Usnic Acid Isolated from Usnea antarctica (Du Rietz) Reduced In Vitro Angiogenesis in VEGF- and bFGF-Stimulated HUVECs and Ex Ovo in Quail Chorioallantoic Membrane (CAM) Assay
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Collection, Extraction, and Characterization of Usnic Acid
2.3. Cell Culture
2.4. Methyl–Thiazole–Tetrazolium (MTT) Assay
2.5. Scratch Assay (Wound Healing)
2.6. Tube Formation Assay
2.7. Fibrin Bead Sprouting Assay
2.8. Ex Ovo CAM Assay
2.9. Alcian Blue Staining of CAM Membrane
2.10. Statistical Analyses
3. Results
3.1. Analysis of Usnic Acid
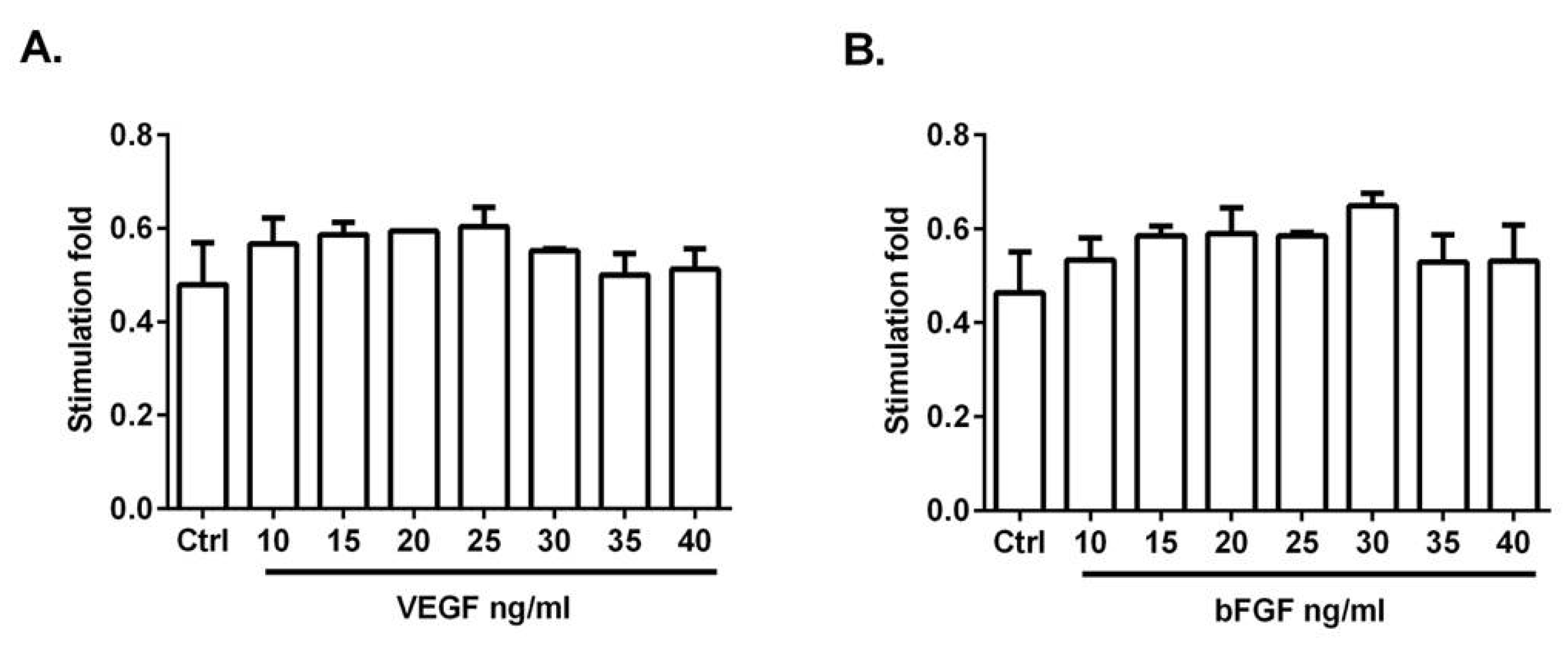
3.2. VEGF and bFGF Promote HUVEC Proliferation
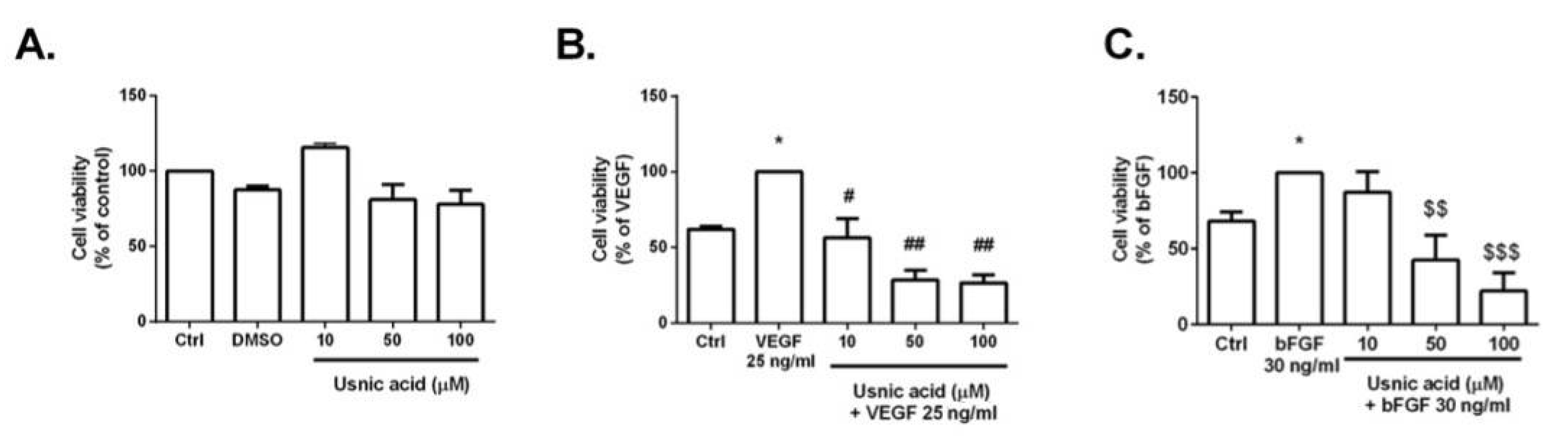
3.3. MTT Assay
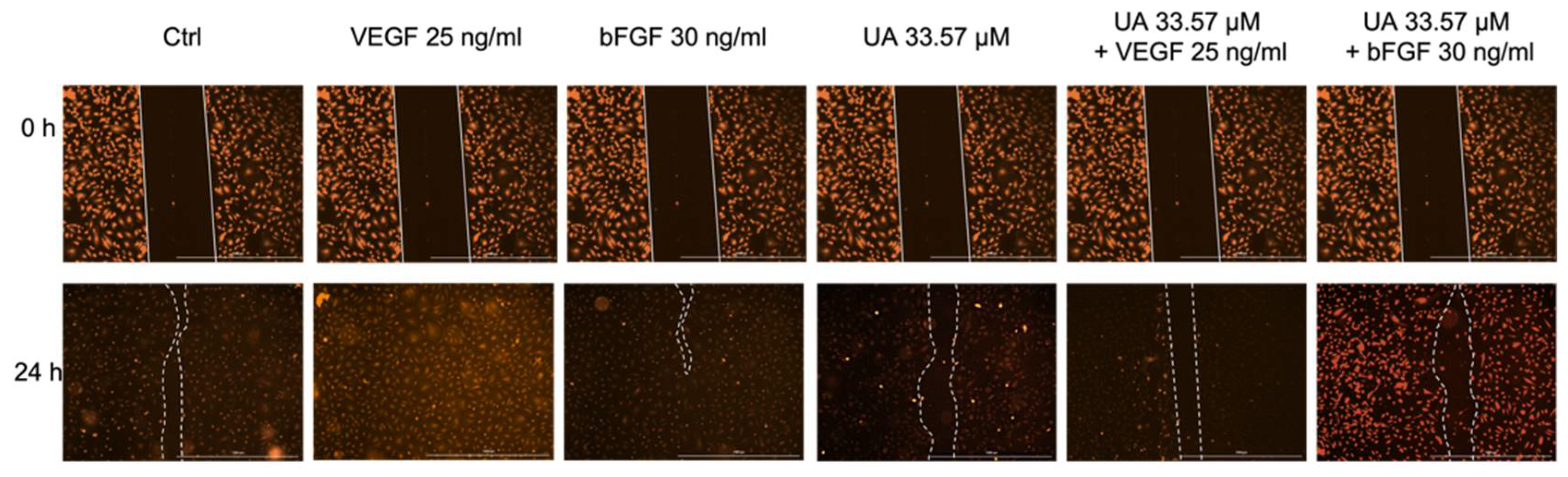
3.4. Usnic Acid Inhibited the Migration of VEGF- and bFGF-Stimulated HUVECs
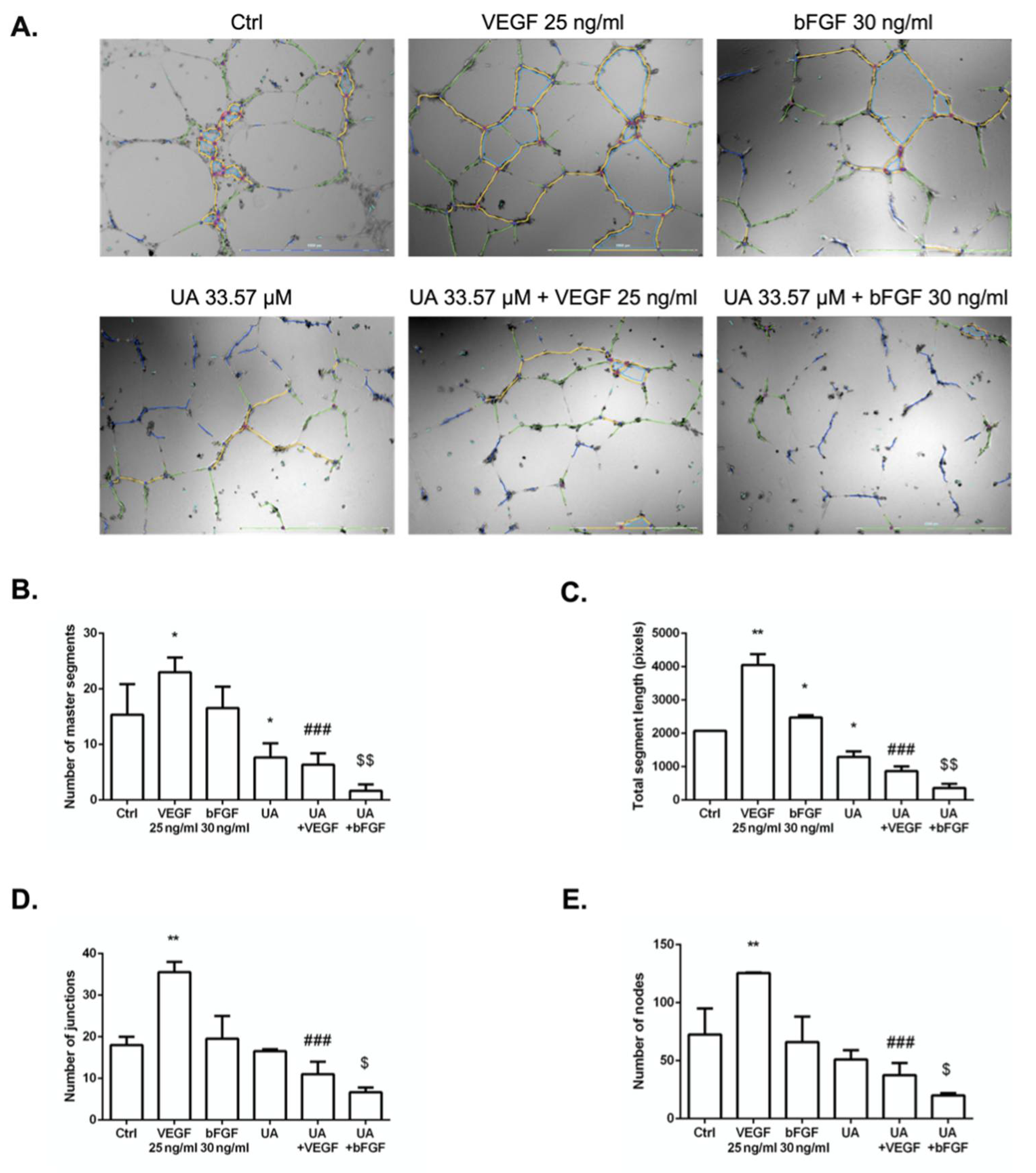
3.5. Usnic Acid Blocked the Formation of HUVECs Tubule in the Presence of VEGF and bFGF
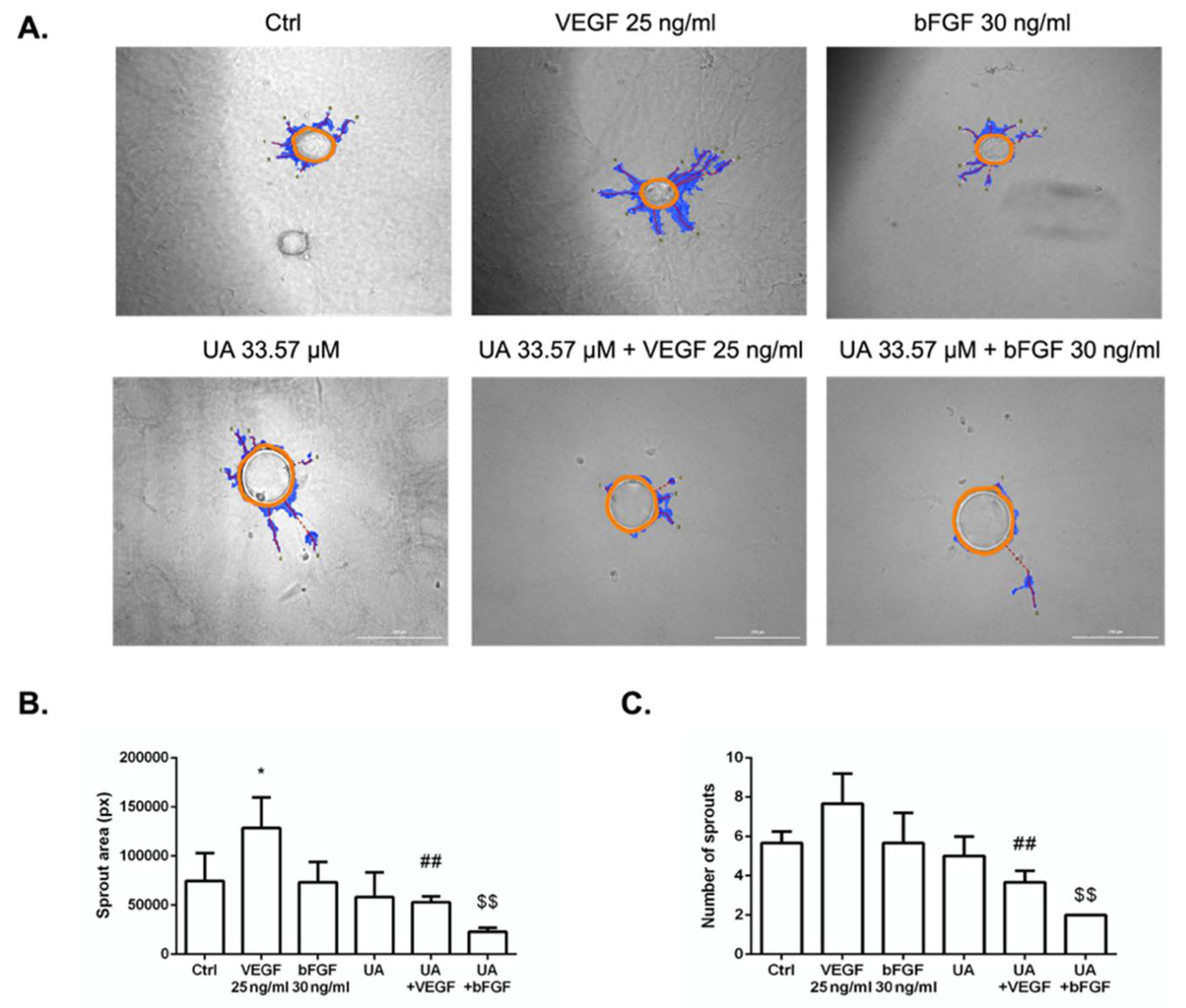
3.6. Fibrin Bead Sprouting Assay
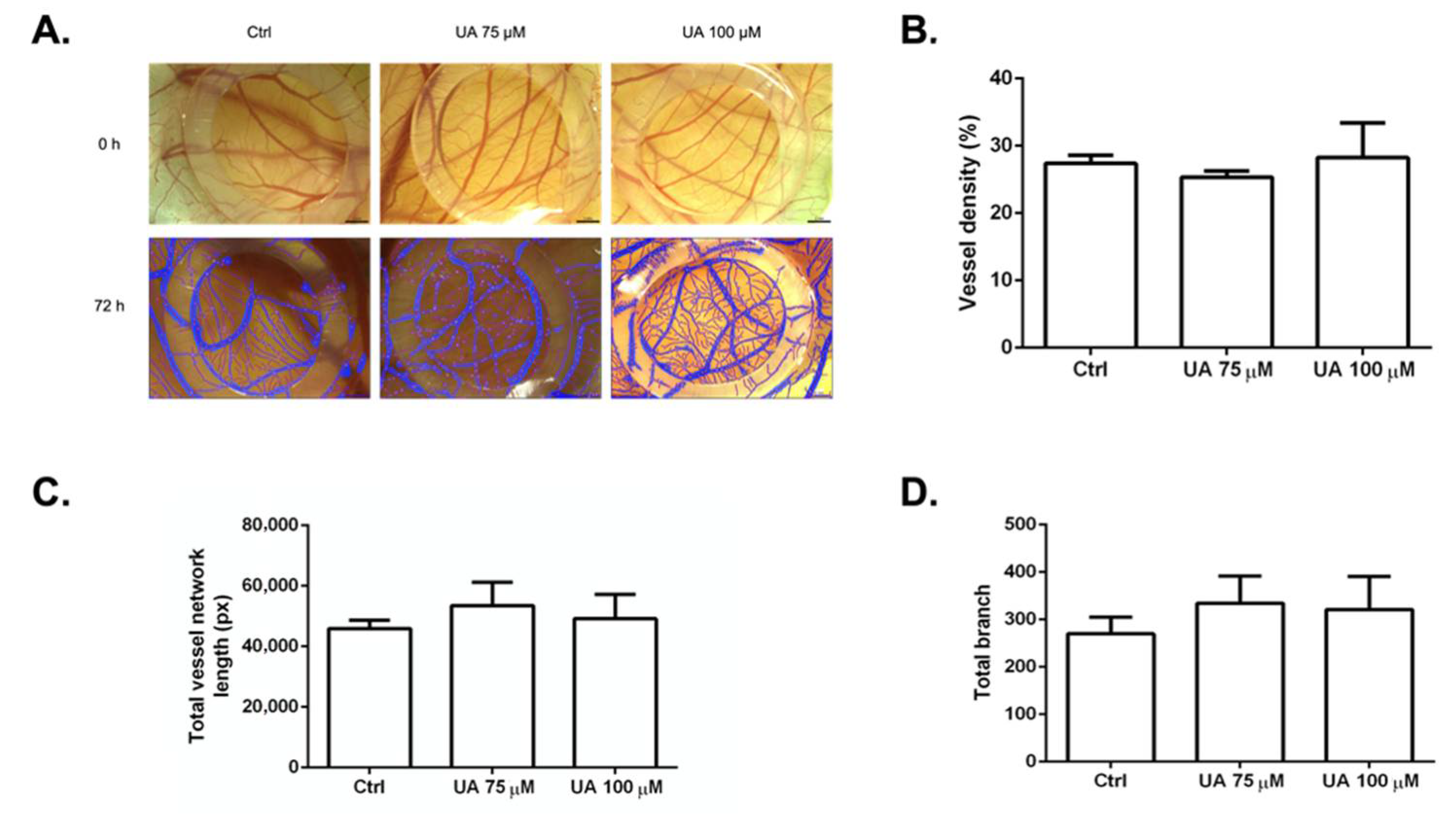
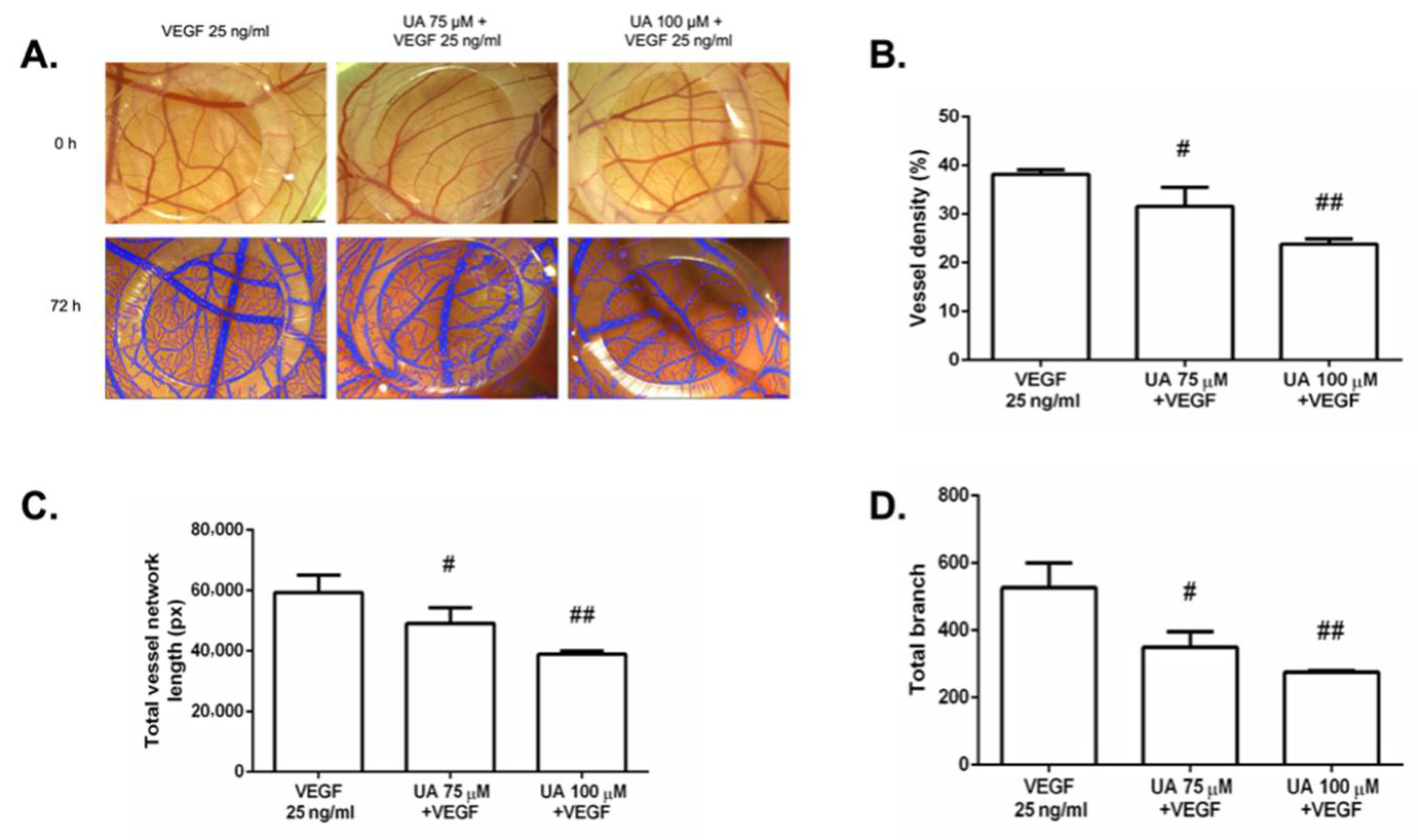
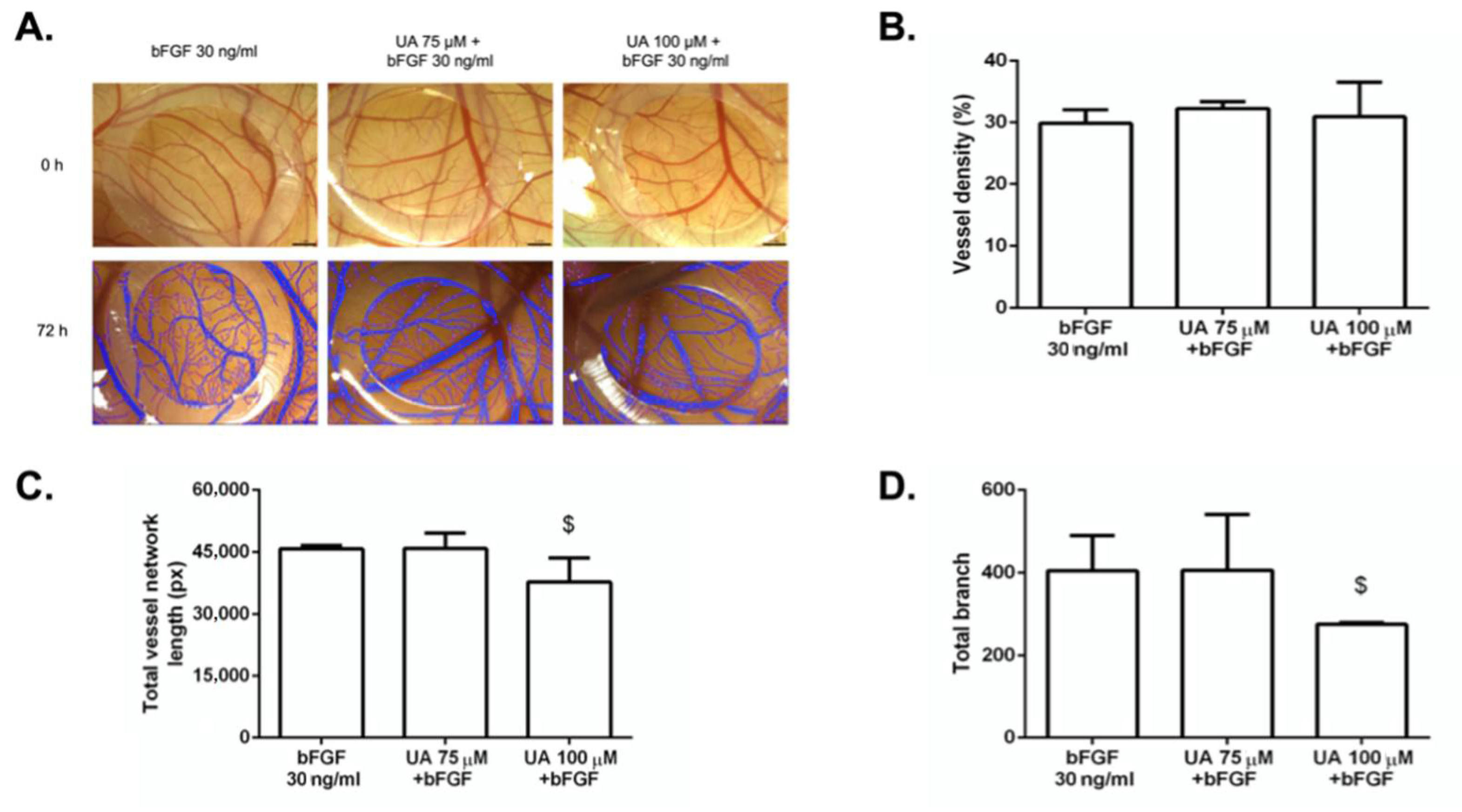
3.7. The Anti-Angiogenic Effect of UA on Vascularization of Quail CAM Model
3.8. Histological Examination of CAM Treated with Usnic Acid
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, M.-C.; Lee, C.-F.; Huang, W.-H.; Chou, T.-C. Magnolol Suppresses Hypoxia-Induced Angiogenesis via Inhibition of HIF-1α/VEGF Signaling Pathway in Human Bladder Cancer Cells. Biochem. Pharmacol. 2013, 85, 1278–1287. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Molecular Mechanisms and Clinical Applications of Angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, L.M.; Parris, E.E.; Folkman, J. Tumor Angiogenesis: Therapeutic Implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [CrossRef]
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E.; et al. Bevacizumab plus Irinotecan, Fluorouracil, and Leucovorin for Metastatic Colorectal Cancer. N. Engl. J. Med. 2004, 350, 2335–2342. [Google Scholar] [CrossRef]
- Folkman, J.; Merler, E.; Abernathy, C.; Williams, G. Isolation of a Tumor Factor Responsible for Angiogenesis. J. Exp. Med. 1971, 133, 275–288. [Google Scholar] [CrossRef]
- Cao, Y.; Arbiser, J.; D’Amato, R.J.; D’Amore, P.A.; Ingber, D.E.; Kerbel, R.; Klagsbrun, M.; Lim, S.; Moses, M.A.; Zetter, B.; et al. Forty-Year Journey of Angiogenesis Translational Research. Sci. Transl. Med. 2011, 3, 114rv3. [Google Scholar] [CrossRef]
- Ferrara, N. VEGF and the Quest for Tumour Angiogenesis Factors. Nat. Rev. Cancer 2002, 2, 795–803. [Google Scholar] [CrossRef]
- Relf, M.; LeJeune, S.; Scott, P.A.; Fox, S.; Smith, K.; Leek, R.; Moghaddam, A.; Whitehouse, R.; Bicknell, R.; Harris, A.L. Expression of the Angiogenic Factors Vascular Endothelial Cell Growth Factor, Acidic and Basic Fibroblast Growth Factor, Tumor Growth Factor Beta-1, Platelet-Derived Endothelial Cell Growth Factor, Placenta Growth Factor, and Pleiotrophin in Human Primary Breast Cancer and Its Relation to Angiogenesis. Cancer Res. 1997, 57, 963–969. [Google Scholar]
- Katoh, M.; Nakagama, H. FGF Receptors: Cancer Biology and Therapeutics: CANCER BIOLOGY AND THERAPEUTICS ON FGF RECEPTORS. Med. Res. Rev. 2014, 34, 280–300. [Google Scholar] [CrossRef]
- Al-Abd, A.M.; Alamoudi, A.J.; Abdel-Naim, A.B.; Neamatallah, T.A.; Ashour, O.M. Anti-Angiogenic Agents for the Treatment of Solid Tumors: Potential Pathways, Therapy and Current Strategies—A Review. J. Adv. Res. 2017, 8, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Riganti, C.; Contino, M. New Strategies to Overcome Resistance to Chemotherapy and Immune System in Cancer. IJMS 2019, 20, 4783. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Annese, T.; Ruggieri, S.; Tamma, R.; Crivellato, E. Limitations of Anti-Angiogenic Treatment of Tumors. Transl. Oncol. 2019, 12, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.-Y.; Zhang, L.-L.; Ding, J.; Lu, J.-J. Anticancer Drug Discovery from Chinese Medicinal Herbs. Chin. Med. 2018, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Koparal, A.T.; Ulus, G.; Zeytinoğlu, M.; Tay, T.; Türk, A.O. Angiogenesis Inhibition by a Lichen Compound Olivetoric Acid. Phytother. Res. 2010, 24, 754–758. [Google Scholar] [CrossRef]
- Song, Y.; Dai, F.; Zhai, D.; Dong, Y.; Zhang, J.; Lu, B.; Luo, J.; Liu, M.; Yi, Z. Usnic Acid Inhibits Breast Tumor Angiogenesis and Growth by Suppressing VEGFR2-Mediated AKT and ERK1/2 Signaling Pathways. Angiogenesis 2012, 15, 421–432. [Google Scholar] [CrossRef]
- Varol, M. Natural Small-Molecules Obtained From Lichens as a Novel Source of Anti-Angiogenic Agents. J. Appl. Pharm. 2015, 8, 1. [Google Scholar] [CrossRef]
- Varol, M. Anti-Breast Cancer and Anti-Angiogenic Potential of a Lichen-Derived Small-Molecule: Barbatolic Acid. Cytotechnology 2018, 70, 1565–1573. [Google Scholar] [CrossRef]
- Varol, M. Parietin as an Efficient and Promising Anti-Angiogenic and Apoptotic Small-Molecule from Xanthoria Parietina. Rev. Bras. Farmacogn. 2019, 29, 728–734. [Google Scholar] [CrossRef]
- Koparal, A.T. Anti-Angiogenic and Antiproliferative Properties of the Lichen Substances (-)-Usnic Acid and Vulpinic Acid. Z. Für Nat. C 2015, 70, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Kranner, I.; Cram, W.J.; Zorn, M.; Wornik, S.; Yoshimura, I.; Stabentheiner, E.; Pfeifhofer, H.W. Antioxidants and Photoprotection in a Lichen as Compared with Its Isolated Symbiotic Partners. Proc. Natl. Acad. Sci. USA 2005, 102, 3141–3146. [Google Scholar] [CrossRef] [PubMed]
- Paudel, B.; Bhattarai, H.D.; Lee, J.S.; Hong, S.G.; Shin, H.W.; Yim, J.H. Antibacterial Potential of Antarctic Lichens against Human Pathogenic Gram-Positive Bacteria: ANTIBACTERIAL ACTIVITY OF ANTARCTIC LICHENS. Phytother. Res. 2008, 22, 1269–1271. [Google Scholar] [CrossRef] [PubMed]
- Araújo, A.A.S.; de Melo, M.G.D.; Rabelo, T.K.; Nunes, P.S.; Santos, S.L.; Serafini, M.R.; Santos, M.R.V.; Quintans-Júnior, L.J.; Gelain, D.P. Review of the Biological Properties and Toxicity of Usnic Acid. Nat. Prod. Res. 2015, 29, 2167–2180. [Google Scholar] [CrossRef]
- Galanty, A.; Paśko, P.; Podolak, I. Enantioselective Activity of Usnic Acid: A Comprehensive Review and Future Perspectives. Phytochem Rev 2019, 18, 527–548. [Google Scholar] [CrossRef]
- Ingólfsdóttir, K. Usnic Acid. Phytochemistry 2002, 61, 729–736. [Google Scholar] [CrossRef]
- Luzina, O.A.; Salakhutdinov, N.F. Biological Activity of Usnic Acid and Its Derivatives: Part 2. Effects on Higher Organisms. Molecular and Physicochemical Aspects. Russ. J. Bioorg. Chem. 2016, 42, 249–268. [Google Scholar] [CrossRef]
- Morris Kupchan, S.; Kopperman, H.L. L-Usnic Acid: Tumor Inhibitor Isolated from Lichens. Experientia 1975, 31, 625. [Google Scholar] [CrossRef]
- Elečko, J.; Vilková, M.; Frenák, R.; Routray, D.; Ručová, D.; Bačkor, M.; Goga, M. A Comparative Study of Isolated Secondary Metabolites from Lichens and Their Antioxidative Properties. Plants 2022, 11, 1077. [Google Scholar] [CrossRef]
- Kahl, R.; Kappus, H. Toxikologie der synthetischen Antioxidantien BHA und BHT im Vergleich mit dem natürlichen Antioxidans Vitamin E. Z. Für Lebensm.-Unters. Forsch. 1993, 196, 329–338. [Google Scholar] [CrossRef]
- Goga, M.; Elečko, J.; Marcinčinová, M.; Ručová, D.; Bačkorová, M.; Bačkor, M. Lichen Metabolites: An Overview of Some Secondary Metabolites and Their Biological Potential. In Bioactive Molecules in Food; Mérillon, J.-M., Ramawat, K.G., Eds.; Reference Series in Phytochemistry; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–36. ISBN 978-3-319-54528-8. [Google Scholar]
- Salgado, F.; Albornoz, L.; Cortéz, C.; Stashenko, E.; Urrea-Vallejo, K.; Nagles, E.; Galicia-Virviescas, C.; Cornejo, A.; Ardiles, A.; Simirgiotis, M.; et al. Secondary Metabolite Profiling of Species of the Genus Usnea by UHPLC-ESI-OT-MS-MS. Molecules 2017, 23, 54. [Google Scholar] [CrossRef] [PubMed]
- Gauslaa, Y.; Solhaug, K.A. Fungal Melanins as a Sun Screen for Symbiotic Green Algae in the Lichen Lobaria Pulmonaria. Oecologia 2001, 126, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Feige, G.B.; Lumbsch, H.T.; Huneck, S.; Elix, J.A. Identification of Lichen Substances by a Standardized High-Performance Liquid Chromatographic Method. J. Chromatogr. A 1993, 646, 417–427. [Google Scholar] [CrossRef]
- Bussolino, F.; Mantovani, A.; Persico, G. Molecular Mechanisms of Blood Vessel Formation. Trends Biochem. Sci. 1997, 22, 251–256. [Google Scholar] [CrossRef]
- Mompéo, B.; Engele, J.; Spanel-Borowski, K. Endothelial Cell Influence on Dorsal Root Ganglion Cell Formation. J. Neurocytol. 2003, 32, 123–129. [Google Scholar] [CrossRef]
- Niu, G.; Chen, X. Vascular Endothelial Growth Factor as an Anti-Angiogenic Target for Cancer Therapy. CDT 2010, 11, 1000–1017. [Google Scholar] [CrossRef]
- Potente, M.; Gerhardt, H.; Carmeliet, P. Basic and Therapeutic Aspects of Angiogenesis. Cell 2011, 146, 873–887. [Google Scholar] [CrossRef]
- Petrovic, N. Targeting Angiogenesis in Cancer Treatments: Where Do We Stand? J. Pharm. Pharm. Sci. 2016, 19, 226–238. [Google Scholar] [CrossRef]
- Lin, Z.; Zhang, Q.; Luo, W. Angiogenesis Inhibitors as Therapeutic Agents in Cancer: Challenges and Future Directions. Eur. J. Pharmacol. 2016, 793, 76–81. [Google Scholar] [CrossRef]
- Fukumura, D.; Kloepper, J.; Amoozgar, Z.; Duda, D.G.; Jain, R.K. Enhancing Cancer Immunotherapy Using Antiangiogenics: Opportunities and Challenges. Nat. Rev. Clin. Oncol. 2018, 15, 325–340. [Google Scholar] [CrossRef]
- Montemagno, C.; Pagès, G. Resistance to Anti-Angiogenic Therapies: A Mechanism Depending on the Time of Exposure to the Drugs. Front. Cell Dev. Biol. 2020, 8, 584. [Google Scholar] [CrossRef]
- Bergers, G.; Hanahan, D. Modes of Resistance to Anti-Angiogenic Therapy. Nat. Rev. Cancer 2008, 8, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Brooks, A.N.; Kilgour, E.; Smith, P.D. Molecular Pathways: Fibroblast Growth Factor Signaling: A New Therapeutic Opportunity in Cancer. Clin. Cancer Res. 2012, 18, 1855–1862. [Google Scholar] [CrossRef]
- Xin, X.; Yang, S.; Ingle, G.; Zlot, C.; Rangell, L.; Kowalski, J.; Schwall, R.; Ferrara, N.; Gerritsen, M.E. Hepatocyte Growth Factor Enhances Vascular Endothelial Growth Factor-Induced Angiogenesis in Vitro and in Vivo. Am. J. Pathol. 2001, 158, 1111–1120. [Google Scholar] [CrossRef]
- Huang, M.; Lu, J.-J.; Ding, J. Natural Products in Cancer Therapy: Past, Present and Future. Nat. Prod. Bioprospect. 2021, 11, 5–13. [Google Scholar] [CrossRef]
- Stouch, T.R.; Gudmundsson, O. Progress in Understanding the Structure–Activity Relationships of P-Glycoprotein. Adv. Drug Deliv. Rev. 2002, 54, 315–328. [Google Scholar] [CrossRef]
- Wu, C.-P.; Ohnuma, S.; Ambudkar, S.V. Discovering Natural Product Modulators to Overcome Multidrug Resistance in Cancer Chemotherapy. CPB 2011, 12, 609–620. [Google Scholar] [CrossRef]
- Bačkorová, M.; Jendželovský, R.; Kello, M.; Bačkor, M.; Mikeš, J.; Fedoročko, P. Lichen Secondary Metabolites Are Responsible for Induction of Apoptosis in HT-29 and A2780 Human Cancer Cell Lines. Toxicol. Vitr. 2012, 26, 462–468. [Google Scholar] [CrossRef]
- Galanty, A.; Koczurkiewicz, P.; Wnuk, D.; Paw, M.; Karnas, E.; Podolak, I.; Węgrzyn, M.; Borusiewicz, M.; Madeja, Z.; Czyż, J.; et al. Usnic Acid and Atranorin Exert Selective Cytostatic and Anti-Invasive Effects on Human Prostate and Melanoma Cancer Cells. Toxicol. Vitr. 2017, 40, 161–169. [Google Scholar] [CrossRef]
- Song, Y.; Yu, Z.; Song, B.; Guo, S.; Lei, L.; Ma, X.; Su, Y. Usnic Acid Inhibits Hypertrophic Scarring in a Rabbit Ear Model by Suppressing Scar Tissue Angiogenesis. Biomed. Pharmacother. 2018, 108, 524–530. [Google Scholar] [CrossRef]
- Draut, H.; Rehm, T.; Begemann, G.; Schobert, R. Antiangiogenic and Toxic Effects of Genistein, Usnic Acid, and Their Copper Complexes in Zebrafish Embryos at Different Developmental Stages. Chem. Biodivers. 2017, 14, e1600302. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, C.; Quesada, A.R.; Medina, M.A. Evaluation of the Anti-Angiogenic Effect of Aloe-Emodin. Cell. Mol. Life Sci. 2006, 63, 3083–3089. [Google Scholar] [CrossRef]
- Deryugina, E.I.; Quigley, J.P. Chapter 2 Chick Embryo Chorioallantoic Membrane Models to Quantify Angiogenesis Induced by Inflammatory and Tumor Cells or Purified Effector Molecules. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2008; Volume 444, pp. 21–41. ISBN 978-0-12-374313-8. [Google Scholar]
- Irvin, M.W.; Zijlstra, A.; Wikswo, J.P.; Pozzi, A. Techniques and Assays for the Study of Angiogenesis. Exp. Biol. Med. (Maywood) 2014, 239, 1476–1488. [Google Scholar] [CrossRef] [PubMed]
- Bellairs, R.; Osmond, M. Atlas of Chick Development; Elsevier: Burlington, VT, USA, 2005; ISBN 978-0-08-045475-7. [Google Scholar]
- Ausprunk, D.H.; Knighton, D.R.; Folkman, J. Differentiation of Vascular Endothelium in the Chick Chorioallantois: A Structural and Autoradiographic Study. Dev. Biol. 1974, 38, 237–248. [Google Scholar] [CrossRef]
- Makanya, A.N.; Dimova, I.; Koller, T.; Styp-Rekowska, B.; Djonov, V. Dynamics of the Developing Chick Chorioallantoic Membrane Assessed by Stereology, Allometry, Immunohistochemistry and Molecular Analysis. PLoS ONE 2016, 11, e0152821. [Google Scholar] [CrossRef]
- Kwak, H.-J.; Park, M.-J.; Park, C.-M.; Moon, S.-I.; Yoo, D.-H.; Lee, H.-C.; Lee, S.-H.; Kim, M.-S.; Lee, H.-W.; Shin, W.-S.; et al. Emodin Inhibits Vascular Endothelial Growth Factor-A-Induced Angiogenesis by Blocking Receptor-2 (KDR/Flk-1) Phosphorylation. Int. J. Cancer 2006, 118, 2711–2720. [Google Scholar] [CrossRef]
- Breslin, S.; O’Driscoll, L. Three-Dimensional Cell Culture: The Missing Link in Drug Discovery. Drug Discov. Today 2013, 18, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Bissell, M.J.; Rizki, A.; Mian, I.S. Tissue Architecture: The Ultimate Regulator of Breast Epithelial Function. Curr. Opin. Cell Biol. 2003, 15, 753–762. [Google Scholar] [CrossRef]
- Galanty, A.; Popiół, J.; Paczkowska-Walendowska, M.; Studzińska-Sroka, E.; Paśko, P.; Cielecka-Piontek, J.; Pękala, E.; Podolak, I. (+)-Usnic Acid as a Promising Candidate for a Safe and Stable Topical Photoprotective Agent. Molecules 2021, 26, 5224. [Google Scholar] [CrossRef]
- Galanty, A.; Paśko, P.; Podolak, I.; Zagrodzki, P. Optimization of Usnic Acid Extraction Conditions Using Fractional Factorial Design. Lichenol. 2020, 52, 397–401. [Google Scholar] [CrossRef]
- Baell, J.B.; Holloway, G.A. New Substructure Filters for Removal of Pan Assay Interference Compounds (PAINS) from Screening Libraries and for Their Exclusion in Bioassays. J. Med. Chem. 2010, 53, 2719–2740. [Google Scholar] [CrossRef] [PubMed]
- Bisson, J.; McAlpine, J.B.; Friesen, J.B.; Chen, S.-N.; Graham, J.; Pauli, G.F. Can Invalid Bioactives Undermine Natural Product-Based Drug Discovery? J. Med. Chem. 2016, 59, 1671–1690. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrová, K.; Bačkorová, M.; Demčišáková, Z.; Petrovová, E.; Goga, M.; Vilková, M.; Frenák, R.; Bačkor, M.; Mojžiš, J.; Kello, M. Usnic Acid Isolated from Usnea antarctica (Du Rietz) Reduced In Vitro Angiogenesis in VEGF- and bFGF-Stimulated HUVECs and Ex Ovo in Quail Chorioallantoic Membrane (CAM) Assay. Life 2022, 12, 1444. https://doi.org/10.3390/life12091444
Petrová K, Bačkorová M, Demčišáková Z, Petrovová E, Goga M, Vilková M, Frenák R, Bačkor M, Mojžiš J, Kello M. Usnic Acid Isolated from Usnea antarctica (Du Rietz) Reduced In Vitro Angiogenesis in VEGF- and bFGF-Stimulated HUVECs and Ex Ovo in Quail Chorioallantoic Membrane (CAM) Assay. Life. 2022; 12(9):1444. https://doi.org/10.3390/life12091444
Chicago/Turabian StylePetrová, Klaudia, Miriam Bačkorová, Zuzana Demčišáková, Eva Petrovová, Michal Goga, Mária Vilková, Richard Frenák, Martin Bačkor, Ján Mojžiš, and Martin Kello. 2022. "Usnic Acid Isolated from Usnea antarctica (Du Rietz) Reduced In Vitro Angiogenesis in VEGF- and bFGF-Stimulated HUVECs and Ex Ovo in Quail Chorioallantoic Membrane (CAM) Assay" Life 12, no. 9: 1444. https://doi.org/10.3390/life12091444
APA StylePetrová, K., Bačkorová, M., Demčišáková, Z., Petrovová, E., Goga, M., Vilková, M., Frenák, R., Bačkor, M., Mojžiš, J., & Kello, M. (2022). Usnic Acid Isolated from Usnea antarctica (Du Rietz) Reduced In Vitro Angiogenesis in VEGF- and bFGF-Stimulated HUVECs and Ex Ovo in Quail Chorioallantoic Membrane (CAM) Assay. Life, 12(9), 1444. https://doi.org/10.3390/life12091444









