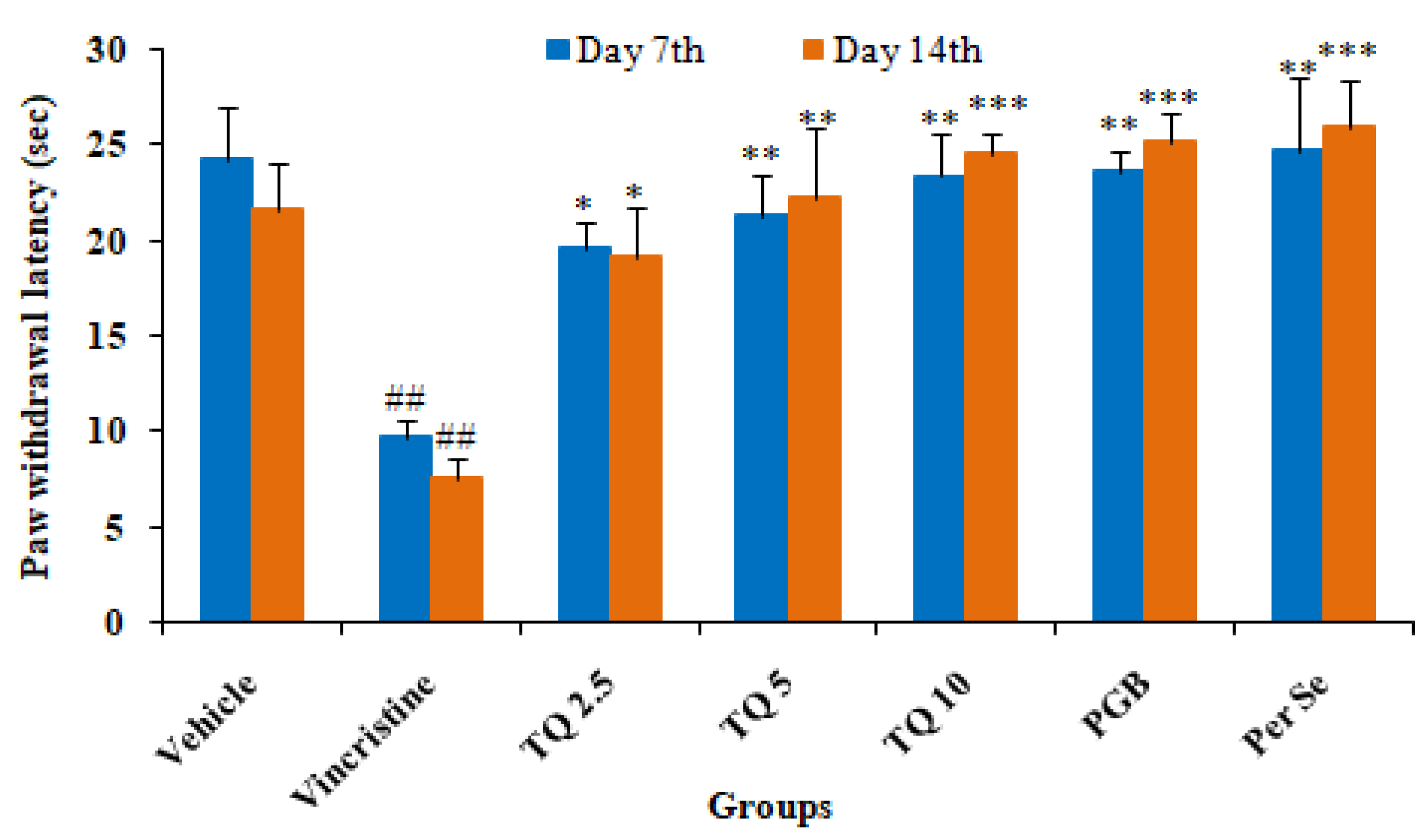
3.1.3. Effect of Thymoquinone on Cold Plate Induced Algesia in Mice
3.1.4. Effect of Thymoquinone on Formalin Test in Mice
3.2. Biochemical Assessments
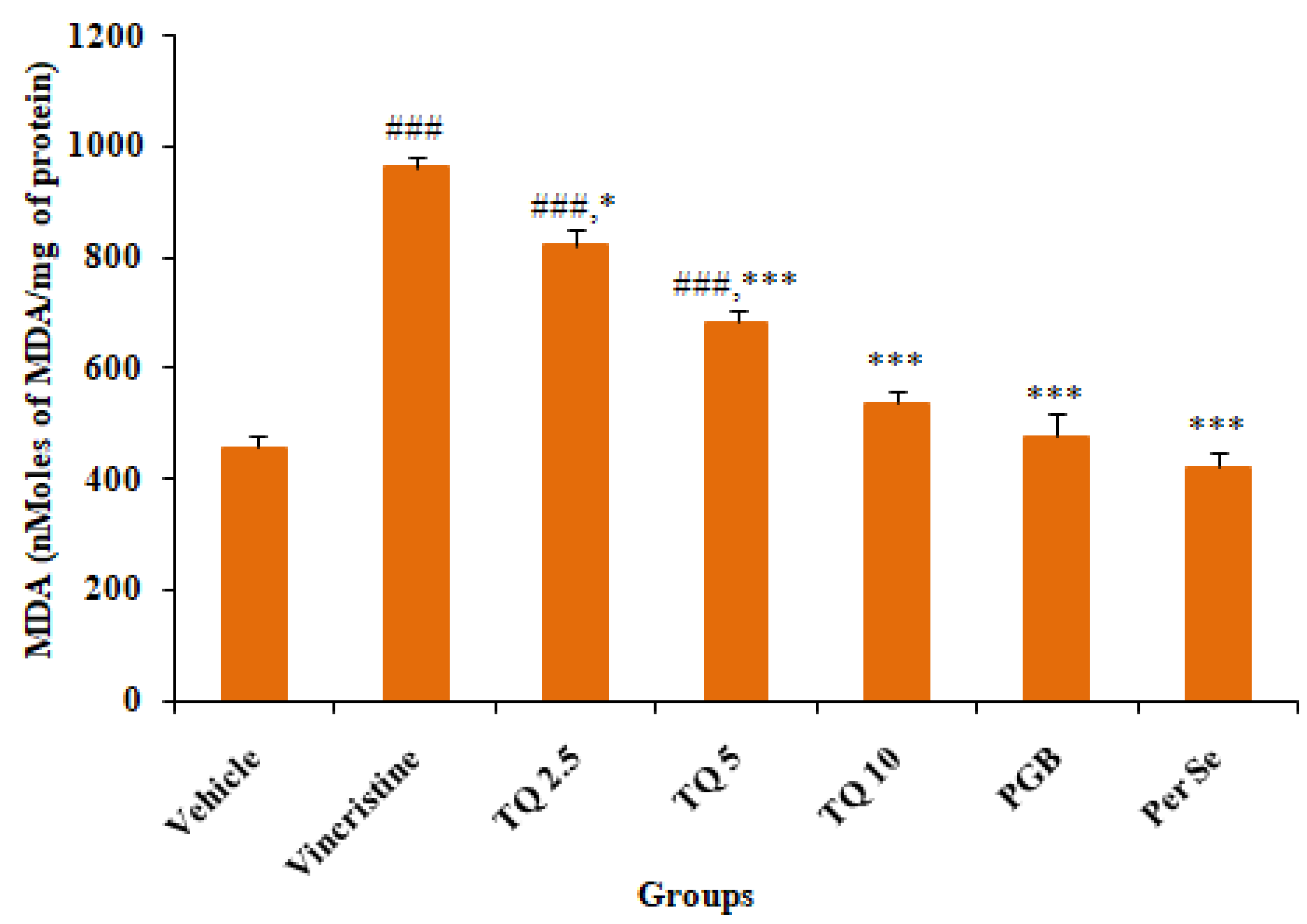
3.2.1. Effect of Thymoquinone on MDA Level in Sciatic Nerve Tissue
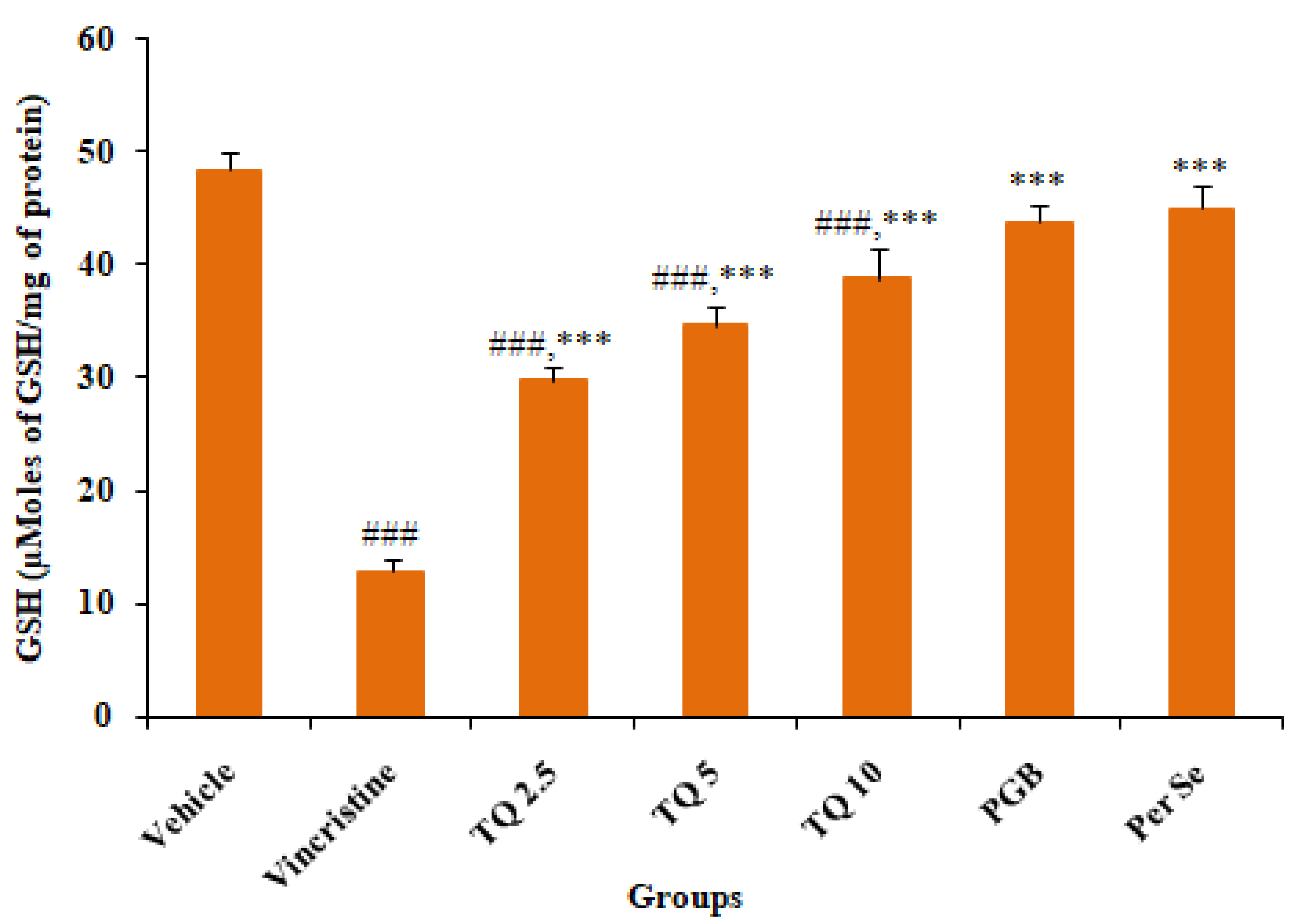
3.2.2. Effect of Reduced Glutathione (GSH) Level in Sciatic Nerve Tissue
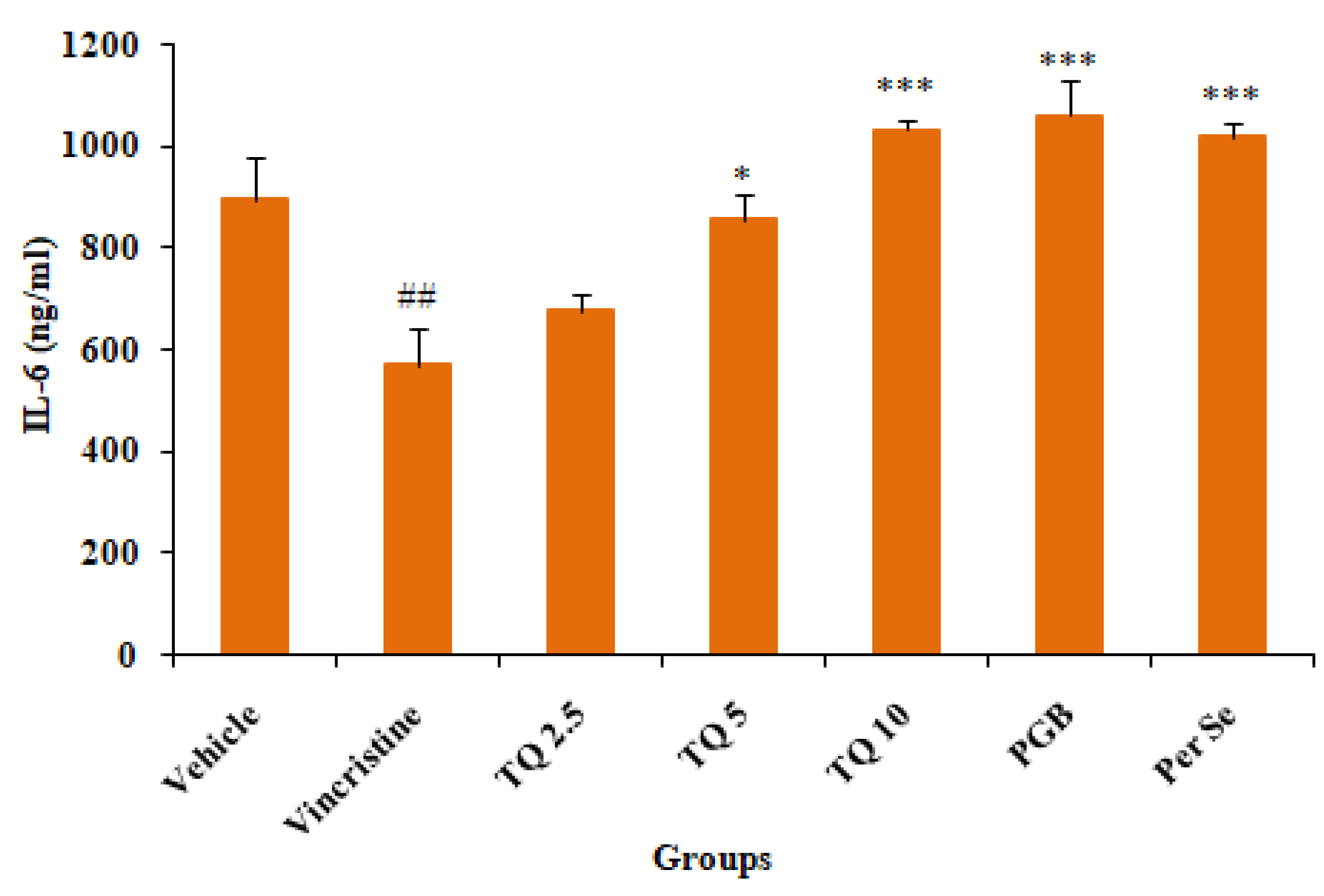
3.2.3. Effect of Thymoquinone on IL-6 Level in Serum
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaley, T.J.; DeAngelis, L.M. Therapy of Chemotherapy-induced Peripheral Neuropathy. Br. J. Haematol. 2009, 145, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Seretny, M.; Currie, G.L.; Sena, E.S.; Ramnarine, S.; Grant, R.; MacLeod, M.R.; Colvin, L.A.; Fallon, M. Incidence, Prevalence, and Predictors of Chemotherapy-Induced Peripheral Neuropathy: A Systematic Review and Meta-Analysis. Pain 2014, 155, 2461–2470. [Google Scholar] [CrossRef] [Green Version]
- Geisler, S.; Doan, R.A.; Strickland, A.; Huang, X.; Milbrandt, J.; DiAntonio, A. Prevention of Vincristine-Induced Peripheral Neuropathy by Genetic Deletion of SARM1 in Mice. Brain 2016, 139, 3092–3108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanner, K.D.; Levine, J.D.; Topp, K.S. Microtubule Disorientation and Axonal Swelling in Unmyelinated Sensory Axons during Vincristine-induced Painful Neuropathy in Rat. J. Comp. Neurol. 1998, 395, 481–492. [Google Scholar] [CrossRef]
- Barton, D.L.; Wos, E.J.; Qin, R.; Mattar, B.I.; Green, N.B.; Lanier, K.S.; Bearden, J.D.; Kugler, J.W.; Hoff, K.L.; Reddy, P.S. A Double-Blind, Placebo-Controlled Trial of a Topical Treatment for Chemotherapy-Induced Peripheral Neuropathy: NCCTG Trial N06CA. Support. Care Cancer 2011, 19, 833–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schloss, J.; Colosimo, M.; Vitetta, L. New Insights into Potential Prevention and Management Options for Chemotherapy-Induced Peripheral Neuropathy. Asia-Pac. J. Oncol. Nurs. 2016, 3, 73–85. [Google Scholar] [CrossRef]
- Gong, S.-S.; Li, Y.-X.; Zhang, M.-T.; Du, J.; Ma, P.-S.; Yao, W.-X.; Zhou, R.; Niu, Y.; Sun, T.; Yu, J.-Q. Neuroprotective Effect of Matrine in Mouse Model of Vincristine-Induced Neuropathic Pain. Neurochem. Res. 2016, 41, 3147–3159. [Google Scholar] [CrossRef]
- Shati, A.A. Sub-Chronic Administration of Vincristine Sulfate Induces Renal Damage and Apoptosis in Rats via Induction of Oxidative Stress and Activation of Raf1-MEK1/2-Erk1/2 Signal Transduction. Int. J. Morphol. 2019, 37, 273–283. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S. Interleukin-6 Trans-Signaling: A Pathway With Therapeutic Potential for Diabetic Retinopathy. Front. Physiol. 2021, 12, 611. [Google Scholar] [CrossRef]
- Robinson, R.; Srinivasan, M.; Shanmugam, A.; Ward, A.; Ganapathy, V.; Bloom, J.; Sharma, A.; Sharma, S. Interleukin-6 Trans-Signaling Inhibition Prevents Oxidative Stress in a Mouse Model of Early Diabetic Retinopathy. Redox Biol. 2020, 34, 101574. [Google Scholar] [CrossRef]
- Lavoie Smith, E.M.; Barton, D.L.; Qin, R.; Steen, P.D.; Aaronson, N.K.; Loprinzi, C.L. Assessing Patient-Reported Peripheral Neuropathy: The Reliability and Validity of the European Organization for Research and Treatment of Cancer QLQ-CIPN20 Questionnaire. Qual. Life Res. 2013, 22, 2787–2799. [Google Scholar] [CrossRef] [Green Version]
- Dworkin, R.H.; Malone, D.C.; Panarites, C.J.; Armstrong, E.P.; Pham, S.V. Impact of Postherpetic Neuralgia and Painful Diabetic Peripheral Neuropathy on Health Care Costs. J. Pain 2010, 11, 360–368. [Google Scholar] [CrossRef]
- Abdelrahman, K.M.; Hackshaw, K. V Nutritional Supplements for the Treatment of Neuropathic Pain. Biomedicines 2021, 9, 674. [Google Scholar] [CrossRef]
- Ellis, R.J.; Toperoff, W.; Vaida, F.; Van Den Brande, G.; Gonzales, J.; Gouaux, B.; Bentley, H.; Atkinson, J.H. Smoked Medicinal Cannabis for Neuropathic Pain in HIV: A Randomized, Crossover Clinical Trial. Neuropsychopharmacology 2009, 34, 672–680. [Google Scholar] [CrossRef] [Green Version]
- Zareba, G. Phytotherapy for Pain Relief. Drugs Today 2009, 45, 445–467. [Google Scholar] [CrossRef]
- Babu, A.; Prasanth, K.G.; Balaji, B. Effect of Curcumin in Mice Model of Vincristine-Induced Neuropathy. Pharm. Biol. 2015, 53, 838–848. [Google Scholar] [CrossRef]
- Anwar, M.J.; Alenezi, S.K.; Azam, F.; Mahmood, D.; Imam, F.; Alharbi, K.S. Nigella Sativa Oil Alleviates Doxorubicin-Induced Cardiomyopathy and Neurobehavioral Changes in Mice: In Vivo and in-Silico Study. Asian Pac. J. Trop. Biomed. 2022, 12, 312. [Google Scholar] [CrossRef]
- Ince, S.; Kucukkurt, I.; Demirel, H.H.; Turkmen, R.; Zemheri, F.; Akbel, E. The Role of Thymoquinone as Antioxidant Protection on Oxidative Stress Induced by Imidacloprid in Male and Female Swiss Albino Mice. Toxicol. Environ. Chem. 2013, 95, 318–329. [Google Scholar] [CrossRef]
- Jakaria, M.; Cho, D.-Y.; Haque, E.; Karthivashan, G.; Kim, I.-S.; Ganesan, P.; Choi, D.-K. Neuropharmacological Potential and Delivery Prospects of Thymoquinone for Neurological Disorders. Oxid. Med. Cell. Longev. 2018, 2018, 1209801. [Google Scholar] [CrossRef]
- Suddek, G.M. Protective Role of Thymoquinone against Liver Damage Induced by Tamoxifen in Female Rats. Can. J. Physiol. Pharmacol. 2014, 92, 640–644. [Google Scholar] [CrossRef]
- De Sousa, D.P.; Nóbrega, F.F.F.; Santos, C.C.M.P.; Benedito, R.B.; Vieira, Y.W.; Uliana, M.P.; Brocksom, T.J.; Almeida, R.N. de Anticonvulsant Activity of Thymoquinone and Its Structural Analogues. Rev. Bras. Farmacogn. 2011, 21, 427–431. [Google Scholar] [CrossRef] [Green Version]
- Gilhotra, N.; Dhingra, D. Thymoquinone produced antianxiety-like effects in mice through modulation of GABA and NO levels. Pharmacol. Rep. 2011, 63, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Aquib, M.; Najmi, A.K.; Akhtar, M. Antidepressant Effect of Thymoquinone in Animal Models of Depression. Drug Res. 2015, 65, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Rashid, A.K.; Abul, K.N.; Arshad, H.K.; Darpan, G.; Mohd, A. Ameliorating Effects of Thymoquinone in Rodent Models of Schizophrenia. African J. Pharm. Pharmacol. 2014, 8, 413–421. [Google Scholar] [CrossRef] [Green Version]
- Çelik, F.; Göçmez, C.; Karaman, H.; Kamaşak, K.; Kaplan, İ.; Akıl, E.; Tufek, A.; Guzel, A.; Uzar, E. Therapeutic Effects of Thymoquinone in a Model of Neuropathic Pain. Curr. Ther. Res. 2014, 76, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Kanter, M. Effects of Nigella Sativa and Its Major Constituent, Thymoquinone on Sciatic Nerves in Experimental Diabetic Neuropathy. Neurochem. Res. 2008, 33, 87–96. [Google Scholar] [CrossRef]
- Fouad, I.A.; Sharaf, N.M.; Abdelghany, R.M.; El Sayed, N.S.E.D. Neuromodulatory Effect of Thymoquinone in Attenuating Glutamate-Mediated Neurotoxicity Targeting the Amyloidogenic and Apoptotic Pathways. Front. Neurol. 2018, 9, 236. [Google Scholar] [CrossRef] [Green Version]
- Kuribara, H.; Higuchi, Y.; Tadokoro, S. Effects of Central Depressants on Rota-Rod and Traction Performances in Mice. Jpn. J. Pharmacol. 1977, 27, 117–126. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, V.; Kuhad, A.; Chopra, K. Emblica Officinalis Corrects Functional, Biochemical and Molecular Deficits in Experimental Diabetic Neuropathy by Targeting the Oxido-nitrosative Stress Mediated Inflammatory Cascade. Phyther. Res. 2011, 25, 1527–1536. [Google Scholar] [CrossRef]
- Beyreuther, B.; Callizot, N.; Stöhr, T. Antinociceptive Efficacy of Lacosamide in a Rat Model for Painful Diabetic Neuropathy. Eur. J. Pharmacol. 2006, 539, 64–70. [Google Scholar] [CrossRef]
- Khalilzadeh, O.; Anvari, M.; Khalilzadeh, A.; Sahebgharani, M.; Zarrindast, M.R. Involvement of Amlodipine, Diazoxide, and Glibenclamide in Development of Morphine Tolerance in Mice. Int. J. Neurosci. 2008, 118, 503–518. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Sedlak, J.; Lindsay, R.H. Estimation of Total, Protein-Bound, and Nonprotein Sulfhydryl Groups in Tissue with Ellman’s Reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef]
- Geis, C.; Beyreuther, B.K.; Stöhr, T.; Sommer, C. Lacosamide Has Protective Disease Modifying Properties in Experimental Vincristine Neuropathy. Neuropharmacology 2011, 61, 600–607. [Google Scholar] [CrossRef]
- Areti, A.; Yerra, V.G.; Naidu, V.G.M.; Kumar, A. Oxidative Stress and Nerve Damage: Role in Chemotherapy Induced Peripheral Neuropathy. Redox Biol. 2014, 2, 289–295. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Taylor, C.P.; Weber, M.; Piechan, J.; Prior, F.; Bian, F.; Cui, M.; Hoffman, D.; Donevan, S. Pregabalin Is a Potent and Selective Ligand for A2δ-1 and A2δ-2 Calcium Channel Subunits. Eur. J. Pharmacol. 2011, 667, 80–90. [Google Scholar] [CrossRef]
- Jones, M.R.; Urits, I.; Wolf, J.; Corrigan, D.; Colburn, L.; Peterson, E.; Williamson, A.; Viswanath, O. Drug-Induced Peripheral Neuropathy: A Narrative Review. Curr. Clin. Pharmacol. 2020, 15, 38–48. [Google Scholar]
- Gilli, F.; Royce, D.B.; Pachner, A.R. Measuring Progressive Neurological Disability in a Mouse Model of Multiple Sclerosis. J. Vis. Exp. 2016, 117, e54616. [Google Scholar] [CrossRef]
- Mo, M.; Erdelyi, I.; Szigeti-Buck, K.; Benbow, J.H.; Ehrlich, B.E. Prevention of Paclitaxel-Induced Peripheral Neuropathy by Lithium Pretreatment. FASEB J. 2012, 26, 4696. [Google Scholar] [CrossRef] [Green Version]
- Aley, K.O.; Reichling, D.B.; Levine, J.D. Vincristine Hyperalgesia in the Rat: A Model of Painful Vincristine Neuropathy in Humans. Neuroscience 1996, 73, 259–265. [Google Scholar] [CrossRef]
- Üstün, R.; Oğuz, E.K.; Şeker, A.; Korkaya, H. Thymoquinone Prevents Cisplatin Neurotoxicity in Primary DRG Neurons. Neurotoxicology 2018, 69, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, S.S.; Oryan, S.; Izadpanah, E.; Hassanzadeh, K. Thymoquinone Exerts Neuroprotective Effect in Animal Model of Parkinson’s Disease. Toxicol. Lett. 2017, 276, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Gülşen, İ.; Ak, H.; Kara, M.; Gokalp, A.; Akyol, V.; Koçak, Ö.; Rağbetli, M. The Acute Effects of Thymoquinone on Acute Peripheral Nerve Injury: An Experimental Study. Ulus Travma Acil Cerrahi Derg. 2016, 22, 526–530. [Google Scholar] [PubMed]
- Verma, R.; Sharma, J.; Singh, N.; Jaggi, A.S. Investigating the Possible Pain Attenuating Mechanisms of Pregabalin in Chronic Constriction Injury-Induced Neuropathic Pain in Rats. Int. J. Neurosci. 2019, 129, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Tjølsen, A.; Berge, O.-G.; Hunskaar, S.; Rosland, J.H.; Hole, K. The Formalin Test: An Evaluation of the Method. Pain 1992, 51, 5–17. [Google Scholar] [CrossRef]
- Salinas-Abarca, A.B.; Avila-Rojas, S.H.; Barragán-Iglesias, P.; Pineda-Farias, J.B.; Granados-Soto, V. Formalin Injection Produces Long-Lasting Hypersensitivity with Characteristics of Neuropathic Pain. Eur. J. Pharmacol. 2017, 797, 83–93. [Google Scholar] [CrossRef]
- Shibata, M.; Ohkubo, T.; Takahashi, H.; Inoki, R. Modified Formalin Test: Characteristic Biphasic Pain Response. Pain 1989, 38, 347–352. [Google Scholar] [CrossRef]
- Le Bars, D.; Gozariu, M.; Cadden, S.W. Animal Models of Nociception. Pharmacol. Rev. 2001, 53, 597–652. [Google Scholar]
- Tian, F.; Liu, R.; Fan, C.; Sun, Y.; Huang, X.; Nie, Z.; Zhao, X.; Pu, X. Effects of Thymoquinone on Small-Molecule Metabolites in a Rat Model of Cerebral Ischemia Reperfusion Injury Assessed Using MALDI-MSI. Metabolites 2020, 10, 27. [Google Scholar] [CrossRef] [Green Version]
- Kartha, S.; Yan, L.; Weisshaar, C.L.; Ita, M.E.; Shuvaev, V.V.; Muzykantov, V.R.; Tsourkas, A.; Winkelstein, B.A.; Cheng, Z. Superoxide Dismutase-Loaded Porous Polymersomes as Highly Efficient Antioxidants for Treating Neuropathic Pain. Adv. Healthc. Mater. 2017, 6, 1700500. [Google Scholar] [CrossRef]
- Wu, G.; Fang, Y.Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef] [Green Version]
- Richner, M.; Ferreira, N.; Dudele, A.; Jensen, T.S.; Vaegter, C.B.; Gonçalves, N.P. Functional and Structural Changes of the Blood-Nerve-Barrier in Diabetic Neuropathy. Front. Neurosci. 2019, 12, 1038. [Google Scholar] [CrossRef]
- Hashem, K.S.; Abdelazem, A.Z.; Mohammed, M.A.; Nagi, A.M.; Aboulhoda, B.E.; Mohammed, E.T.; Abdel-Daim, M.M. Thymoquinone Alleviates Mitochondrial Viability and Apoptosis in Diclofenac-Induced Acute Kidney Injury (AKI) via Regulating Mfn2 and MiR-34a MRNA Expressions. Environ. Sci. Pollut. Res. 2021, 28, 10100–10113. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Alnuqaydan, A.M.; Alsahli, M.A.; Khan, A.A.; Rahmani, A.H. Thymoquinone, the Most Prominent Constituent of Nigella Sativa, Attenuates Liver Damage in Streptozotocin-Induced Diabetic Rats via Regulation of Oxidative Stress, Inflammation and Cyclooxygenase-2 Protein Expression. Appl. Sci. 2021, 11, 3223. [Google Scholar] [CrossRef]
- Cox, A.A.; Sagot, Y.; Hedou, G.; Grek, C.; Wilkes, T.; Vinik, A.I.; Ghatnekar, G. Low-Dose Pulsatile Interleukin-6 as a Treatment Option for Diabetic Peripheral Neuropathy. Front. Endocrinol. 2017, 8, 89. [Google Scholar] [CrossRef]
- Bolin, L.M.; Verity, A.N.; Silver, J.E.; Shooter, E.M.; Abrams, J.S. Interleukin-6 Production by Schwann Cells and Induction in Sciatic Nerve Injury. J. Neurochem. 1995, 64, 850–858. [Google Scholar] [CrossRef]
- Murphy, P.G.; Grondin, J.; Altares, M.; Richardson, P.M. Induction of Interleukin-6 in Axotomized Sensory Neurons. J. Neurosci. 1995, 15, 5130–5138. [Google Scholar] [CrossRef] [Green Version]
- Gadient, R.A.; Otten, U.H. Interleukin-6 (IL-6)—A Molecule with Both Beneficial and Destructive Potentials. Prog. Neurobiol. 1997, 52, 379–390. [Google Scholar] [CrossRef]
- Forcina, L.; Miano, C.; Scicchitano, B.M.; Rizzuto, E.; Berardinelli, M.G.; De Benedetti, F.; Pelosi, L.; Musarò, A. Increased circulating levels of interleukin-6 affect the redox balance in skeletal muscle. Oxid. Med. Cell. Longev. 2019, 2019, 3018584. [Google Scholar] [CrossRef]
| Treatment (n = 5) | Dose | Acute Phase | Delayed Phase | ||
|---|---|---|---|---|---|
| No. of Flinches | Licking Duration (Sec) | No. of Flinches | Licking Duration (Sec) | ||
| Vehicle | 10 mL/kg, p.o. | 4.4 ± 0.812 | 44.2 ± 8.49 | 1.4 ± 0.509 | 11.82 ± 3.251 |
| Vincristine | 0.1 mg/kg, sc | 161.0 ± 4.701 ### | 254.29 ± 27.55 ### | 38.2 ± 6.98 ### | 34.47 ± 2.741 ### |
| TQ 2.5 | 2.5 mg/kg, p.o. | 134.8 ± 9.521 ### | 226.76 ± 18.81 ### | 21.4 ± 5.202 #,* | 23.96 ± 1.172 ##,* |
| TQ 5 | 5 mg/kg, p.o. | 106.2 ± 4.454 ###,** | 172.9 ± 18.50 ###,* | 19.0 ± 2.51 #,* | 20.21 ± 0.785 *** |
| TQ 10 | 10 mg/kg, p.o. | 80.8 ± 5.544 ###,*** | 147.43 ± 8.03 ##,** | 12.4 ± 2.315 *** | 14.32 ± 1.261 *** |
| PGB | 10 mg/kg, p.o. | 78.0 ± 17.595 ###,*** | 142.77 ± 18.08 ##,** | 8.4 ± 1.887 *** | 12.14 ± 1.599 *** |
| Per se | 10 mg/kg, p.o. | 80.0 ± 5.568 ###,*** | 132.2 ± 16.18 #,*** | 5.6 ± 1.965 *** | 16.14 ± 2.018 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alenezi, S.K. The Ameliorative Effect of Thymoquinone on Vincristine-Induced Peripheral Neuropathy in Mice by Modulating Cellular Oxidative Stress and Cytokine. Life 2023, 13, 101. https://doi.org/10.3390/life13010101
Alenezi SK. The Ameliorative Effect of Thymoquinone on Vincristine-Induced Peripheral Neuropathy in Mice by Modulating Cellular Oxidative Stress and Cytokine. Life. 2023; 13(1):101. https://doi.org/10.3390/life13010101
Chicago/Turabian StyleAlenezi, Sattam Khulaif. 2023. "The Ameliorative Effect of Thymoquinone on Vincristine-Induced Peripheral Neuropathy in Mice by Modulating Cellular Oxidative Stress and Cytokine" Life 13, no. 1: 101. https://doi.org/10.3390/life13010101
APA StyleAlenezi, S. K. (2023). The Ameliorative Effect of Thymoquinone on Vincristine-Induced Peripheral Neuropathy in Mice by Modulating Cellular Oxidative Stress and Cytokine. Life, 13(1), 101. https://doi.org/10.3390/life13010101






