Cordyceps militaris Inhibited Angiotensin-Converting Enzyme through Molecular Interaction between Cordycepin and ACE C-Domain
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Cordyceps militaris Ethanolic Extract
2.2. UHPLC-FD Analysis
2.3. Measurement of ACE Inhibitory Activity
2.4. Molecular Docking between ACE C-Domain and Cordycepin
2.5. Statistical Analysis
3. Results
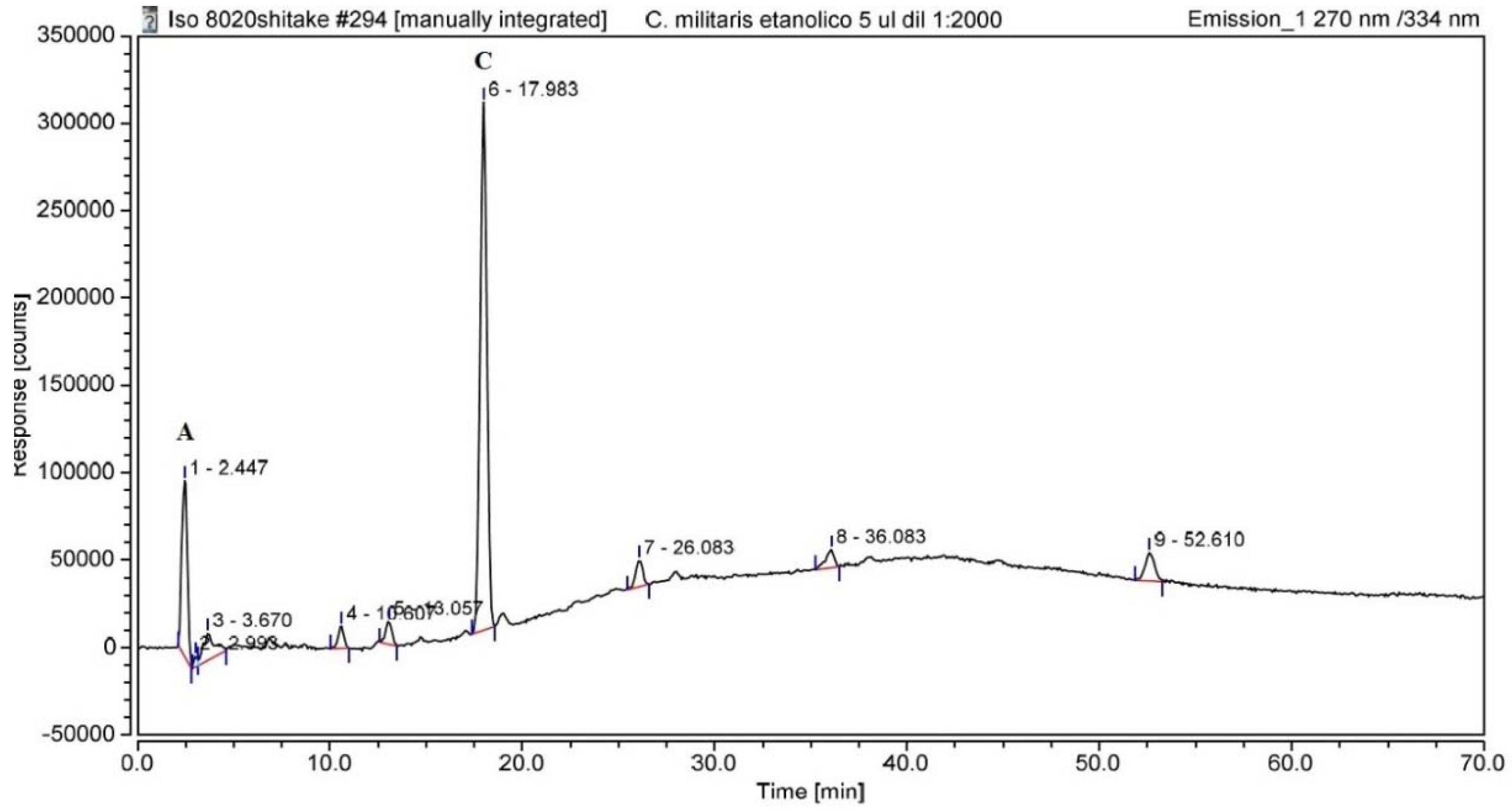
3.1. UHPLC Analysis
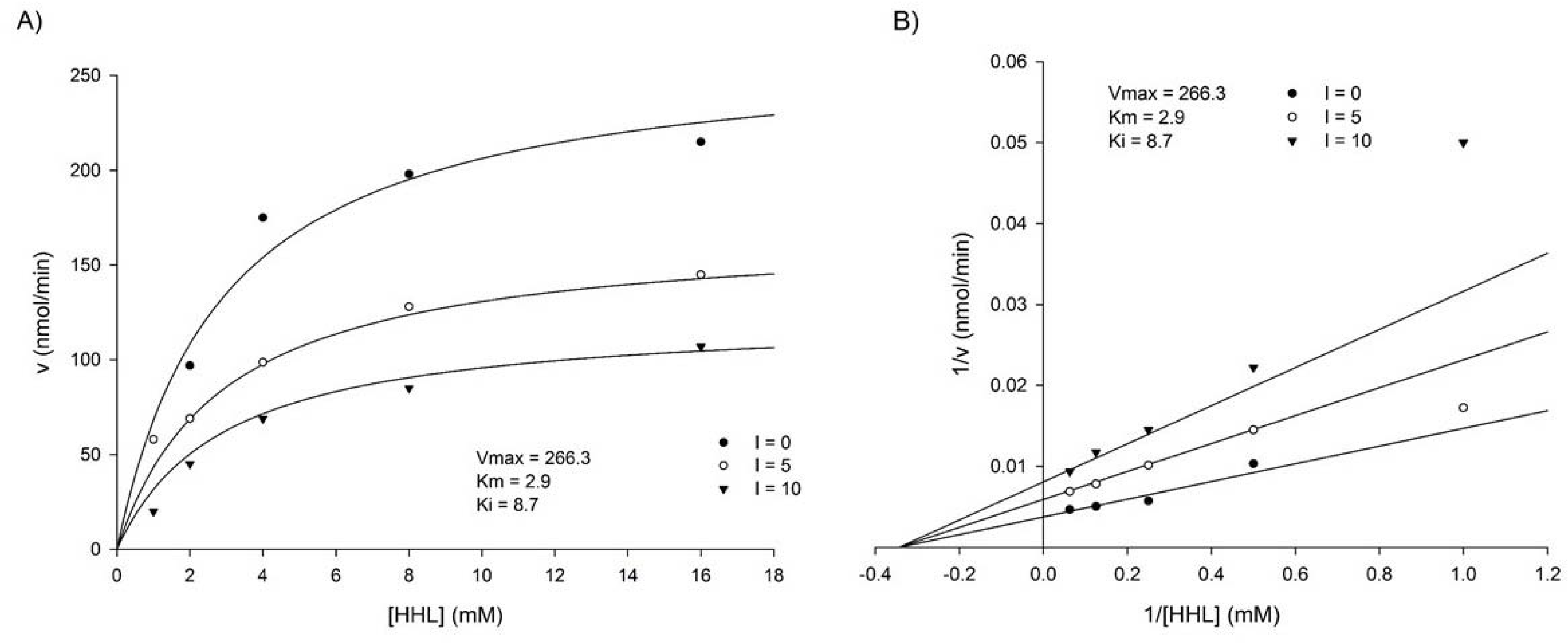
3.2. Kinetics of ACE Inhibition
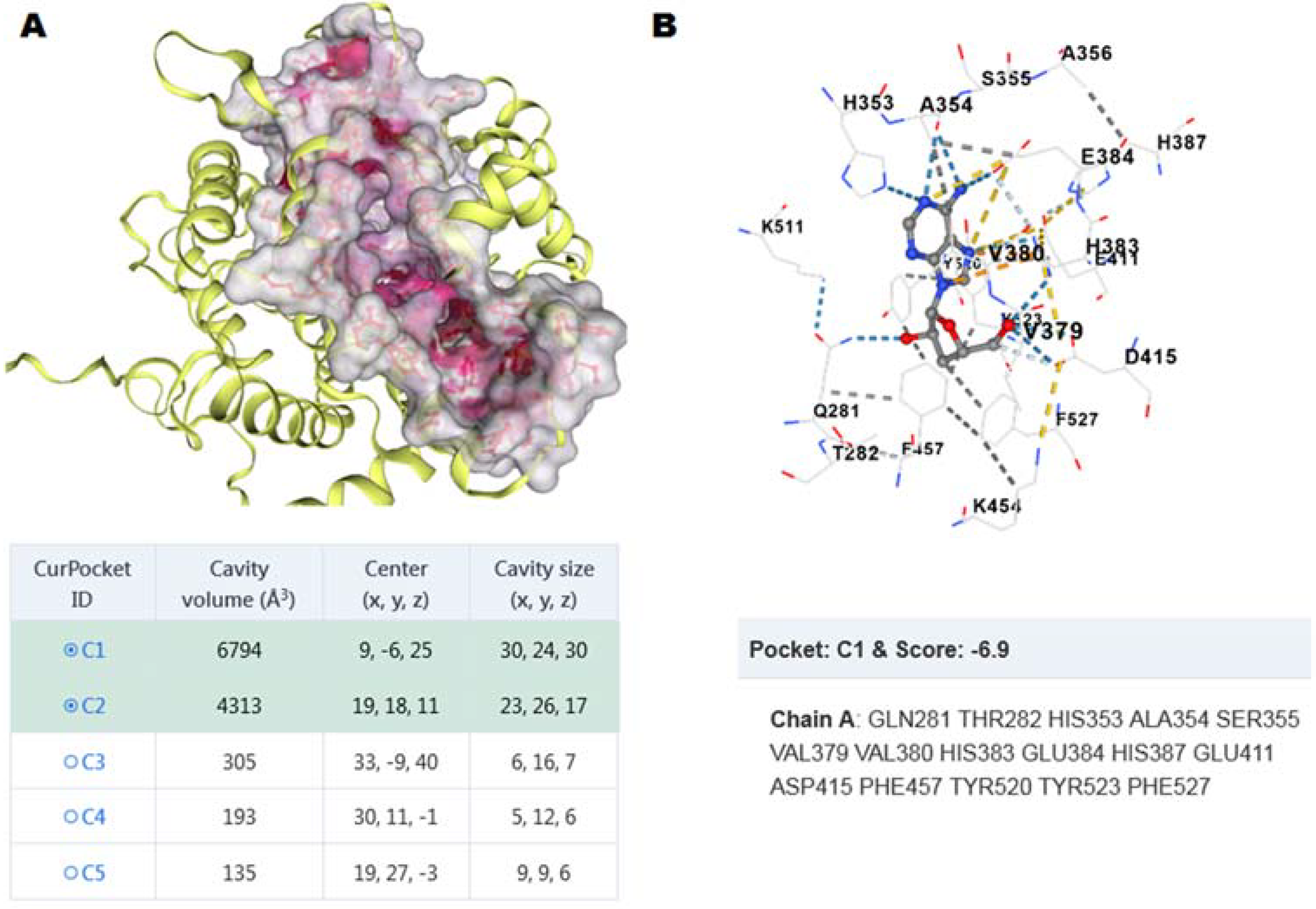
3.3. Molecular Docking between ACE C-Domain and Cordycepin
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- INEGI. Características de Las Defunciones Registradas En México Durante 2018; Comunicado De Prensa Núm. 538/19; Instituto Nacional de Estadistica y Geografia (INEGI): México City, Mexico, 2019; pp. 1–65.
- Alcocer, L.; Álvarez-López, H.; Borrayo-Sánchez, G.; Cardona-Muñoz, E.; Chávez-Mendoza, A. Hypertension as a Persistent Public Health Problem. A Position Paper from Alliance for a Healthy Heart, Mexico. Ann. Clin. Hypertens. 2019, 3, 9–30. [Google Scholar] [CrossRef]
- Sparks, M.A.; Crowley, S.D.; Gurley, S.B.; Mirotsou, M.; Coffman, T.M. Classical Renin-Angiotensin System in Kidney Physiology. Compr. Physiol. 2014, 4, 1201–1228. [Google Scholar] [CrossRef]
- Campbell, D.J. The Renin–Angiotensin and the Kallikrein–Kinin Systems. Int. J. Biochem. Cell Biol. 2003, 35, 784–791. [Google Scholar] [CrossRef]
- Fernández-Musoles, R.; Manzanares, P.; Burguete, M.C.; Alborch, E.; Salom, J.B. In Vivo Angiotensin I-Converting Enzyme Inhibition by Long-Term Intake of Antihypertensive Lactoferrin Hydrolysate in Spontaneously Hypertensive Rats. Food Res. Int. 2013, 54, 627–632. [Google Scholar] [CrossRef]
- Aluko, R.E. Food Protein-Derived Renin-Inhibitory Peptides: In Vitro and in Vivo Properties. J. Food Biochem. 2019, 43, e12648. [Google Scholar] [CrossRef]
- Cheung, I.W.Y.; Nakayama, S.; Hsu, M.N.K.; Samaranayaka, A.G.P.; Li-Chan, E.C.Y. Angiotensim-I Converting Enzyme Inhibitory Activity of Hydrolysates from Oat (Avena Sativa) Proteins by in Silico and in Vitro Analyses. J. Agric. Food Chem. 2009, 57, 9234–9242. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Masuda, M.; Sakurai, A.; Sakakibara, M. Medicinal Uses of the Mushroom Cordyceps Militaris: Current State and Prospects. Fitoterapia 2010, 81, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Ho, K.J.; Hsieh, Y.J.; Wang, L.T.; Mau, J.L. Contents of Lovastatin, γ-Aminobutyric Acid and Ergothioneine in Mushroom Fruiting Bodies and Mycelia. LWT—Food Sci. Technol. 2012, 47, 274–278. [Google Scholar] [CrossRef]
- Chan, J.S.L.; Barseghyan, G.S.; Asatiani, M.D.; Wasser, S.P. Chemical Composition and Medicinal Value of Fruiting Bodies and Submerged Cultured Mycelia of Caterpillar Medicinal Fungus Cordyceps Militaris CBS-132098 (Ascomycetes). Int. J. Med. Mushrooms 2015, 17, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Cohen, N.; Cohen, J.; Asatiani, M.D.; Varshney, V.K.; Yu, H.T.; Yang, Y.C.; Li, Y.H.; Mau, J.L.; Wasser, S.P. Chemical Composition and Nutritional and Medicinal Value of Fruit Bodies and Submerged Cultured Mycelia of Culinary-Medicinal Higher Basidiomycetes Mushrooms. Int. J. Med. Mushrooms 2014, 16, 273–291. [Google Scholar] [CrossRef] [PubMed]
- Hur, H. Chemical Ingredients of Cordyceps Militaris. Mycobiology 2008, 36, 233. [Google Scholar] [CrossRef] [PubMed]
- Williamson, R.D.; McCarthy, F.P.; Manna, S.; Groarke, E.; Kell, D.B.; Kenny, L.C.; McCarthy, C.M. L-(+)-Ergothioneine Significantly Improves the Clinical Characteristics of Preeclampsia in the Reduced Uterine Perfusion Pressure Rat Model. Hypertension 2020, 75, 561–568. [Google Scholar] [CrossRef]
- Maaliki, D.; Shaito, A.A.; Pintus, G.; El-Yazbi, A.; Eid, A.H. Flavonoids in Hypertension: A Brief Review of the Underlying Mechanisms. Curr. Opin. Pharmacol. 2019, 45, 57–65. [Google Scholar] [CrossRef]
- Cushman, D.W.; Cheung, H.S. Spectrophotometric Assay and Properties of the Angiotensin-Converting Enzyme of Rabbit Lung. Biochem. Pharmacol. 1971, 20, 1637–1648. [Google Scholar] [CrossRef]
- Riniker, S.; Landrum, G.A. Better Informed Distance Geometry: Using What We Know to Improve Conformation Generation. J. Chem. Inf. Model. 2015, 55, 2562–2574. [Google Scholar] [CrossRef]
- Shelley, J.C.; Cholleti, A.; Frye, L.L.; Greenwood, J.R.; Timlin, M.R.; Uchimaya, M. Epik: A Software Program for PK(a) Prediction and Protonation State Generation for Drug-like Molecules. J. Comput. Aided. Mol. Des. 2007, 21, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, X.; Gan, J.; Chen, S.; Xiao, Z.-X.; Cao, Y. CB-Dock2: Improved Protein–Ligand Blind Docking by Integrating Cavity Detection, Docking and Homologous Template Fitting. Nucleic Acids Res. 2022, 50, W159–W164. [Google Scholar] [CrossRef] [PubMed]
- Adasme, M.F.; Linnemann, K.L.; Bolz, S.N.; Kaiser, F.; Salentin, S.; Haupt, V.J.; Schroeder, M. PLIP 2021: Expanding the Scope of the Protein-Ligand Interaction Profiler to DNA and RNA. Nucleic Acids Res. 2021, 49, W530–W534. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, Z.; Bao, D.; Li, B.; Yin, X.; Wu, Y.; Chen, H.; Tang, G.; Li, N.; Zou, G. Improving Hypoxia Adaption Causes Distinct Effects on Growth and Bioactive Compounds Synthesis in an Entomopathogenic Fungus Cordyceps Militaris. Front. Microbiol. 2021, 12, 1624. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.; Lee, H.H.; Park, I.; Seo, Y.S. Development of High Cordycepin-Producing Cordyceps Militaris Strains. Mycobiology 2017, 45, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Kontogiannatos, D.; Koutrotsios, G.; Xekalaki, S.; Zervakis, G.I. Biomass and Cordycepin Production by the Medicinal Mushroom Cordyceps Militaris—A Review of Various Aspects and Recent Trends towards the Exploitation of a Valuable Fungus. J. Fungi 2021, 7, 986. [Google Scholar] [CrossRef] [PubMed]
- Schwager, S.L.; Carmona, A.K.; Sturrock, E.D. A High-Throughput Fluorimetric Assay for Angiotensin I-Converting Enzyme. Nat. Protoc. 2006, 1, 1961–1964. [Google Scholar] [CrossRef] [PubMed]
- Won, K.J.; Lee, S.C.; Lee, C.K.; Lee, H.M.; Lee, S.H.; Fang, Z.; Choi, O.B.; Jin, M.; Kim, J.; Park, T.; et al. Cordycepin Attenuates Neointimal Formation by Inhibiting Reactive Oxygen Species-Mediated Responses in Vascular Smooth Muscle Cells in Rats. J. Pharmacol. Sci. 2009, 109, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Lian, Z.Q.; Zhu, P.; Zhu, H.B. Lipid-Lowering Effect of Cordycepin (3′-Deoxyadenosine) from Cordyceps Militaris on Hyperlipidemic Hamsters and Rats. Yaoxue Xuebao 2011, 46, 669–676. [Google Scholar]
- Uen, W.-C.; Shi, Y.-C.; Choong, C.-Y.; Tai, C.-J. Cordycepin Suppressed Lipid Accumulation via Regulating AMPK Activity and Mitochondrial Fusion in Hepatocytes. J. Food Biochem. 2018, 42, e12569. [Google Scholar] [CrossRef]
- Caballero, J. Considerations for Docking of Selective Angiotensin-Converting Enzyme Inhibitors. Molecules 2020, 25, 295. [Google Scholar] [CrossRef] [PubMed]
- Nussberger, J.; Cugno, M.; Amstutz, C.; Cicardi, M.; Pellacani, A.; Agostoni, A. Plasma Bradykinin in Angio-Oedema. Lancet 1998, 351, 1693–1697. [Google Scholar] [CrossRef]
- Bernstein, K.E.; Shen, X.Z.; Gonzalez-Villalobos, R.A.; Billet, S.; Okwan-Duodu, D.; Ong, F.S.; Fuchs, S. Different in Vivo Functions of the Two Catalytic Domains of Angiotensin-Converting Enzyme (ACE). Curr. Opin. Pharmacol. 2011, 11, 105–111. [Google Scholar] [CrossRef]
- Fuchs, S.; Xiao, H.D.; Hubert, C.; Michaud, A.; Campbell, D.J.; Adams, J.W.; Capecchi, M.R.; Corvol, P.; Bernstein, K.E. Angiotensin-Converting Enzyme C-Terminal Catalytic Domain Is the Main Site of Angiotensin I Cleavage in Vivo. Hypertension 2008, 51, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Yin, C. The Anti-Inflammatory Peptide Ac-SDKP: Synthesis, Role in ACE Inhibition, and Its Therapeutic Potential in Hypertension and Cardiovascular Diseases. Pharmacol. Res. 2018, 134, 268–279. [Google Scholar] [CrossRef]
- Messerli, F.H.; Nussberger, J. Vasopeptidase Inhibition and Angio-Oedema. Lancet 2000, 356, 608–609. [Google Scholar] [CrossRef]
- Cotton, J.; Hayashi, M.A.F.; Cuniasse, P.; Vazeux, G.; Ianzer, D.; De Camargo, A.C.M.; Dive, V. Selective Inhibition of the C-Domain of Angiotensin I Converting Enzyme by Bradykinin Potentiating Peptides. Biochemistry 2002, 41, 6065–6071. [Google Scholar] [CrossRef] [PubMed]
- Sharma, U.; Rhaleb, N.E.; Pokharel, S.; Harding, P.; Rasoul, S.; Peng, H.; Carretero, O.A. Novel Anti-Inflammatory Mechanisms of N-Acetyl-Ser-Asp-Lys-Pro in Hypertension-Induced Target Organ Damage. Am. J. Physiol. Hear. Circ. Physiol. 2008, 294, H1226–H1232. [Google Scholar] [CrossRef] [PubMed]
- Fong, P.; Tong, H.H.Y.; Chao, C.M. In Silico Prediction of Tyrosinase and Adenylyl Cyclase Inhibitors from Natural Compounds. Nat. Prod. Commun. 2014, 9, 189–194. [Google Scholar] [CrossRef]
- Forero, D.P.; Masatani, C.; Fujimoto, Y.; Coy-Barrera, E.; Peterson, D.G.; Osorio, C. Spermidine Derivatives in Lulo (Solanum Quitoense Lam.) Fruit: Sensory (Taste) versus Biofunctional (ACE-Inhibition) Properties. J. Agric. Food Chem. 2016, 64, 5375–5383. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdez-Solana, M.A.; Corral-Guerrero, I.A.; Téllez-Valencia, A.; Avitia-Domínguez, C.; Meza-Velázquez, J.A.; de Casa, A.G.; Sierra-Campos, E. Cordyceps militaris Inhibited Angiotensin-Converting Enzyme through Molecular Interaction between Cordycepin and ACE C-Domain. Life 2022, 12, 1450. https://doi.org/10.3390/life12091450
Valdez-Solana MA, Corral-Guerrero IA, Téllez-Valencia A, Avitia-Domínguez C, Meza-Velázquez JA, de Casa AG, Sierra-Campos E. Cordyceps militaris Inhibited Angiotensin-Converting Enzyme through Molecular Interaction between Cordycepin and ACE C-Domain. Life. 2022; 12(9):1450. https://doi.org/10.3390/life12091450
Chicago/Turabian StyleValdez-Solana, Mónica A., Iván A. Corral-Guerrero, Alfredo Téllez-Valencia, Claudia Avitia-Domínguez, Jorge A. Meza-Velázquez, Atahualpa Guzmán de Casa, and Erick Sierra-Campos. 2022. "Cordyceps militaris Inhibited Angiotensin-Converting Enzyme through Molecular Interaction between Cordycepin and ACE C-Domain" Life 12, no. 9: 1450. https://doi.org/10.3390/life12091450
APA StyleValdez-Solana, M. A., Corral-Guerrero, I. A., Téllez-Valencia, A., Avitia-Domínguez, C., Meza-Velázquez, J. A., de Casa, A. G., & Sierra-Campos, E. (2022). Cordyceps militaris Inhibited Angiotensin-Converting Enzyme through Molecular Interaction between Cordycepin and ACE C-Domain. Life, 12(9), 1450. https://doi.org/10.3390/life12091450





