Simple Summary
Neurodegenerative diseases are complex neurological disorders with a high incidence worldwide in older people, increasing hospital visits and requiring expensive treatments. As a precursor phase of neurodegenerative diseases, cognitive impairment needs to be studied to understand the factors that influence its development and improve patients’ quality of life. The present review compiles possible factors and biomarkers for diagnosing mild cognitive impairment based on the most recent studies involving miRNAs. These molecules can direct the gene expression in multiple cells, affecting their behavior under certain conditions, such as stressing factors. This review encourages further research into biomarkers that identify cognitive impairment in cellular models such as astrocytes, which are brain cells capable of maintaining the optimal conditions for the central nervous system functioning.
Abstract
The importance of miRNAs in cellular processes and their dysregulation has taken significant importance in understanding different pathologies. Due to the constant increase in the prevalence of neurodegenerative diseases (ND) worldwide and their economic impact, mild cognitive impairment (MCI), considered a prodromal phase, is a logical starting point to study this public health problem. Multiple studies have established the importance of miRNAs in MCI, including astrocyte regulation during stressful conditions. Additionally, the protection mechanisms exerted by astrocytes against some damage in the central nervous system (CNS) lead to astrocytic reactivation, in which a differential expression of miRNAs has been shown. Nevertheless, excessive reactivation can cause neurodegeneration, and a clear pattern defining the equilibrium point between a neuroprotective or detrimental astrocytic phenotype is unknown. Therefore, the miRNA expression has gained significant attention to understand the maintenance of brain balance and improve the diagnosis and treatment at earlier stages in the ND. Here, we provide a comprehensive review of the emerging role of miRNAs in cellular processes that contribute to the loss of cognitive function, including lipotoxicity, which can induce chronic inflammation, also considering the fundamental role of astrocytes in brain homeostasis.
1. Introduction
Patients with mild cognitive impairment (MCI) present a cognitive decline at a greater level than expected by age without reaching dementia criteria [1]. MCI has been considered a risk factor for developing dementias, including neurodegenerative diseases (ND), such as Alzheimer’s (AD) and Parkinson’s (PD) diseases [2,3]. For example, AD has been recognized as a pathology that progresses for decades, initially manifesting as MCI [4]. However, epidemiological investigations report that 25 to 50% of PD patients presented with MCI, depending on the population and clinical setting [2,5]. In the case of all dementias, MCI is considered a prodromal stage due to the time it takes for this disease to present the first symptoms. It has been reported that the progression from MCI to dementia increases between 80 and 90% after six years [1]. About 50 million people have dementia worldwide, and it is estimated that there will be 102 million more by 2050. In addition, their treatment costs about USD 1 trillion annually [6].
Although the diagnosis of ND is carried out by clinical criteria based on the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), in which a significant cognitive decline affecting independence in everyday activities is highlighted, this sign is observed when the disease is already advanced. In contrast, in MCI, there is a modest cognitive decline without affecting everyday activities [7]. However, the diagnosis of MCI is performed through neuropsychological assessment tools such as the Mini-Mental Status Examination (MMSE) or the Montreal Cognitive Assessment (MoCA) [8,9,10]. Unfortunately, the first one does not have recommended specificity and sensitivity, while the outcome of the second one depends on educational level, cultural norms, and other aspects. Moreover, neuroimaging AD biomarkers and proteins in cerebrospinal fluid have been used but have not been validated in the context of MCI [4]. Therefore, identifying new biomarkers becomes imperative to improve MCI diagnosis and prognosis, preventing other ND.
Following the above, it is crucial to overcome the reductionist paradigm approach to the study of MCI, considering the participation of multiple brain cells in its development; for example, the astrocytes. These glial cells maintain the equilibrium of brain physiological characteristics [11]. Nevertheless, factors such as cellular senescence could induce function loss, causing neurodegeneration. During injury or inflammation, reactive astrocytes may cause a homeostatic disturbance [12]. Interestingly, it has been found that astrocytes play an important role in cognitive functions [13].
Due to the fundamental role performed by the astrocytes, the molecules that regulate the activation of the astrocytic metabolic pathways and pro- or anti-inflammatory mechanisms could be potential biomarkers for ND. Among these molecules are the miRNAs expressed by astrocytes, which might be suitable candidate biomarkers because they are stable in blood and cerebrospinal fluid, making them easily detected [14]. In addition, these non-coding RNAs represent an epigenetic mechanism that leads to gene silencing, affecting protein levels without altering the DNA sequence [15].
The lipidome is the set of lipids necessary for cell metabolism [16]. However, an excessive increase in lipids leads to diseases due to lipotoxicity. This toxicity of lipids is characterized by metabolic dysfunction generating mitochondrial and endoplasmic reticulum stress, autophagy, and inflammation [17]. It is important to highlight that inflammation is a particular feature of neurodegeneration [18]. Additionally, inflammation can affect astrocytes and thus lose their functionality [19]. Hence, lipotoxicity could play a key role in understanding ND by analyzing the presence of high fatty acids in astrocytes and other cells. Furthermore, the central nervous system (CNS) is significantly affected by the lipotoxic effects of dyslipidemia, which develops MCI and AD [20]. For example, it has been shown that when mice are exposed to a high concentration of lipids, only one day is needed to lose performance in memory tasks [20].
Remarkably, lipotoxicity triggered by high concentrations of saturated lipids such as palmitic acid (PA) has been related to the progression of neurodegeneration in diseases such as AD and PD [21]. It has also been associated with cognitive decline and generation of pro-inflammatory responses, causing loss of astrocytic function, where the miRNAs are possible mechanisms involved [22]. It should be noted that the expression of miRNAs in mice can be modified by diets rich in fatty acids [23]. Thus, excess lipids in the brain could be considered a starting point for the dysregulation of miRNAs and genes involved in different cellular processes.
Accordingly, a better understanding of the role of miRNAs in the development of ND will allow us to improve our knowledge of these diseases, thus enabling their diagnosis and prevention. The following sections will focus on the biological role that miRNA dysregulation exerts in the CNS, their importance in stressing conditions such as lipotoxicity, and their possible role as biomarkers in ND progression. Additionally, astrocytes will be highlighted as fundamental cells in these miRNAs’ expression and influence.
2. Non-Coding RNAs as Key Epigenetic Factors
Classically, epigenetics is defined as the mechanisms by which the genotype produces the phenotype, and its study is mainly centered on transcription factor activation, DNA methylation, and histone modifications [24,25]. Nevertheless, cell expression control is more complex than that, and factors such as non-coding RNAs also play an important role in the process [26]. For example, long non-coding RNA BACE1-AS is strongly related to AD, and studies have proposed it as a potential biomarker [27]. BACE1-AS can transcriptionally silence miR-214-3p, promoting the expression of ATG5 and inducing neuronal damage through Aβ1-42 production [28]. In addition, the expression of BACE1-AS increases the β-secretase 1 (BACE1) levels by sequestrating BACE1-targeting miRNAs [29,30]. BACE1 is reported as the protein responsible for amyloid plaque formation, and its relationship with AD pathogenesis has been documented [31]. Moreover, miRNAs are also crucial elements in controlling gene expression at the transcriptional level. For instance, miR-1, miR-22p, miR-26b-3p and miR-28-3p have a strong relationship with ND, including AD, PD, Huntington’s (HD), and amyotrophic lateral sclerosis (ALS) [32].
miRNAs: Biogenesis and Functions
Back in the human genome project time, only 1.9% of the human DNA had a defined or predicted function [33]. Currently, we are discovering new miRNAs controlling hundreds of metabolic events daily, slowly improving our knowledge about the human genome. Nevertheless, we are far from completely knowing the direct relationship between a miRNA and a developmental process or disease [34].
The miRNA biogenesis itself is a clue to understanding how miRNAs can exert control over processes such as neurogenesis and neurodegeneration. First, RNA polymerase II or III synthesizes the primary miRNA (pri-miRNA), a hairpin loop RNA capped at the 5′ end and polyadenylated at the 3′ end. Next, pri-miRNA must be excised by the nuclear endoribonuclease Drosha in complex with the dsRNA-binding protein DGCR8, releasing an ~70 nt stem-loop precursor miRNA (pre-miRNA) with a 30 overhang. Then, the Exportin-5 and Ran-GTP complex translocates the pre-miRNA to the cytoplasm, where it is processed by the endoribonuclease Dicer, yielding an ~22 nt RNA duplex. After that, an Argonaute protein conserves the guide strand (complementary to de target mRNA), and the passenger strand is released and degraded [35,36]. Finally, the guide strand directs the mRNA degradation by Argonaute proteins through complementarity to the transcript’s untranslated (UTR) or even coding sequence (CDS) regions [37,38,39]. Each point in this process represents a regulation possibility, where even the final size of the miRNAs can affect their target specificity and, therefore, change the way a gene is expressed [40]. Hence, the epigenetic function of miRNAs has been demonstrated to be complex, and its understanding could reveal multiple critical points in some diseases.
For instance, cell differentiation can be directed through determined miRNA expression [41]. That is the case of pluripotency reprogramming using the miR-290 cluster, let-7, and miR-130/miR-301/miR-721 [42,43,44], and neuronal reprogramming into different phenotypes through let-7, miR-9-5p/3p, miR-124, and miR-218 [45]. Additionally, miRNAs can mediate the differentiation, expansion, inflammation, and apoptosis of the CNS cells, which could promote or inhibit MCI and ND [46,47].
Recently, the miRNA expression has attracted the attention of researchers in the ND field, finding some direct correlations between their presence/absence or augmentation/diminution throughout the disease progression [32,48].
3. The Crosstalk between MCI Factors and miRNA Dysregulation
As established before, miRNA expression responds to environmental and internal factors, which allows us to affirm that miRNA dysregulation is a multifactorial process. For example, miRNA dysregulation can be a consequence of determinate events disrupting cerebral homeostasis, including blood-brain barrier (BBB) damage, neuroinflammation, and reduced blood flow to the brain, among others. However, miRNAs can also be the cause of those events, representing a complex control loop that we need to understand to elucidate the association between MCI and ND development [49,50,51].
In the BBB context, an erythrocyte-derived miRNA, miR-451a, can reach the CNS when this barrier is damaged [52]. Interestingly, miR-451a promotes neuronal differentiation by inhibiting cellular proliferation [53], and its entrance to the CNS could lead to a differentiation imbalance. Furthermore, the cardiac overexpression of miR-1, a muscle-enriched miRNA, attenuates the synaptic vesicle exocytosis. The diminished synaptic activity could explain the relationship between cognitive deterioration and cardiac diseases, called “cardiogenic dementia” [54]. Another miRNA that alters synaptic activity is miR-128, which regulates the expression of two synaptic transmission proteins, SNAP-25 and synaptotagmin 1 (Syt1). However, an interesting experiment with mice demonstrated that miR-128 effects could be reversed by exposing the individuals to an enriched environment that offers the opportunity to express the full range of species-typical behavioral patterns [55].
Chronic low-grade inflammation is associated with increased odds of developing MCI [56]. The expression of some miRNAs has been related to an inflammatory scenario in the brain. For example, miR-31-5p is responsible for repressing the Numb expression, a Notch pathway negative regulator, degrading the Notch intracellular domain [57]. Interestingly, Notch defines whether a cell acquires neural potential during neurogenesis and if the progeny will display neural or glial fates [58]. Furthermore, the downregulation of miR-31-5p can inhibit the M1 phenotype in microglia and neuroinflammation [57]. Moreover, the attenuation of miR-31-5p expression via CircARF3 improved BBB integrity and decreased neuronal apoptosis and microglial activation in a subarachnoid hemorrhage rat model by inactivating the MyD88-NF-κB pathway [59]. Additionally, miR-15a/16-1 is associated with vascular cognitive impairment and dementia, and its genetic deletion improves the related cognitive and sensorimotor deficits of this disease. Noteworthy, miR-15a/16-1 inhibits AKT3 and IL-10RA expression, known anti-inflammatory proteins [60].
Moreover, opioid abuse can trigger neuroinflammation, making it a possible cause of MCI. That is the case of morphine-mediated microglial activation, where miR-138 is released by astrocytes, activating the TLR7 receptor in BV-2 microglial cells. These findings were confirmed in vivo by administrating morphine in wild-type mice, resulting in increased microglial activation in the thalamus, which has been related to MCI and dementia development in humans [61,62,63]. Interestingly, miR-124 and its target, IQGAP1, have shown a regulative role in addition and cognitive impairment in morphine-dependent patients [64]. Furthermore, ethanol drinking also causes neuroinflammation and brain damage by TLR4 activation. Murine astrocyte treatment with ethanol increased miR-146a, miR-182, and miR-200b levels in extracellular vesicles. This change increased the expression of the inflammatory-related proteins TLR4, NFκB-p65, IL-1R, caspase-1, and NLRP3, reducing neuronal survival [65].
Ischemia is a mechanism derived from impaired blood flow to the brain, causing an acute brain injury. The lack of oxygen and glucose supply induces the loss of neurons and gliosis, and the extent of neuronal damage depends on the area size and duration of the hypoperfusion [66]. In this sense, an ischemic lesion can increase the risk of developing MCI and, after that, some dementia [67]. For that reason, tracking miRNA dysregulation in ischemic injuries can shed light on the process of developing MCI. For instance, miR-143-3p is aberrantly expressed in patients with ischemic stroke, playing an essential role in angiogenesis [68,69]. Its reduction via the circular RNA circ_0025984 protects astrocytes from ischemia-induced autophagy and apoptosis [69]. Furthermore, miR-125a-5p, miR-125b-5p, and miR-143-3p were found to be upregulated in ischemic patients and patients with transient ischemic attacks. In an interesting way, miR-125b-5p and miR-143-3p return to control levels two days after the lesion, making them good candidates for ischemic biomarkers [70].
According to this, astrocytic miRNA expression has a fundamental role in the ND progression and in processes that trigger MCI, such as BBB disruption, ischemia, and inflammation.
4. Structure and Function of Astrocytes
Astrocytes, also known as astroglia or astroglial cells, are the most abundant type of glial cell in the brain [71], being diverse in their ability to regulate and support several functions in the CNS [72]. For example, these cells maintain brain homeostasis and neuronal metabolism, supporting the neurons and the microarchitecture of the brain parenchyma [12]. Additionally, astrocytes regulate the concentrations of ions, neurotransmitters, and metabolites, controlling neural development, plasticity, and synaptogenesis [73,74,75]. Even more, it has been recently suggested that astrocytes play a role in the regulation of the sleep/wake cycle through Ca2+ signaling [76,77]. Furthermore, astrocytes also play a role in immune defense by, for example, recruiting neutrophils or neuronal protection and barrier formation during infection [78,79,80,81]. In addition, mature astrocytes also activate neurogenesis and support neuronal differentiation and maturation, which is a promising approach for repairing spinal cord injury [82,83,84].
Considering the heterogeneity of this cell population, it is not surprising that their disorders are related to a wide range of neuropathologies. For example, brain diseases are characterized by the active inflammatory state of the astrocytes, which presents a high expression of the glial fibrillary acidic protein (GFAP) [85,86], and many other molecular markers [87]. In particular, the loss of astrocyte function due to cellular senescence could have implications for neurodegenerative disorders, such as AD and HD, and the aging brain [88]. Additionally, the disease severity depends on the astrocytic Ca2+ signals driving the induction and the progression of the inflammatory state [12].
While their role in ND was thought to be deleterious, it is now believed to be more complex, depending on the stage of the disease [89,90]. Similarly, various other factors have demonstrated importance, such as the microenvironment and localization in which the astrocyte is found [91]. Therefore, astrocytes are rapidly becoming an exciting area considering their potential as targets in ND.
miRNA Role in Astrocytes
As mentioned previously, astrocytes play a critical role in the homeostasis of the central nervous system. Both the regulation of their function and dysfunction can be mediated by miRNAs. For this reason, the expression of miRNAs in astrocytes is related to different pathologies such as AD and PD, among others [92]. Several studies have evaluated the expression profile of miRNAs in body fluids, but few have been conducted on astrocytes. Evaluating the miRNA expression directly in these cells could be useful to diagnose ND, assessing the biological processes that trigger these pathologies from the reactive astrocytes and promoting the finding of novel therapeutic targets. For instance, reactive gliosis is a pathological hallmark of ND, presented as a response to damage such as neurodegeneration. Those neuronal lesions, in turn, can be induced by the loss of the functions of the astroglia [93].
The expression of miRNAs in astrocytes depends on the brain regions where astrocytes are located and cerebral maturity. The fetal brain has a different miRNA profile in comparison with the adult brain. Likewise, there is a differential expression of miRNAs between white matter and gray matter [94]. Additionally, astrocytes are a suitable study object because they represent most brain cells and perform relevant functions [95].
Moreover, it has been found that the differential expression of miRNAs can contribute to the loss of cellular functions in astrocytes, triggering ND development [96]. For example, miR-125b and miR-449 are involved in structural function in astrocytes, attracting attention due to the assumption that their dysregulation would affect different functions and damage the physical structuring of the brain [94]. Furthermore, astrocytes have the ability to synthesize cholesterol and provide it to neurons [97], but in aged astrocytes, it has been observed dysregulation in cholesterol synthesis [98]. Interestingly, the miR-335 was found to be overexpressed in cultures of aged astrocytes, which has been associated with altered cholesterol metabolism in astrocytes and cognitive function [98].
Furthermore, CCL5 is associated with the regulation of glutamatergic transmission [99], which is secreted by astrocytes under pathological conditions [100]. CCL5 is controlled by miR-324-5p [101]. Therefore, it is coherent to infer that this miRNA affects the synapse processing in astrocytes. Another example is miR-137, which targets glutamate transporter in neurons [102], whose function is to regulate glutamate concentrations at the synapses [103]. As astrocytes are releasers of these transporters [104], there is a possibility that miR-137 affects the tripartite synapse. Moreover, miR-223 controls the NMDA-induced calcium flux in the neuronal hippocampus [105], which could be related to controlling glutamate amounts.
Another key molecule is GFAP, which is part of the formation, dynamics, and structure of the cytoskeleton of astrocytes, and it is highly expressed in the process of astrogliosis [106]. Moreover, in AD, the upregulation of the cytoskeleton is a feature of reactive astrocytes [107], which could, in turn, result in the deregulation of GFAP. Regarding miRNAs, miR-145 is reported as a regulator of the cellular dynamics of astrocytes, being a clue in astrogliosis [108]. Moreover, astrogliosis is characterized by vimentin and GFAP increase due to astrocyte proliferation. Interestingly, there is a significant correlation between the increase of these globular proteins and the over-expression of miRNA-125b [109].
In addition, different miRNAs have been involved in pathophysiological events during the development of ND by activating glial cells, including astrocytes. That is the case of miR-155, a promoter of pro-inflammatory cytokines through the modulation of SOCS-1 [110]. Similarly, miR-9 targets MCPIP1, contributing to the degradation of cytokines such as IL-6 and IL-1β [111]. Additionally, in a study where the astrocytes were treated with lipopolysaccharide (LPS), miR-181 was downregulated, triggering an increased release of TNF-α [112]. As these molecules contribute to the development of neuroinflammation, it suggests that miR-155, miR-9, and miR-181 would affect the immune response in cells such as astrocytes and thus influence the evolution from MCI to AD.
However, we cannot ignore the protective and homeostatic role of astroglia, which is also modulated by miRNA expression. For example, the astrocyte-derived miR-873a-5p promotes a microglial M2 phenotype after a traumatic brain injury, which means a less inflammatory and more repairer phenotype [113]. Interestingly, astrocytes can also modify the miRNA expression in response to inflammation. For instance, a study demonstrated that in the presence of IL-1β and TNF-α, astrocyte-derived extracellular vesicles contained higher concentrations of miR-125a-5p and miR-16-5p. These two miRNAs target NTKR3 and its downstream effector Bcl2, associated with neuronal growth and activity. In contrast, in the presence of ATP, the astrocyte-derived extracellular vesicles had the opposite effect. Additionally, this ATP challenge increased astrocytic intracellular levels of let-7f, miR-100, miR-23a, and miR-145. These changes adjust the activity of target neurons, controlling the transcriptional programs related to synaptic stability and neuronal excitability [114]. Note of worth, miR-16-5p has been associated with cell cycle G1/S phase arrest and apoptosis by targeting Bcl2, impairing fracture healing [115].
Some miRNAs are related to protection against the ischemia-triggered processes. For instance, miR-29a, enriched in astrocytes, protects neurons from forebrain ischemia by reducing the expression of the pro-apoptotic PUMA. Additionally, this miRNA decreased cell injury and improved mitochondrial function after ischemia-like stresses in vitro [116]. In addition, the loss of miR-29b contributes to neural cell death and infarct size after acute ischemic stroke. miR-29b is required for glutathione and 12-lipoxygenase-dependent arachidonic acid metabolism, leading to neurodegeneration [117]. Similarly, miR-17-5p showed neuronal protection from hypoxic-ischemic brain damage in neonatal rats. This miRNA inhibits BNIP2, a member of pro-apoptotic BNIP families, reducing neuronal apoptosis and inflammation [118]. In addition, miR-92b-3p is also associated with the protective function of astrocytes during oxygen and glucose deprivation through exosome release. This effect was tested using rat embryo neurons, which showed higher viability when exposed to miR-92b-3p-containing exosomes [119]. Interestingly, miR-92b-3p is downregulated after acute spinal cord injury, but its upregulation leads to PTEN inhibition and AKT phosphorylation, related to neurite growth and functional recovery [120].
It is worth mentioning that astrogliosis also has a beneficial role that is essential for functions in the brain, although it has detrimental outcomes under peculiar circumstances [121]. There are some miRNAs involved in both processes. For example, miR-21 has an elevated expression in astrogliosis when an injury in the spinal cord occurs. In contrast, in healthy conditions, this miRNA is diminished [122]. Similarly, miR-181 increases its levels under the influence of cytokine IL-10 [112], suggesting that this miRNA promotes inflammation. On the flip side, miR-190 reduces neuroinflammation, according to a murine model study [123]. Therefore, it is possible to infer that dysregulation in these and other miRNAs would result in a bigger injury or inefficient containment of some trauma.
Considering all the information above, we suggest that the regulation and dysregulation of miRNAs in astrocytes must be studied more in the development of MCI and ND.
5. Lipids in the Brain
Lipids are considered fundamental for the functioning of the brain [124]. For example, they have functions at the structural level in the lipid bilayer of the cell membrane and other organelles. In addition, they act as a source of energy storage in the CNS [16]. Furthermore, lipids contribute to the maintenance of physiological conditions, such as synapse and neuron formation, as well as cell signaling, which are relevant for various neurological disorders [125]. Dysregulation of lipidic composition could be triggered by different disorders, such as obesity [124]. For example, an imbalance of palmitate in human CSF has been observed in obese people with amnestic MCI; remarkably, the palmitate injection induced memory loss in mice [126].
In addition, the brain represents an important lipid reservoir together with adipose tissue [127]. Moreover, within the brain, specific cells, such as neurons and glial cells, have a connection with lipid metabolism [128], which work together as a metabolic unit by releasing/consuming lipids as an energy resource. In that context, astrocytes accumulate lipid droplets necessary for energy production and membrane synthesis [129]. Moreover, lipid droplets are also formed as a stress mechanism by astrocytes [130], protecting the CNS from oxidative stress and lipotoxicity [131].
Lipid droplets are also associated with high lipid diets since a high exogenous number of fatty acids contributes to their formation [130]. In addition to this, lipid droplets can be triggered as a cause and consequence of inflammatory processes [132]. In this way, it is related to ND, such as AD, suggesting lipid droplets are responses to lipid accumulation and neuroinflammation [133]. Therefore, it is also necessary to explore miRNA in cellular models such as glial cells and astrocytes under different experimental conditions, including lipotoxicity, to understand the cellular mechanisms that lead to the development of these pathologies.
As obesity and metabolic dysregulation are risk factors for cognitive decline [134], high lipid concentration could take part in the loss of cognition in older adults. Therefore, analyzing the behavior of miRNAs under these conditions might be a suitable alternative for the early identification of MCI. Furthermore, it has been proposed that the connection between the adipose tissue and the CNS, along with signals and cellular intermediaries, contributes to cognitive decline and neurodegeneration [135].
Adipogenesis, characterized by the formation of lipid droplets, is modulated by different miRNAs, such as miR-146a-3p, miR-4495, miR-4663, miR-6069, and miR-675-3p [136]. Interestingly, miR-146a was previously associated with cognitive impairment [137].
As a feature of cognitive decline and ND, neuroinflammation and some adipokines, such as adiponectin and leptin, play a part in cognitive functions [138,139]. Thus, higher levels of leptin have been observed in people suffering from cognitive impairment [134], whereas higher levels of adiponectin improve cognition [140,141]. Moreover, according to the KEGG database, there are diseases related to leptin in the JAK-STAT signaling pathway, among which obesity and MCI can be highlighted. According to the miRDB database, miR-146-5p modulates the LEPROT gene, which is associated with the overlapping transcription of the leptin receptor, whose function is associated with the immune response in the CNS [142]. Additionally, miR-378 is involved in adiponectin regulation [143], and this miRNA expression is deregulated in disorders such as AD, affecting neurogenesis [144].
Palmitic acid (PA), the most common fatty acid in the human body, has been reported to be increased in the CSF of obese people, leading to altered cognitive properties in mice [126]. It has been shown that, at healthy levels, PA plays an essential role in memory and learning and is usually acquired through diet [145]. In addition, PA induces the expression of α-synuclein, tyrosine hydroxylase, dopamine, and serotonin, which play a vital role in the development and reduction of ND [21]. Regulation of α-synuclein expression is given by miR-7, and the decrease of this miRNA is related to PD [146]. Additionally, miR-16 regulates serotonin transporters [147] and β-amyloid protein, which control cell death in an in vitro model of AD [148]. Moreover, insulin resistance increases the likelihood of MCI development [149], and some studies have observed miRNAs that prompt insulin resistance caused by high levels of PA, such as miR-3180-3p and miR-4632-5p [150], which could be related to cognitive impairment. In fact, miR-3180 has been reported to be downregulated in AD from the human cerebral cortex sample [151]. Additionally, in an in vitro model of astrocytes, high PA levels affect the expression of two miRNAs, miR-125a and miR-155-5p, which are associated with the regulation of inflammatory genes [152]. Interestingly, in microglial cells, high PA concentrations increased the miR-124 expression, which was associated with a reduced inflammatory process in these cells [153].
6. The Biomarker Potential of miRNAs in CNS
MCI is a multifactorial disease, including age, genetics, and educational level [154], and different miRNAs could be implied in the outcome of those factors. For example, differentially expressed miRNAs related to aging, including miR-146a-5p, miR-30a-3p, miR-148a-3p, miR-130b-3p, miR-181a-5p, and miR-192-5p, have been suggested as candidates for biomarkers of cognition [155].
Many miRNAs dysregulated in AD participate in distinct biological processes in the brain. For instance, miR-146a, enriched in microglia and neurons, is involved in the activation of glial cells and inflammatory processes [137,156]. Interestingly, miR-146a-5p is associated with a risk of loss of cognitive function [155]. Therefore, it would be interesting to relate specific miRNAs expressed in MCI to understand better the origin of the disease’s progression. In addition, miR-148a-3p is overexpressed under neurodegenerative conditions, altering the extracellular matrix. This condition would affect cellular functions such as cell movement and multiplication [155].
Recent studies have highlighted the miRNA impact on ND. For instance, a work identified a miRNA signature composed of 3 miRNAs (miR-181a-5p, miR-146a-5p, and miR-148a-3p), which alter cellular processes involved in cognition in experimental models [155]. In another study, miR-567 was upregulated in CSF, blood, and serum samples from MCI-AD patients. This work suggested this miRNA as an early development biomarker of AD due to its expression was higher in patients than in healthy people [157]. Additionally, the overexpression of miR-142-3p in the hippocampus or plasma from AD and MCI-AD patients was confirmed through a meta-analysis that included 18 studies with 1027 participants. This study showed the miR-142-3p biomarker potential with a sensitivity and specificity of 100% and 77%, respectively. The same data compilation also found miR-483-5p, which reached 100% sensitivity, and miR-107, with a 79% sensitivity for AD and 98% for MCI [46]. In addition to its high sensitivity, miR-483-5p showed the highest expression among the altered miRNAs in AD [158], reaffirming its potential role in preventing AD.
Research related to PD diagnosis has also been conducted, with the critical limitation of a few participants. A study involving 75 PD patients and 73 normal controls observed a reduction in miR-153 and miR-223 levels in the PD cases compared to control and PD-treated subjects [159]. The Additionally, the detection in CSF of the miRNA combination let-7f-5p, miR-27a-3p, miR-125a-5p, miR-151a-3p, and miR-423-5p showed 90% sensitivity and 80% specificity in a study with 40 PD patients and 40 matched control individuals [160].
Otherwise, miRNA dysregulation has also been detected in ND progression, even showing a differential expression in their preclinical and successive stages. For that reason, miRNAs are starting to attract interest with diagnosis and prognosis ends.
For example, miR-92a-3p, miR-181c-5p, and miR-210-3p have been upregulated in AD. Interestingly, MCI subjects that progress to AD showed higher levels of these miRNAs than before developing the disease. The study included 38 healthy donors, 56 AD, 26 MCI, and 27 frontotemporal dementia patients, proposing miR-92a-3p, miR-181c-5p, and miR-210-3p as potential biomarkers for AD [161]. Additionally, a more recent study with three different cohorts and 269 participants found a correlation between β-amyloid (Aβ) load and miR-27a-3p, miR-27b-3p, and miR-324-5p. Moreover, miR-195-5p and miR-335-5p were consistently upregulated in MCI, cognitively normal Aβ-positive, and AD individuals. More interestingly, miR-122-5p levels increased as the disease progressed, while miR-27b-3p, miR-29c-3p, miR-143-3p, and miR-324-5p decreased [162]. Remarkably, miR-195 inhibition ameliorates mitochondrial dysfunction in an AD mice model, improving cognitive function [163]. In addition, miR-384 has been proposed as an AD biomarker, which can be detected in exosomes in plasma blood in conjunction with other marker proteins. This method showed high power for detecting subjective cognitive decline, MCI, and AD [164].
Additionally, miR-9, miR-29a, miR-34a, miR-125b, miR-146a, and miR-29b, were evaluated in CSF due to their presence in the human brain and their relationship with AD processes. After assessing them through RT-qPCR, these miRNAs were proposed as landmarks in AD [165]. Moreover, miR-206 was found in blood plasma from patients with MCI, who also progressed to dementia over four years [166]. Therefore, these studies give us an idea of the importance of evaluating the expression of miRNAs in the MCI.
Nevertheless, there are also miRNAs with a neuroprotective role in AD. For example, the neuron-expressed miR-132-3p was found to be progressively downregulated as the disease evolved. miR-132-3p targets FOXO1a, Tau, EP300, and SIRT1, with the first protein being increased in late-onset AD patients [48]. Additionally, miR-132-3p-targeted FOXO3a induces apoptosis in cultured primary neurons, demonstrating a neuroprotective role of this miRNA [167]. Moreover, let-7a-5p has demonstrated a regenerative role for the neurons in the spinal cord in a rat model. let-7a-5p inhibits the expression of HMGA2, finally downregulating the TGF-β/Smad signaling pathway [168]. TGF-β is associated with the lesions after spinal cord injury and the formation of glial scars [169,170]. In addition, miR-107 directs BACE1 degradation, increasing cell survival, reducing lactate dehydrogenase leakage, inhibiting apoptosis, and reducing Aβ production in AD. In fact, miR-107 is a current study target for pharmacologic treatment development [171].
Furthermore, some miRNAs are dysregulated in ALS patients. For example, in a study with 48 ALS patients, 16 disease mimics, and 24 age- and sex-matched healthy controls, miR-16-5p, miR-21-5p, and miR-92a-3p were downregulated while miR-206 was upregulated only in the ALS patients [172].
In the case of multiple sclerosis, severity can be followed by the expression of some miRNAs. That is the case of miR-375 and miR-629-5p, which positively correlate with brain atrophy, while miR-143-3p, miR-142-5p, miR-181c-3p, and miR-181c-5p demonstrate a protective correlation [173]. Moreover, the upregulation of miR-155, miR-153, miR-361-5p, miR-4668-5p, miR-8071, miR-197-5p, miR-145, miR-181, miR-199a, miR-1183, miR-129-2-3p, and miR-143-3p and the downregulation of miR-134, miR-0067835, and miR-153 have been proposed as prognostic biomarkers of Mesial Temporal Lobe Epilepsy in potentially epileptogenic patients [174]. Noteworthy, miR-155 has been detected in the neurovascular unit of individuals with multiple sclerosis, and its upregulation is related to the loss of BBB function during neuroinflammation [175].
7. miRNAs Altered in MCI and ND: Related Biological Processes
The mentioned miRNAs are associated with different characteristic signaling pathways in the development of neurodegeneration, such as inflammation, insulin resistance, angiogenesis, and cellular senescence, through the control of several genes. Figure 1 shows a simplified summary of pathways and cellular processes of several discussed miRNAs related to neurodegenerative processes. According to the KEGG database, these miRNAs control several genes in common. That is the case of the PTEN gene, which is regulated by miR30a-30 and miR-130b-3p and involved in IGF-1/mTOR inhibition and PDK1/2activation. IGF is a gene that contributes to brain development and immune response; under normal physiological conditions, this gene is essential for brain growth and proper functioning of the central and peripheral nervous system [176].
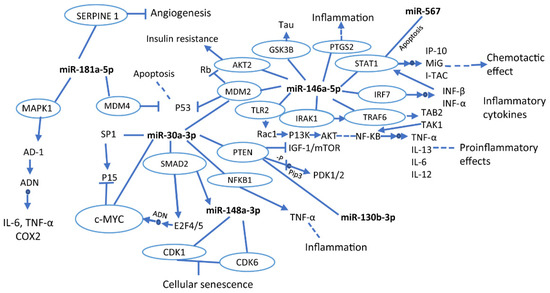
Figure 1.
Interactions of miRNAs in signaling pathways and cellular processes. The blue circles indicate genes that are being modulated by miRNAs. The full arrows indicate production or direct activation of a particular process, while the dotted arrows show that the pathway is activated, but different processes occur prior to its termination. Likewise, the small blue circles in the middle of the arrows indicate direct action on the track. Moreover, the line terminated by another line perpendicularly means inhibition. This graphic was made based on the KEGG database.
The MDM2 gene is also regulated by miR-30a-3p and miR-146a-5p. This gene inhibits the production of p53 and Rb. The p53 is involved in the promotion of neuronal plasticity [177]. Thus, the lack of control of p53 may lead to the loss of neuronal communication, which could trigger cognitive impairment. Not having been studied yet, it could be an object of study.
The main cellular mechanisms related to these miRNAs are inflammatory processes, chemotactic effects, senescence, and cell death. Inflammatory processes are characteristic events of early AD or MCI development, and it is a crucial factor in the progression of the disease. Therefore, it has been detected as the earliest biomarker of the disease [178]. Furthermore, apoptosis is a relevant process associated with neuronal loss of neurodegeneration development [179]. In addition, cellular senescence could induce the loss of function of brain cells such as astrocytes, causing neurodegenerative disorders [12]. Finally, chemotactic activity has been reported to represent an early response to β-amyloid deposition, which suggests that miR-146a-5p in MCI could play an essential role in the early detection of AD [180].
Table 1 presents a summary of the miRNAs associated with ND; it shows their function and the diseases in which they were studied. Noteworthy, the miR-125b, miR-143-3p, miR-155, miR-181c-5p, and miR-92a-3p are related to more than two ND, which might suggest their biomarker potential in the early stages of illnesses. Inflammation, synapse reduction/augmentation, apoptosis/cell survival, and neurodegeneration are the most common functions involved with these miRNAs. All studies considered in this review were conducted using human, rat, or murine models.

Table 1.
miRNA role in neurodegenerative diseases.
8. Conclusions
Epigenetics plays an essential role in the regulation of different cell functions, which are affected due to gene silencing through the down/upregulation of miRNAs, among other mechanisms [202]. In addition, these miRNAs are involved in biological processes such as apoptosis, cell differentiation, and proliferation, whose dysregulation can contribute to the development of pathologies [15].
Figure 2 summarizes the four main biological processes controlled by miRNAs discussed here, which are divided into those triggered and those impaired by miRNA expression. The need to study differential expression of miRNAs in specific cells such as astrocytes is evident since several miRNAs are related to the balance or imbalance in their functions, with a probable consequence in developing MCI and ND. For instance, some of the miRNAs involved in MCI have been highlighted, such as miR-146a, which is differentially expressed in glial cell activation, cognitive processes, and ND [137]. Additionally, miR-125 is reported as a structural modulator of astrocytes, and, in turn, it has been involved in MCI development [109]. Therefore, a strong hypothesis highlights these miRNAs as important study molecules in these neuronal pathologies such as MCI. Furthermore, one of the mechanisms inducing inflammation in astrocytes is related to a high concentration of lipids that alter insulin resistance. The miR-146a is also related to insulin resistance as a modulator of the AKT2 gene that controls that resistance. Moreover, from an inflammatory and immune response point of view, both miRNAs (mir-146a and miR-125b) are highly expressed. Then, substantial evidence allows us to point them out as possible markers under pathological conditions and inflammatory processes presented in the MCI, which requires further studies.
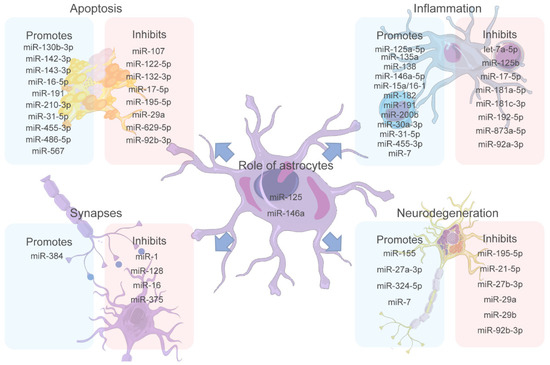
Figure 2.
The miRNA role in significant factors triggering MCI. miRNAs play an important role in the modulation of the different physiological processes of the central nervous system (CNS) affecting different cells. Apoptosis, inflammation, synapses, and neurodegeneration are the most studied processes in MCI regarding how they are affected by miRNA dysregulation. Recent studies have revealed miRNAs that promote or inhibit each of these processes, making them potential targets for biomarkers and treatment targets. Additionally, miR-125 and miR-146a are proposed as miRNAs of great importance since they are expressed by astrocytes, essential cells for brain homeostasis, and have been shown to affect all the other processes mentioned. Figure 2 was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license.
To date, differential expression of miRNAs in ND has started to be studied, but it has become pivotal to study them in MCI due to the necessity of an earlier understanding and diagnosis of these diseases. Hence, finding innovative models to improve biomarker and therapeutic target detection is essential. Many studies have been conducted under a simplistic view, focusing only on detecting the miRNAs in fluids or considering the neuron alone. Different miRNAs in MCI in human, mouse, and rat models have mostly been found in CSF and plasma samples. A more holistic view, taking into account other brain cells and their crosstalk, is required to improve the current knowledge. In this context, astrocytes are of great interest due to their fundamental role in the CNS and their involvement in the pathology of ND. Thus, these cells are considered key to the understanding of neurological disorders.
Considering the findings about miRNAs, studying and understanding the expression and interaction of these molecules in the different cellular pathways involved in MCI will allow us to understand early neurodegenerative stages. In addition, miRNAs present undeniable potential as biomarkers and could be used as pharmacological targets. Notably, multiple studies have demonstrated that a large number of miRNAs can affect biological processes and pathways, and a single miRNA can control the function of different genes that, in turn, cause physiological changes, altering homeostasis.
Author Contributions
Conceptualization, A.E.R., N.G.-J., P.J.P.-R., A.F.A.-P. and J.G.; Writing—original draft, A.E.R., N.G.-J. and M.A.T.; Writing—review & editing, A.E.R., N.G.-J., Y.G.-G., A.P., P.J.P.-R., A.F.A.-P. and J.G.; Visualization, N.G.-J.; Supervision, J.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by SGR Bogotá Colombia BPIN 2020000100357 and Minciencias IDs 8845 to J.G.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Eshkoor, S.A.; Hamid, T.A.; Mun, C.Y.; Ng, C.K. Mild Cognitive Impairment and Its Management in Older People. Clin. Interv. Aging 2015, 10, 687–693. [Google Scholar] [CrossRef]
- Foltynie, T.; Brayne, C.E.G.; Robbins, T.W.; Barker, R.A. The Cognitive Ability of an Incident Cohort of Parkinson’s Patients in the UK. The CamPaIGN Study. Brain 2004, 127, 550–560. [Google Scholar] [CrossRef]
- Mitolo, M.; Stanzani-Maserati, M.; Capellari, S.; Testa, C.; Rucci, P.; Poda, R.; Oppi, F.; Gallassi, R.; Sambati, L.; Rizzo, G.; et al. Predicting Conversion from Mild Cognitive Impairment to Alzheimer’s Disease Using Brain 1 H-MRS and Volumetric Changes: A Two- Year Retrospective Follow-up Study. NeuroImage Clin. 2019, 23, 101843. [Google Scholar] [CrossRef]
- Ogonowski, N.; Salcidua, S.; Leon, T.; Chamorro-Veloso, N.; Valls, C.; Avalos, C.; Bisquertt, A.; Rentería, M.E.; Orellana, P.; Duran-Aniotz, C. Systematic Review: MicroRNAs as Potential Biomarkers in Mild Cognitive Impairment Diagnosis. Front. Aging Neurosci. 2022, 13, 959. [Google Scholar] [CrossRef]
- Aarsland, D.; Bronnick, K.; Williams-Gray, C.; Weintraub, D.; Marder, K.; Kulisevsky, J.; Burn, D.; Barone, P.; Pagonabarraga, J.; Allcock, L.; et al. Mild Cognitive Impairment in Parkinson Disease: A Multicenter Pooled Analysis. Neurology 2010, 75, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia Prevention, Intervention, and Care: 2020 Report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Sachdev, P.S.; Blacker, D.; Blazer, D.G.; Ganguli, M.; Jeste, D.V.; Paulsen, J.S.; Petersen, R.C. Classifying Neurocognitive Disorders: The DSM-5 Approach. Nat. Rev. Neurol. 2014, 10, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”. A Practical Method for Grading the Cognitive State of Patients for the Clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool for Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Arevalo-Rodriguez, I.; Smailagic, N.; Roqué-Figuls, M.; Ciapponi, A.; Sanchez-Perez, E.; Giannakou, A.; Pedraza, O.L.; Bonfill Cosp, X.; Cullum, S. Mini-Mental State Examination (MMSE) for the Early Detection of Dementia in People with Mild Cognitive Impairment (MCI). Cochrane Database Syst. Rev. 2021, 7, CD010783g. [Google Scholar] [CrossRef]
- Schönfeld, P.; Reiser, G. How the Brain Fights Fatty Acids’ Toxicity. Neurochem. Int. 2021, 148, 105050. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, R.; Fusco, R.; Cuzzocrea, S. Astrocytes: Role and Functions in Brain Pathologies. Front. Pharmacol. 2019, 10, 1114. [Google Scholar] [CrossRef] [PubMed]
- Santello, M.; Toni, N.; Volterra, A. Astrocyte Function from Information Processing to Cognition and Cognitive Impairment. Nat. Neurosci. 2019, 22, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, N.; Fehlmann, T.; Kern, F.; Gogol, M.; Maetzler, W.; Deutscher, S.; Gurlit, S.; Schulte, C.; von Thaler, A.K.; Deuschle, C.; et al. Machine Learning to Detect Alzheimer’s Disease from Circulating Non-Coding RNAs. Genom. Proteom. Bioinform. 2019, 17, 430–440. [Google Scholar] [CrossRef]
- Yao, Q.; Chen, Y.; Zhou, X. The Roles of MicroRNAs in Epigenetic Regulation. Curr. Opin. Chem. Biol. 2019, 51, 11–17. [Google Scholar] [CrossRef]
- Adibhatla, R.; Hatcher, J. Role of Lipids in Brain Injury and Diseases. Futur. Lipidol. 2007, 8, 403–422. [Google Scholar] [CrossRef]
- Yoon, H.; Shaw, J.L.; Haigis, M.C.; Greka, A. Lipid Metabolism in Sickness and in Health: Emerging Regulators of Lipotoxicity. Mol. Cell 2021, 81, 3708–3730. [Google Scholar] [CrossRef]
- Gayen, M.; Bhomia, M.; Balakathiresan, N.; Knollmann-Ritschel, B. Exosomal MicroRNAs Released by Activated Astrocytes as Potential Neuroinflammatory Biomarkers. Int. J. Mol. Sci. 2020, 21, 2312. [Google Scholar] [CrossRef]
- Soung, A.; Klein, R.S. Astrocytes: Initiators of and Responders to Inflammation. In Glia in Health and Disease; Spohr, T., Ed.; IntechOpen: London, UK, 2019; ISBN 978-1-78985-254-7. [Google Scholar]
- Tan, B.L.; Norhaizan, M.E. Effect of High-Fat Diets on Oxidative Stress, Cellular Inflammatory Response and Cognitive Function. Nutrients 2019, 11, 2579. [Google Scholar] [CrossRef]
- Schommer, J.; Marwarha, G.; Nagamoto-Combs, K.; Ghribi, O. Palmitic Acid-Enriched Diet Increases α-Synuclein and Tyrosine Hydroxylase Expression Levels in the Mouse Brain. Front. Neurosci. 2018, 12, 552. [Google Scholar] [CrossRef]
- Macdonald-Ramos, K.; Martínez-Ibarra, A.; Monroy, A.; Miranda-Ríos, J.; Cerbón, M. Effect of Dietary Fatty Acids on Microrna Expression Related to Metabolic Disorders and Inflammation in Human and Animal Trials. Nutrients 2021, 13, 1830. [Google Scholar] [CrossRef] [PubMed]
- Nuthikattu, S.; Milenkovic, D.; Rutledge, J.; Villablanca, A. Lipotoxic Injury Differentially Regulates Brain Microvascular Gene Expression in Male Mice. Nutrients 2020, 12, 1771. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, G.; Heard, E. Advances in Epigenetics Link Genetics to the Environment and Disease. Nature 2019, 571, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Waddington, C.H. The Epigenotype. 1942. Int. J. Epidemiol. 2012, 41, 10–13. [Google Scholar] [CrossRef]
- Bender, J. DNA Methylation and Epigenetics. Annu. Rev. Plant Biol. 2004, 55, 41–68. [Google Scholar] [CrossRef]
- Fotuhi, S.N.; Khalaj-Kondori, M.; Hoseinpour Feizi, M.A.; Talebi, M. Long Non-Coding RNA BACE1-AS May Serve as an Alzheimer’s Disease Blood-Based Biomarker. J. Mol. Neurosci. 2019, 69, 351–359. [Google Scholar] [CrossRef]
- Zhou, Y.; Ge, Y.; Liu, Q.; Li, Y.X.; Chao, X.; Guan, J.J.; Diwu, Y.C.; Zhang, Q. LncRNA BACE1-AS Promotes Autophagy-Mediated Neuronal Damage Through The MiR-214-3p/ATG5 Signalling Axis In Alzheimer’s Disease. Neuroscience 2021, 455, 52–64. [Google Scholar] [CrossRef]
- Li, F.; Wang, Y.; Yang, H.; Xu, Y.; Zhou, X.; Zhang, X.; Xie, Z.; Bi, J. The Effect of BACE1-AS on β-Amyloid Generation by Regulating BACE1 MRNA Expression. BMC Mol. Biol. 2019, 20, 23. [Google Scholar] [CrossRef]
- Zeng, T.; Ni, H.; Yu, Y.; Zhang, M.; Wu, M.; Wang, Q.; Wang, L.; Xu, S.; Xu, Z.; Xu, C.; et al. BACE1-AS Prevents BACE1 MRNA Degradation through the Sequestration of BACE1-Targeting MiRNAs. J. Chem. Neuroanat. 2019, 98, 87–96. [Google Scholar] [CrossRef]
- Das, B.; Yan, R. Role of BACE1 in Alzheimer’s Synaptic Function. Transl. Neurodegener. 2017, 6, 23. [Google Scholar] [CrossRef]
- García-Fonseca, Á.; Martin-Jimenez, C.; Barreto, G.E.; Pachón, A.F.A.; González, J. The Emerging Role of Long Non-Coding Rnas and Micrornas in Neurodegenerative Diseases: A Perspective of Machine Learning. Biomolecules 2021, 11, 1132. [Google Scholar] [CrossRef] [PubMed]
- International Human Genome Sequencing Consortium Finishing the Euchromatic Sequence of the Human Genome. Nature 2004, 431, 931–945. [CrossRef] [PubMed]
- Bhatti, G.K.; Khullar, N.; Sidhu, I.S.; Navik, U.S.; Reddy, A.P.; Reddy, P.H.; Bhatti, J.S. Emerging Role of Non-coding RNA in Health and Disease. Metab. Brain Dis. 2021, 36, 1. [Google Scholar] [CrossRef] [PubMed]
- Yates, L.A.; Norbury, C.J.; Gilbert, R.J.C. The Long and Short of MicroRNA. Cell 2013, 153, 516–519. [Google Scholar] [CrossRef] [PubMed]
- Grosshans, H.; Slack, F.J. Micro-RNAs: Small Is Plentiful. J. Cell Biol. 2002, 156, 17. [Google Scholar] [CrossRef]
- Good, L. Translation Repression by Antisense Sequences. Cell. Mol. Life Sci. 2003, 60, 854–861. [Google Scholar] [CrossRef]
- Lytle, J.R.; Yario, T.A.; Steitz, J.A. Target MRNAs Are Repressed as Efficiently by MicroRNA-Binding Sites in the 5′ UTR as in the 3′ UTR. Proc. Natl. Acad. Sci. USA 2007, 104, 9667–9672. [Google Scholar] [CrossRef]
- Hausser, J.; Syed, A.P.; Bilen, B.; Zavolan, M. Analysis of CDS-Located MiRNA Target Sites Suggests That They Can Effectively Inhibit Translation. Genome Res. 2013, 23, 604–615. [Google Scholar] [CrossRef]
- Fukunaga, R.; Han, B.W.; Hung, J.H.; Xu, J.; Weng, Z.; Zamore, P.D. Dicer Partner Proteins Tune the Length of Mature MiRNAs in Flies and Mammals. Cell 2012, 151, 533–546. [Google Scholar] [CrossRef]
- Pascale, E.; Caiazza, C.; Paladino, M.; Parisi, S.; Passaro, F.; Caiazzo, M. MicroRNA Roles in Cell Reprogramming Mechanisms. Cells 2022, 11, 940. [Google Scholar] [CrossRef]
- Judson, R.L.; Babiarz, J.E.; Venere, M.; Blelloch, R. Embryonic Stem Cell-Specific MicroRNAs Promote Induced Pluripotency. Nat. Biotechnol. 2009, 27, 459–461. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced Pluripotent Stem Cell Lines Derived from Human Somatic Cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef] [PubMed]
- Marión, R.M.; Strati, K.; Li, H.; Murga, M.; Blanco, R.; Ortega, S.; Fernandez-Capetillo, O.; Serrano, M.; Blasco, M.A. A P53-Mediated DNA Damage Response Limits Reprogramming to Ensure IPS Cell Genomic Integrity. Nature 2009, 460, 1149–1153. [Google Scholar] [CrossRef] [PubMed]
- Sempere, L.F.; Freemantle, S.; Pitha-Rowe, I.; Moss, E.; Dmitrovsky, E.; Ambros, V. Expression Profiling of Mammalian MicroRNAs Uncovers a Subset of Brain-Expressed MicroRNAs with Possible Roles in Murine and Human Neuronal Differentiation. Genome Biol. 2004, 5, R13. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Han, M.; Liu, W.; Tao, J.; Chen, L. Circulating MicroRNAs as Diagnostic Biomarkers of Clinical Cognitive Impairment: A Meta-Analysis. Am. J. Alzheimers. Other Dis. Demen. 2020, 35, 1533317520951686. [Google Scholar] [CrossRef]
- Maciotta, S.; Meregalli, M.; Torrente, Y. The Involvement of MicroRNAs in Neurodegenerative Diseases. Front. Cell. Neurosci. 2013, 7, 265. [Google Scholar] [CrossRef]
- Lau, P.; Bossers, K.; Janky, R.; Salta, E.; Frigerio, C.S.; Barbash, S.; Rothman, R.; Sierksma, A.S.R.; Thathiah, A.; Greenberg, D.; et al. Alteration of the MicroRNA Network during the Progression of Alzheimer’s Disease. EMBO Mol. Med. 2013, 5, 1613–1634. [Google Scholar] [CrossRef]
- Almutairi, M.M.A.; Gong, C.; Xu, Y.G.; Chang, Y.; Shi, H. Factors Controlling Permeability of the Blood-Brain Barrier. Cell. Mol. Life Sci. 2016, 73, 57–77. [Google Scholar] [CrossRef]
- Carini, G.; Musazzi, L.; Bolzetta, F.; Cester, A.; Fiorentini, C.; Ieraci, A.; Maggi, S.; Popoli, M.; Veronese, N.; Barbon, A. The Potential Role of MiRNAs in Cognitive Frailty. Front. Aging Neurosci. 2021, 13, 777. [Google Scholar] [CrossRef]
- Arora, T.; Prashar, V.; Singh, R.; Barwal, T.S.; Changotra, H.; Sharma, A.; Parkash, J. Dysregulated MiRNAs in Progression and Pathogenesis of Alzheimer’s Disease. Mol. Neurobiol. 2022, 59, 6107–6124. [Google Scholar] [CrossRef]
- Koopaei, N.N.; Chowdhury, E.A.; Jiang, J.; Noorani, B.; da Silva, L.; Bulut, G.; Hakimjavadi, H.; Chamala, S.; Bickel, U.; Schmittgen, T.D. Enrichment of the Erythrocyte MiR-451a in Brain Extracellular Vesicles Following Impairment of the Blood-Brain Barrier. Neurosci. Lett. 2021, 751, 135829. [Google Scholar] [CrossRef] [PubMed]
- Trattnig, C.; Üçal, M.; Tam-Amersdorfer, C.; Bucko, A.; Zefferer, U.; Grünbacher, G.; Absenger-Novak, M.; Öhlinger, K.A.; Kraitsy, K.; Hamberger, D.; et al. MicroRNA-451a Overexpression Induces Accelerated Neuronal Differentiation of Ntera2/D1 Cells and Ablation Affects Neurogenesis in MicroRNA-451a-/- Mice. PLoS ONE 2018, 13, e0207575. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.J.; Yan, M.L.; Wang, Q.; Mao, M.; Su, D.; Sun, L.L.; Li, K.X.; Qu, Y.; Sun, Q.; Zhang, X.Y.; et al. Overexpression of MiR-1 in the Heart Attenuates Hippocampal Synaptic Vesicle Exocytosis by the Posttranscriptional Regulation of SNAP-25 through the Transportation of Exosomes. Cell Commun. Signal. 2018, 16, 91. [Google Scholar] [CrossRef] [PubMed]
- Shvarts-Serebro, I.; Sheinin, A.; Gottfried, I.; Adler, L.; Schottlender, N.; Ashery, U.; Barak, B. MiR-128 as a Regulator of Synaptic Properties in 5xFAD Mice Hippocampal Neurons. J. Mol. Neurosci. 2021, 71, 2593–2607. [Google Scholar] [CrossRef] [PubMed]
- Businaro, R.; Corsi, M.; Asprino, R.; Di Lorenzo, C.; Laskin, D.; Corbo, R.M.; Ricci, S.; Pinto, A. Modulation of Inflammation as a Way of Delaying Alzheimer’s Disease Progression: The Diet’s Role. Curr. Alzheimer Res. 2018, 15, 363–380. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, J.; Li, Y.; Cui, L.; Wu, K.; Luo, H. Astaxanthin Inhibits Microglia M1 Activation against Inflammatory Injury Triggered by Lipopolysaccharide through Down-Regulating MiR-31-5p. Life Sci. 2021, 267, 118943. [Google Scholar] [CrossRef]
- Bray, S.J. Notch Signalling: A Simple Pathway Becomes Complex. Nat. Rev. Mol. Cell Biol. 2006, 7, 678–689. [Google Scholar] [CrossRef]
- Cai, L.; Ge, B.; Xu, S.; Chen, X.; Yang, H. Up-Regulation of CircARF3 Reduces Blood-Brain Barrier Damage in Rat Subarachnoid Hemorrhage Model via MiR-31-5p/MyD88/NF-ΚB Axis. Aging 2021, 13, 21345–21363. [Google Scholar] [CrossRef]
- Zhou, C.; Sun, P.; Xu, Y.; Chen, Y.; Huang, Y.; Hamblin, M.H.; Foley, L.; Hitchens, T.K.; Li, S.; Yin, K.J. Genetic Deficiency of MicroRNA-15a/16-1 Confers Resistance to Neuropathological Damage and Cognitive Dysfunction in Experimental Vascular Cognitive Impairment and Dementia. Adv. Sci. 2022, 9, e2104986. [Google Scholar] [CrossRef]
- Liao, K.; Niu, F.; Hu, G.; Yang, L.; Dallon, B.; Villarreal, D.; Buch, S. Morphine-mediated Release of MiR-138 in Astrocyte-derived Extracellular Vesicles Promotes Microglial Activation. J. Extracell. Vesicles 2020, 10, e12027. [Google Scholar] [CrossRef]
- Fan, Z.; Dani, M.; Femminella, G.D.; Wood, M.; Calsolaro, V.; Veronese, M.; Turkheimer, F.; Gentleman, S.; Brooks, D.J.; Hinz, R.; et al. Parametric Mapping Using Spectral Analysis for 11 C-PBR28 PET Reveals Neuroinflammation in Mild Cognitive Impairment Subjects. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1432–1441. [Google Scholar] [CrossRef]
- Cagnin, A.; Brooks, D.J.; Kennedy, A.M.; Gunn, R.N.; Myers, R.; Turkheimer, F.E.; Jones, T.; Banati, R.B. In-Vivo Measurement of Activated Microglia in Dementia. Lancet 2001, 358, 461–467. [Google Scholar] [CrossRef]
- Shi, J.; Chi, Y.; Wang, X.; Zhang, Y.; Tian, L.; Chen, Y.; Chen, C.; Dong, Y.; Sang, H.; Chen, M.; et al. MiR-124 Regulates IQGAP1 and Participates in the Relationship Between Morphine Dependence Susceptibility and Cognition. Front. Psychiatry 2022, 13, 845357. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, F.; Montesinos, J.; Ureña-Peralta, J.R.; Guerri, C.; Pascual, M. TLR4 Participates in the Transmission of Ethanol-Induced Neuroinflammation via Astrocyte-Derived Extracellular Vesicles. J. Neuroinflammation 2019, 16, 136. [Google Scholar] [CrossRef] [PubMed]
- Béjot, Y.; Garnier, P. Cerebral Ischemia. In Hormesis in Health and Disease; Rattan, S.I., Le Bourg, É., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 185–200. ISBN 9781482205466. [Google Scholar]
- Ponirakis, G.; Elsotouhy, A.; Al Hamad, H.; Vattoth, S.; Petropoulos, I.N.; Khan, A.; Gad, H.; Al-Khayat, F.; Chandran, M.; Ramadan, M.; et al. Association of Cerebral Ischemia With Corneal Nerve Loss and Brain Atrophy in MCI and Dementia. Front. Neurosci. 2021, 15, 763. [Google Scholar] [CrossRef] [PubMed]
- Climent, M.; Quintavalle, M.; Miragoli, M.; Chen, J.; Condorelli, G.; Elia, L. TGFβ Triggers MiR-143/145 Transfer From Smooth Muscle Cells to Endothelial Cells, Thereby Modulating Vessel Stabilization. Circ. Res. 2015, 116, 1753–1764. [Google Scholar] [CrossRef]
- Zhou, D.; Huang, Z.; Zhu, X.; Hong, T.; Zhao, Y. Circular RNA 0025984 Ameliorates Ischemic Stroke Injury and Protects Astrocytes Through MiR-143-3p/TET1/ORP150 Pathway. Mol. Neurobiol. 2021, 58, 5937–5953. [Google Scholar] [CrossRef]
- Tiedt, S.; Prestel, M.; Malik, R.; Schieferdecker, N.; Duering, M.; Kautzky, V.; Stoycheva, I.; Böck, J.; Northoff, B.H.; Klein, M.; et al. RNA-Seq Identifies Circulating MIR-125a-5p, MIR-125b-5p, and MIR-143-3p as Potential Biomarkers for Acute Ischemic Stroke. Circ. Res. 2017, 121, 970–980. [Google Scholar] [CrossRef]
- Tower, D.B.; Young, O.M. The Activities of Butyrylcholinesterase and Carbonic Anhydrase, the Rate of Anaerobic Glycolysis, and the Question of a Constant Density of Glial Cells in Cerebral Cortices of Various Mammalian Species from Mouse to Whale. J. Neurochem. 1973, 20, 269–278. [Google Scholar] [CrossRef]
- Phatnani, H.; Maniatis, T. Astrocytes in Neurodegenerative Disease. Cold Spring Harb. Perspect. Biol. 2015, 7, a020628. [Google Scholar] [CrossRef]
- Hamilton, N.B.; Attwell, D. Do Astrocytes Really Exocytose Neurotransmitters? Nat. Rev. Neurosci. 2010, 11, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Pellerin, L.; Magistretti, P.J. Glutamate Uptake into Astrocytes Stimulates Aerobic Glycolysis: A Mechanism Coupling Neuronal Activity to Glucose Utilization. Proc. Natl. Acad. Sci. USA 1994, 91, 10625–10629. [Google Scholar] [CrossRef] [PubMed]
- Magistretti, P.J.; Pellerin, L. Cellular Mechanisms of Brain Energy Metabolism. Relevance to Functional Brain Imaging and to Neurodegenerative Disorders. Ann. N. Y. Acad. Sci. 1996, 777, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Davla, S.; Artiushin, G.; Li, Y.; Chitsaz, D.; Li, S.; Sehgal, A.; van Meyel, D.J. AANAT1 Functions in Astrocytes to Regulate Sleep Homeostasis. Elife 2020, 9, e53994. [Google Scholar] [CrossRef] [PubMed]
- Bojarskaite, L.; Bjørnstad, D.M.; Pettersen, K.H.; Cunen, C.; Hermansen, G.H.; Åbjørsbråten, K.S.; Chambers, A.R.; Sprengel, R.; Vervaeke, K.; Tang, W.; et al. Astrocytic Ca 2+ Signaling Is Reduced during Sleep and Is Involved in the Regulation of Slow Wave Sleep. Nat. Commun. 2020, 11, 3240. [Google Scholar] [CrossRef] [PubMed]
- Drögemüller, K.; Helmuth, U.; Brunn, A.; Sakowicz-Burkiewicz, M.; Gutmann, D.H.; Mueller, W.; Deckert, M.; Schlüter, D. Astrocyte Gp130 Expression Is Critical for the Control of Toxoplasma Encephalitis. J. Immunol. 2008, 181, 2683–2693. [Google Scholar] [CrossRef]
- Cekanaviciute, E.; Dietrich, H.K.; Axtell, R.C.; Williams, A.M.; Egusquiza, R.; Wai, K.M.; Koshy, A.A.; Buckwalter, M.S. Astrocytic TGF-β Signaling Limits Inflammation and Reduces Neuronal Damage during Central Nervous System Toxoplasma Infection. J. Immunol. 2014, 193, 139–149. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Astrocyte Barriers to Neurotoxic Inflammation. Nat. Rev. Neurosci. 2015, 16, 249–263. [Google Scholar] [CrossRef]
- Liu, P.; Wang, X.; Yang, Q.; Yan, X.; Fan, Y.; Zhang, S.; Wei, Y.; Huang, M.; Jiang, L.; Feng, L. Collaborative Action of Microglia and Astrocytes Mediates Neutrophil Recruitment to the CNS to Defend against Escherichia Coli K1 Infection. Int. J. Mol. Sci. 2022, 23, 6540. [Google Scholar] [CrossRef]
- Wang, S.; He, Y.; Zhang, H.; Chen, L.; Cao, L.; Yang, L.; Wang, C.; Pan, Y.; Tang, Q.; Tan, W.; et al. The Neural Stem Cell Properties of PKD2L1 + Cerebrospinal Fluid-Contacting Neurons in Vitro. Front. Cell. Neurosci. 2021, 15, 630882. [Google Scholar] [CrossRef]
- Morgun, A.V.; Osipova, E.D.; Boitsova, E.B.; Shuvaev, A.N.; Malinovskaya, N.A.; Mosiagina, A.I.; Salmina, A.B. Neurogenic Potential of Implanted Neurospheres Is Regulated by Optogenetic Stimulation of Hippocampal Astrocytes Ex Vivo. Bull. Exp. Biol. Med. 2021, 170, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Taga, A.; Dastgheyb, R.; Habela, C.; Joseph, J.; Richard, J.P.; Gross, S.K.; Lauria, G.; Lee, G.; Haughey, N.; Maragakis, N.J. Role of Human-Induced Pluripotent Stem Cell-Derived Spinal Cord Astrocytes in the Functional Maturation of Motor Neurons in a Multielectrode Array System. Stem Cells Transl. Med. 2019, 8, 1272–1285. [Google Scholar] [CrossRef] [PubMed]
- Dozio, V.; Sanchez, J.C. Profiling the Proteomic Inflammatory State of Human Astrocytes Using DIA Mass Spectrometry. J. Neuroinflammation 2018, 15, 331. [Google Scholar] [CrossRef]
- Wareham, L.K.; Liddelow, S.A.; Temple, S.; Benowitz, L.I.; Di Polo, A.; Wellington, C.; Goldberg, J.L.; He, Z.; Duan, X.; Bu, G.; et al. Solving Neurodegeneration: Common Mechanisms and Strategies for New Treatments. Mol. Neurodegener. 2022, 17, 23. [Google Scholar] [CrossRef]
- Zamanian, J.L.; Xu, L.; Foo, L.C.; Nouri, N.; Zhou, L.; Giffard, R.G.; Barres, B.A. Genomic Analysis of Reactive Astrogliosis. J. Neurosci. 2012, 32, 6391–6410. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.; Torres, C. Astrocyte Senescence: Evidence and Significance. Aging Cell 2019, 18, e12937. [Google Scholar] [CrossRef] [PubMed]
- Pekny, M.; Pekna, M. Astrocyte Reactivity and Reactive Astrogliosis: Costs and Benefits. Physiol. Rev. 2014, 94, 1077–1098. [Google Scholar] [CrossRef]
- Pekny, M.; Wilhelmsson, U.; Pekna, M. The Dual Role of Astrocyte Activation and Reactive Gliosis. Neurosci. Lett. 2014, 565, 30–38. [Google Scholar] [CrossRef]
- Dallérac, G.; Zapata, J.; Rouach, N. Versatile Control of Synaptic Circuits by Astrocytes: Where, When and How? Nat. Rev. Neurosci. 2018, 19, 729–743. [Google Scholar] [CrossRef]
- Bai, Y.; Su, X.; Piao, L.; Jin, Z.; Jin, R. Involvement of Astrocytes and MicroRNA Dysregulation in Neurodegenerative Diseases: From Pathogenesis to Therapeutic Potential. Front. Mol. Neurosci. 2021, 14, 556215. [Google Scholar] [CrossRef]
- Kieran, N.W.; Suresh, R.; Dorion, M.F.; MacDonald, A.; Blain, M.; Wen, D.; Fuh, S.C.; Ryan, F.; Diaz, R.J.; Stratton, J.A.; et al. MicroRNA-210 Regulates the Metabolic and Inflammatory Status of Primary Human Astrocytes. J. Neuroinflammation 2022, 19, 10. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.T.S.; Ludwin, S.K.; Fuh, S.C.; Sawaya, R.; Moore, C.S.; Ho, M.K.; Bedell, B.J.; Sarnat, H.B.; Bar-Or, A.; Antel, J.P. MicroRNA Expression Patterns in Human Astrocytes in Relation to Anatomical Location and Age. J. Neuropathol. Exp. Neurol. 2016, 75, 156–166. [Google Scholar] [CrossRef]
- Giovannoni, F.; Quintana, F.J. The Role of Astrocytes in CNS Inflammation. Trends Immunol. 2020, 41, 805–819. [Google Scholar] [CrossRef] [PubMed]
- Chu, A.J.; Williams, J.M. Astrocytic MicroRNA in Ageing, Inflammation, and Neurodegenerative Disease. Front. Physiol. 2022, 12, 826697. [Google Scholar] [CrossRef] [PubMed]
- Mauch, D.H.; Nägier, K.; Schumacher, S.; Göritz, C.; Müller, E.C.; Otto, A.; Pfrieger, F.W. CNS Synaptogenesis Promoted by Glia-Derived Cholesterol. Science 2001, 294, 1354–1357. [Google Scholar] [CrossRef] [PubMed]
- Raihan, O.; Brishti, A.; Molla, M.R.; Li, W.; Zhang, Q.; Xu, P.; Khan, M.I.; Zhang, J.; Liu, Q. The Age-Dependent Elevation of MiR-335-3p Leads to Reduced Cholesterol and Impaired Memory in Brain. Neuroscience 2018, 390, 160–173. [Google Scholar] [CrossRef]
- Pittaluga, A. CCL5-Glutamate Cross-Talk in Astrocyte-Neuron Communication in Multiple Sclerosis. Front. Immunol. 2017, 8, 1079. [Google Scholar] [CrossRef]
- Ambrosini, E.; Remoli, M.E.; Giacomini, E.; Rosicarelli, B.; Serafini, B.; Lande, R.; Aloisi, F.; Coccia, E.M. Astrocytes Produce Dendritic Cell-Attracting Chemokines In Vitro and in Multiple Sclerosis Lesions. J. Neuropathol. Exp. Neurol. 2005, 64, 706–715. [Google Scholar] [CrossRef]
- Sun, C.; Zhu, L.; Ma, R.; Ren, J.; Wang, J.; Gao, S.; Yang, D.; Ning, K.; Ling, B.; Lu, B.; et al. Astrocytic MiR-324-5p Is Essential for Synaptic Formation by Suppressing the Secretion of CCL5 from Astrocytes. Cell Death Dis. 2019, 10, 141. [Google Scholar] [CrossRef]
- Kinser, H.E.; Pincus, Z. MicroRNAs as Modulators of Longevity and the Aging Process. Hum. Genet. 2020, 139, 291–308. [Google Scholar] [CrossRef]
- Vandenberg, R.J.; Ryan, R.M. Mechanisms of Glutamate Transport. Physiol. Rev. 2013, 93, 1621–1657. [Google Scholar] [CrossRef] [PubMed]
- Montana, V.; Ni, Y.; Sunjara, V.; Hua, X.; Parpura, V. Vesicular Glutamate Transporter-Dependent Glutamate Release from Astrocytes. J. Neurosci. 2004, 24, 2633–2642. [Google Scholar] [CrossRef]
- Harraz, M.M.; Eacker, S.M.; Wang, X.; Dawson, T.M.; Dawson, V.L. MicroRNA-223 Is Neuroprotective by Targeting Glutamate Receptors. Proc. Natl. Acad. Sci. USA 2012, 109, 18962–18967. [Google Scholar] [CrossRef] [PubMed]
- McKeon, A.; Benarroch, E.E. Glial Fibrillary Acid Protein: Functions and Involvement in Disease. Neurology 2018, 90, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, J.; Zheng, J.; Qin, S. Reactive Astrocytes in Neurodegenerative Diseases. Aging Dis. 2019, 10, 664–675. [Google Scholar] [CrossRef]
- Wang, C.Y.; Yang, S.H.; Tzeng, S.F. MicroRNA-145 as One Negative Regulator of Astrogliosis. Glia 2015, 63, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Pogue, A.I.; Cui, J.G.; Li, Y.Y.; Zhao, Y.; Culicchia, F.; Lukiw, W.J. Micro RNA-125b (MiRNA-125b) Function in Astrogliosis and Glial Cell Proliferation. Neurosci. Lett. 2010, 476, 18–22. [Google Scholar] [CrossRef]
- Cardoso, A.L.; Guedes, J.R.; Pereira de Almeida, L.; Pedroso de Lima, M.C. MiR-155 Modulates Microglia-Mediated Immune Response by down-Regulating SOCS-1 and Promoting Cytokine and Nitric Oxide Production. Immunology 2012, 135, 73–88. [Google Scholar] [CrossRef]
- Yao, H.; Ma, R.; Yang, L.; Hu, G.; Chen, X.; Duan, M.; Kook, Y.; Niu, F.; Liao, K.; Fu, M.; et al. MiR-9 Promotes Microglial Activation by Targeting MCPIP1. Nat. Commun. 2014, 5, 4386. [Google Scholar] [CrossRef]
- Hutchison, E.R.; Kawamoto, E.M.; Taub, D.D.; Lal, A.; Abdelmohsen, K.; Zhang, Y.; Wood, W.H.; Lehrmann, E.; Camandola, S.; Becker, K.G.; et al. Evidence for MiR-181 Involvement in Neuroinflammatory Responses of Astrocytes. Glia 2013, 61, 1018–1028. [Google Scholar] [CrossRef]
- Long, X.; Yao, X.; Jiang, Q.; Yang, Y.; He, X.; Tian, W.; Zhao, K.; Zhang, H. Astrocyte-Derived Exosomes Enriched with MiR-873a-5p Inhibit Neuroinflammation via Microglia Phenotype Modulation after Traumatic Brain Injury. J. Neuroinflammation 2020, 17, 89. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A.D.; Dastgheyb, R.M.; Yoo, S.W.; Trout, A.; Talbot, C.C.; Hao, H.; Witwer, K.W.; Haughey, N.J. TNFα and IL-1β Modify the MiRNA Cargo of Astrocyte Shed Extracellular Vesicles to Regulate Neurotrophic Signaling in Neurons. Cell Death Dis. 2018, 9, 363. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xiong, Y.; Yan, C.; Chen, L.; Chen, D.; Mi, B.; Liu, G. Downregulation of MicroRNA-16-5p Accelerates Fracture Healing by Promoting Proliferation and Inhibiting Apoptosis of Osteoblasts in Patients with Traumatic Brain Injury. Am. J. Transl. Res. 2019, 11, 4746. [Google Scholar]
- Ouyang, Y.-B.; Xu, L.; Lu, Y.; Sun, X.; Yue, S.; Xiong, X.-X.; Giffard, R.G. Astrocyte Enriched MiR-29a Targets PUMA and Reduces Neuronal Vulnerability to Forebrain Ischemia. Glia 2013, 61, 1784–1794. [Google Scholar] [CrossRef] [PubMed]
- Khanna, S.; Rink, C.; Ghoorkhanian, R.; Gnyawali, S.; Heigel, M.; Wijesinghe, D.S.; Chalfant, C.E.; Chan, Y.C.; Banerjee, J.; Huang, Y.; et al. Loss of MiR-29b Following Acute Ischemic Stroke Contributes to Neural Cell Death and Infarct Size. J. Cereb. Blood Flow Metab. 2013, 33, 1197–1206. [Google Scholar] [CrossRef]
- Du, L.; Jiang, Y.; Sun, Y. Astrocyte-Derived Exosomes Carry MicroRNA-17-5p to Protect Neonatal Rats from Hypoxic-Ischemic Brain Damage via Inhibiting BNIP-2 Expression. Neurotoxicology 2021, 83, 28–39. [Google Scholar] [CrossRef]
- Xu, L.; Cao, H.; Xie, Y.; Zhang, Y.; Du, M.; Xu, X.; Ye, R.; Liu, X. Exosome-Shuttled MiR-92b-3p from Ischemic Preconditioned Astrocytes Protects Neurons against Oxygen and Glucose Deprivation. Brain Res. 2019, 1717, 66–73. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Z.; Jiang, C.; Jiang, X.; Zhang, J. MiR-92b-3p Promotes Neurite Growth and Functional Recovery via the PTEN/AKT Pathway in Acute Spinal Cord Injury. J. Cell. Physiol. 2019, 234, 23043–23052. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Molecular Dissection of Reactive Astrogliosis and Glial Scar Formation. Trends Neurosci. 2009, 32, 638. [Google Scholar] [CrossRef]
- Bhalala, O.G.; Pan, L.; Sahni, V.; McGuire, T.L.; Gruner, K.; Tourtellotte, W.G.; Kessler, J.A. MicroRNA-21 Regulates Astrocytic Response Following Spinal Cord Injury. J. Neurosci. 2012, 32, 17935–17947. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, S.; Chen, J.; Cai, H.; Huang, W.; Zhang, Y.; Wang, L.; Xing, Y. MicroRNA-190 Alleviates Neuronal Damage and Inhibits Neuroinflammation via Nlrp3 in MPTP-Induced Parkinson’s Disease Mouse Model. J. Cell. Physiol. 2019, 234, 23379–23387. [Google Scholar] [CrossRef] [PubMed]
- Bruce, K.D.; Zsombok, A.; Eckel, R.H. Lipid Processing in the Brain: A Key Regulator of Systemic Metabolism. Front. Endocrinol. 2017, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Hussain, G.; Wang, J.; Rasul, A.; Anwar, H.; Imran, A.; Qasim, M.; Zafar, S.; Kamran, S.K.S.; Razzaq, A.; Aziz, N.; et al. Role of Cholesterol and Sphingolipids in Brain Development and Neurological Diseases. Lipids Health Dis. 2019, 18, 26. [Google Scholar] [CrossRef] [PubMed]
- Melo, H.M.; Seixas da Silva, G.d.S.; Sant’Ana, M.R.; Teixeira, C.V.L.; Clarke, J.R.; Miya Coreixas, V.S.; de Melo, B.C.; Fortuna, J.T.S.; Forny-Germano, L.; Ledo, J.H.; et al. Palmitate Is Increased in the Cerebrospinal Fluid of Humans with Obesity and Induces Memory Impairment in Mice via Pro-Inflammatory TNF-α. Cell Rep. 2020, 30, 2180–2194.e8. [Google Scholar] [CrossRef]
- Hamilton, J.A.; Hillard, C.J.; Spector, A.A.; Watkins, P.A. Brain Uptake and Utilization of Fatty Acids, Lipids and Lipoproteins: Application to Neurological Disorders. J. Mol. Neurosci. 2007, 33, 2–11. [Google Scholar] [CrossRef]
- Barber, C.N.; Raben, D.M. Lipid Metabolism Crosstalk in the Brain: Glia and Neurons. Front. Cell. Neurosci. 2019, 13, 212. [Google Scholar] [CrossRef]
- Smolič, T.; Tavčar, P.; Horvat, A.; Černe, U.; Petan, T.; Zorec, R.; Vardjan, N. Stressed Astrocytes Accumulate Lipid Droplets. Glia 2021, 69, E233–E234. [Google Scholar] [CrossRef]
- Ralhan, I.; Chang, C.L.; Lippincott-Schwartz, J.; Ioannou, M.S. Lipid Droplets in the Nervous System. J. Cell Biol. 2021, 220, e202102136. [Google Scholar] [CrossRef]
- Olzmann, J.A.; Carvalho, P. Dynamics and Functions of Lipid Droplets. Nat. Rev. Mol. Cell Biol. 2019, 20, 137–155. [Google Scholar] [CrossRef]
- Bozza, P.T.; Viola, J.P.B. Lipid Droplets in Inflammation and Cancer. Prostaglandins Leukot. Essent. Fat. Acids 2010, 82, 243–250. [Google Scholar] [CrossRef]
- Farmer, B.C.; Walsh, A.E.; Kluemper, J.C.; Johnson, L.A. Lipid Droplets in Neurodegenerative Disorders. Front. Neurosci. 2020, 14, 742. [Google Scholar] [CrossRef] [PubMed]
- Feinkohl, I.; Janke, J.; Slooter, A.J.C.; Winterer, G.; Spies, C.; Pischon, T. Plasma Leptin, but Not Adiponectin, Is Associated with Cognitive Impairment in Older Adults. Psychoneuroendocrinology 2020, 120, 104783. [Google Scholar] [CrossRef] [PubMed]
- Parimisetty, A.; Dorsemans, A.C.; Awada, R.; Ravanan, P.; Diotel, N.; Lefebvre d’Hellencourt, C. Secret Talk between Adipose Tissue and Central Nervous System via Secreted Factors-an Emerging Frontier in the Neurodegenerative Research. J. Neuroinflammation 2016, 13, 67. [Google Scholar] [CrossRef]
- Yi, X.; Liu, J.; Wu, P.; Gong, Y.; Xu, X.; Li, W. The Key MicroRNA on Lipid Droplet Formation during Adipogenesis from Human Mesenchymal Stem Cells. J. Cell. Physiol. 2020, 235, 328–338. [Google Scholar] [CrossRef]
- Karthikeyan, A.; Patnala, R.; Jadhav, S.P.; Eng-Ang, L.; Thameem Dheen, S. MicroRNAs: Key Players in Microglia and Astrocyte Mediated Inflammation in CNS Pathologies. Curr. Med. Chem. 2016, 23, 3528–3546. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.; Kwak, S.; Kim, K.Y.; Kim, H.; Cho, S.Y.; Kim, M.; Lee, J.-Y.; Kim, E. Relationship between Adipokines, Cognition, and Brain Structures in Old Age Depending on Obesity. J. Gerontol. Ser. A 2022, glac021. [Google Scholar] [CrossRef] [PubMed]
- Kempuraj, D.; Thangavel, R.; Selvakumar, G.P.; Zaheer, S.; Ahmed, M.E.; Raikwar, S.P.; Zahoor, H.; Saeed, D.; Natteru, P.A.; Iyer, S.; et al. Brain and Peripheral Atypical Inflammatory Mediators Potentiate Neuroinflammation and Neurodegeneration. Front. Cell. Neurosci. 2017, 11, 216. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Barua, S.; Jeong, Y.J.; Lee, J.E. Adiponectin: The Potential Regulator and Therapeutic Target of Obesity and Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 6419. [Google Scholar] [CrossRef]
- Song, N.; Jeong, D.Y.; Tu, T.H.; Park, B.S.; Yang, H.R.; Kim, Y.J.; Kim, J.K.; Park, J.T.; Yeh, J.Y.; Yang, S.; et al. Adiponectin Controls Nutrient Availability in Hypothalamic Astrocytes. Int. J. Mol. Sci. 2021, 22, 1587. [Google Scholar] [CrossRef]
- Sánchez-Margalet, V.; Martín-Romero, C.; Santos-Alvarez, J.; Goberna, R.; Najib, S.; Gonzalez-Yanes, C. Role of Leptin as an Immunomodulator of Blood Mononuclear Cells: Mechanisms of Action. Clin. Exp. Immunol. 2003, 133, 11–19. [Google Scholar] [CrossRef]
- Ishida, M.; Shimabukuro, M.; Yagi, S.; Nishimoto, S.; Kozuka, C.; Fukuda, D.; Soeki, T.; Masuzaki, H.; Tsutsui, M.; Sata, M. MicroRNA-378 Regulates Adiponectin Expression in Adipose Tissue: A New Plausible Mechanism. PLoS ONE 2014, 9, e111537. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, D.; Zhou, H.; Wu, G.; He, Z.; Liao, W.; Li, Y.; Zhi, Y. MicroRNA-338-5p Alleviates Neuronal Apoptosis via Directly Targeting BCL2L11 in APP/PS1 Mice. Aging 2020, 12, 20728–20741. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, Z.; Moazedi, A.A.; Chinipardaz, R. The Effect of Palmitic Acid on Spatial Learning and Extinction in Adult Male Rat. Pak. J. Biol. Sci. 2007, 10, 2653–2658. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, L.; Wang, Z.; Mata, I. MicroRNAs: Game Changers in the Regulation of α-Synuclein in Parkinson’s Disease. Parkinsons. Dis. 2019, 2019, 1743183. [Google Scholar] [CrossRef]
- Baudry, A.; Mouillet-Richard, S.; Schneider, B.; Launay, J.M.; Kellermann, O. MiR-16 Targets the Serotonin Transporter: A New Facet for Adaptive Responses to Antidepressants. Science 2010, 329, 1537–1541. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, C.F.; Wang, A.H.; Lin, Q.F. MiR-16 Regulates Cell Death in Alzheimer’s Disease by Targeting Amyloid Precursor Protein. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 4020–4027. [Google Scholar]
- Zhao, H.; Wu, C.; Zhang, X.; Wang, L.; Sun, J.; Zhuge, F. Insulin Resistance Is a Risk Factor for Mild Cognitive Impairment in Elderly Adults with T2DM. Open Life Sci. 2019, 14, 255–261. [Google Scholar] [CrossRef]
- Tashiro, E.; Nagasawa, Y.; Itoh, S.; Imoto, M. Involvement of MiR-3180-3p and MiR-4632-5p in Palmitic Acid-Induced Insulin Resistance. Mol. Cell. Endocrinol. 2021, 534, 111371. [Google Scholar] [CrossRef]
- Sun, C.; Liu, J.; Duan, F.; Cong, L.; Qi, X. The Role of the MicroRNA Regulatory Network in Alzheimer’s Disease: A Bioinformatics Analysis. Arch. Med. Sci. 2022, 18, 206. [Google Scholar] [CrossRef] [PubMed]
- González-Giraldo, Y.; Forero, D.A.; Echeverria, V.; Garcia-Segura, L.M.; Barreto, G.E. Tibolone Attenuates Inflammatory Response by Palmitic Acid and Preserves Mitochondrial Membrane Potential in Astrocytic Cells through Estrogen Receptor Beta. Mol. Cell. Endocrinol. 2019, 486, 65–78. [Google Scholar] [CrossRef]
- Yang, C.; Sui, G.; Wang, L.; Chen, Z.; Wang, F. MiR-124 Prevents the Microglial Proinflammatory Response by Inhibiting the Activities of TLR4 and Downstream NLRP3 in Palmitic Acid-Treated BV2 Cells. J. Mol. Neurosci. 2022, 72, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Campbell, N.L.; Unverzagt, F.; LaMantia, M.A.; Khan, B.A.; Boustani, M.A. Risk Factors for the Progression of Mild Cognitive Impairment to Dementia. Clin. Geriatr. Med. 2013, 29, 873–893. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Kaurani, L.; Berulava, T.; Heilbronner, U.; Budde, M.; Centeno, T.P.; Elerdashvili, V.; Zafieriou, M.; Benito, E.; Sertel, S.M.; et al. A MicroRNA Signature That Correlates with Cognition and Is a Target against Cognitive Decline. EMBO Mol. Med. 2021, 13, e13659. [Google Scholar] [CrossRef]
- Maschmeyer, P.; Petkau, G.; Siracusa, F.; Zimmermann, J.; Zügel, F.; Kühl, A.A.; Lehmann, K.; Schimmelpfennig, S.; Weber, M.; Haftmann, C.; et al. Selective Targeting of Pro-Inflammatory Th1 Cells by MicroRNA-148a-Specific Antagomirs in Vivo. J. Autoimmun. 2018, 89, 41–52. [Google Scholar] [CrossRef]
- De Felice, B.; Montanino, C.; Oliva, M.; Bonavita, S.; Di Onofrio, V.; Coppola, C. MicroRNA Expression Signature in Mild Cognitive Impairment Due to Alzheimer’s Disease. Mol. Neurobiol. 2020, 57, 4408–4416. [Google Scholar] [CrossRef]
- Nagaraj, S.; Want, A.; Laskowska-Kaszub, K.; Fesiuk, A.; Vaz, S.; Logarinho, E.; Wojda, U. Candidate Alzheimer’s Disease Biomarker Mir-483-5p Lowers Tau Phosphorylation by Direct Erk1/2 Repression. Int. J. Mol. Sci. 2021, 22, 3653. [Google Scholar] [CrossRef]
- Wu, L.; Xu, Q.; Zhou, M.; Chen, Y.; Jiang, C.; Jiang, Y.; Lin, Y.; He, Q.; Zhao, L.; Dong, Y.; et al. Plasma MiR-153 and MiR-223 Levels as Potential Biomarkers in Parkinson’s Disease. Front. Neurosci. 2022, 16, 865139. [Google Scholar] [CrossRef]
- dos Santos, M.C.T.; Barreto-Sanz, M.A.; Correia, B.R.S.; Bell, R.; Widnall, C.; Perez, L.T.; Berteau, C.; Schulte, C.; Scheller, D.; Berg, D.; et al. MiRNA-Based Signatures in Cerebrospinal Fluid as Potential Diagnostic Tools for Early Stage Parkinson’s Disease. Oncotarget 2018, 9, 17455–17465. [Google Scholar] [CrossRef]
- Siedlecki-Wullich, D.; Català-Solsona, J.; Fábregas, C.; Hernández, I.; Clarimon, J.; Lleó, A.; Boada, M.; Saura, C.A.; Rodríguez-Álvarez, J.; Miñano-Molina, A.J. Altered MicroRNAs Related to Synaptic Function as Potential Plasma Biomarkers for Alzheimer’s Disease. Alzheimers Res. Ther. 2019, 11, 46. [Google Scholar] [CrossRef]
- Guévremont, D.; Tsui, H.; Knight, R.; Fowler, C.J.; Masters, C.L.; Martins, R.N.; Abraham, W.C.; Tate, W.P.; Cutfield, N.J.; Williams, J.M. Plasma MicroRNA Vary in Association with the Progression of Alzheimer’s Disease. Alzheimers Dement. Diagn. Assess. Dis. Monit. 2022, 14, e12251. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, R.; Jiang, L.; Zhou, H.; Wang, Q.; Ma, Y.; Zhang, D.; Qin, Y.; Tian, P.; Zhang, N.; et al. Administration of MiR-195 Inhibitor Enhances Memory Function Through Improving Synaptic Degradation and Mitochondrial Dysfunction of the Hippocampal Neurons in SAMP8 Mice. J. Alzheimers Dis. 2022, 85, 1495–1509. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Meng, S.; Di, W.; Xia, M.; Dong, L.; Zhao, Y.; Ling, S.; He, J.; Xue, X.; Chen, X.; et al. Amyloid-β Protein and MicroRNA-384 in NCAM-Labeled Exosomes from Peripheral Blood Are Potential Diagnostic Markers for Alzheimer’s Disease. CNS Neurosci. Ther. 2022, 28, 1093–1107. [Google Scholar] [CrossRef] [PubMed]
- Kiko, T.; Nakagawa, K.; Tsuduki, T.; Furukawa, K.; Arai, H.; Miyazawa, T. MicroRNAs in Plasma and Cerebrospinal Fluid as Potential Markers for Alzheimer’s Disease. J. Alzheimers Dis. 2014, 39, 253–259. [Google Scholar] [CrossRef]
- Kenny, A.; McArdle, H.; Calero, M.; Rabano, A.; Madden, S.F.; Adamson, K.; Forster, R.; Spain, E.; Prehn, J.H.M.; Henshall, D.C.; et al. Elevated Plasma MicroRNA-206 Levels Predict Cognitive Decline and Progression to Dementia from Mild Cognitive Impairment. Biomolecules 2019, 9, 734. [Google Scholar] [CrossRef]
- Wong, H.K.A.; Veremeyko, T.; Patel, N.; Lemere, C.A.; Walsh, D.M.; Esau, C.; Vanderburg, C.; Krichevsky, A.M. De-Repression of FOXO3a Death Axis by MicroRNA-132 and -212 Causes Neuronal Apoptosis in Alzheimer’s Disease. Hum. Mol. Genet. 2013, 22, 3077–3092. [Google Scholar] [CrossRef]
- Wang, Y.; Han, T.; Guo, R.; Song, P.; Liu, Y.; Wu, Z.; Ai, J.; Shen, C. Micro-RNA Let-7a-5p Derived From Mesenchymal Stem Cell-Derived Extracellular Vesicles Promotes the Regrowth of Neurons in Spinal-Cord-Injured Rats by Targeting the HMGA2/SMAD2 Axis. Front. Mol. Neurosci. 2022, 15, 850364. [Google Scholar] [CrossRef]
- Buss, A.; Pech, K.; Kakulas, B.A.; Martin, D.; Schoenen, J.; Noth, J.; Brook, G.A. TGF-Beta1 and TGF-Beta2 Expression after Traumatic Human Spinal Cord Injury. Spinal Cord 2008, 46, 364–371. [Google Scholar] [CrossRef]
- Song, G.; Yang, R.; Zhang, Q.; Chen, L.; Huang, D.; Zeng, J.; Yang, C.; Zhang, T. TGF-β Secretion by M2 Macrophages Induces Glial Scar Formation by Activating Astrocytes In Vitro. J. Mol. Neurosci. 2019, 69, 324–332. [Google Scholar] [CrossRef]
- Jiao, Y.; Kong, L.; Yao, Y.; Li, S.; Tao, Z.; Yan, Y.; Yang, J. Osthole Decreases Beta Amyloid Levels through Up-Regulation of MiR-107 in Alzheimer’s Disease. Neuropharmacology 2016, 108, 332–344. [Google Scholar] [CrossRef]
- Joilin, G.; Gray, E.; Thompson, A.G.; Bobeva, Y.; Talbot, K.; Weishaupt, J.; Ludolph, A.; Malaspina, A.; Leigh, P.N.; Newbury, S.F.; et al. Identification of a Potential Non-Coding RNA Biomarker Signature for Amyotrophic Lateral Sclerosis. Brain Commun. 2020, 2, fcaa053. [Google Scholar] [CrossRef]
- Regev, K.; Healy, B.C.; Khalid, F.; Paul, A.; Chu, R.; Tauhid, S.; Tummala, S.; Diaz-Cruz, C.; Raheja, R.; Mazzola, M.A.; et al. Association between Serum MicroRNAs and Magnetic Resonance Imaging Measures of Multiple Sclerosis Severity. JAMA Neurol. 2017, 74, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Yakovleva, K.D.; Dmitrenko, D.V.; Panina, I.S.; Usoltseva, A.A.; Gazenkampf, K.A.; Konovalenko, O.V.; Kantimirova, E.A.; Novitsky, M.A.; Nasyrova, R.F.; Shnayder, N.A. Expression Profile of MiRs in Mesial Temporal Lobe Epilepsy: Systematic Review. Int. J. Mol. Sci. 2022, 23, 951. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Ramirez, M.A.; Wu, D.; Pryce, G.; Simpson, J.E.; Reijerkerk, A.; King-Robson, J.; Kay, O.; De Vries, H.E.; Hirst, M.C.; Sharrack, B.; et al. MicroRNA-155 Negatively Affects Blood-Brain Barrier Function during Neuroinflammation. FASEB J. 2014, 28, 2551–2565. [Google Scholar] [CrossRef] [PubMed]
- Mangiola, A.; Vigo, V.; Anile, C.; De Bonis, P.; Marziali, G.; Lofrese, G. Role and Importance of IGF-1 in Traumatic Brain Injuries. Biomed Res. Int. 2015, 2015, 736104. [Google Scholar] [CrossRef]
- Chang, J.R.; Ghafouri, M.; Mukerjee, R.; Bagashev, A.; Chabrashvili, T.; Sawaya, B.E. Role of P53 in Neurodegenerative Diseases. Neurodegener. Dis. 2012, 9, 68–80. [Google Scholar] [CrossRef]
- Bradburn, S.; Murgatroyd, C.; Ray, N. Neuroinflammation in Mild Cognitive Impairment and Alzheimer’s Disease: A Meta-Analysis. Ageing Res. Rev. 2019, 50, 1–8. [Google Scholar] [CrossRef]
- Fricker, M.; Tolkovsky, A.M.; Borutaite, V.; Coleman, M.; Brown, G.C. Neuronal Cell Death. Physiol. Rev. 2018, 98, 813–880. [Google Scholar] [CrossRef]
- McLarnon, J.G. Microglial Chemotactic Signaling Factors in Alzheimer’s Disease. Am. J. Neurodegener. Dis. 2012, 1, 199. [Google Scholar]
- Li, K.; Yan, G.; Huang, H.; Zheng, M.; Ma, K.; Cui, X.; Lu, D.; Zheng, L.; Zhu, B.; Cheng, J.; et al. Anti-Inflammatory and Immunomodulatory Effects of the Extracellular Vesicles Derived from Human Umbilical Cord Mesenchymal Stem Cells on Osteoarthritis via M2 Macrophages. J. Nanobiotechnology 2022, 20, 38. [Google Scholar] [CrossRef]
- Kayano, M.; Higaki, S.; Satoh, J.I.; Matsumoto, K.; Matsubara, E.; Takikawa, O.; Niida, S. Plasma MicroRNA Biomarker Detection for Mild Cognitive Impairment Using Differential Correlation Analysis. Biomark. Res. 2016, 4, 22. [Google Scholar] [CrossRef]
- Zhai, A.; Zhang, Z.; Kong, X. Paeoniflorin Alleviates H 2 O 2-Induced Oxidative Injury Through Down-Regulation of MicroRNA-135a in HT-22 Cells. Neurochem. Res. 2019, 44, 2821–2831. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Wang, B.; Li, W. LncRNA MALAT1 Improves Cerebral Ischemia-Reperfusion Injury and Cognitive Dysfunction by Regulating MiR-142-3p/SIRT1 Axis. Int. J. Neurosci. 2021, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Strafella, C.; Caputo, V.; Termine, A.; Fabrizio, C.; Calvino, G.; Megalizzi, D.; Ruffo, P.; Toppi, E.; Banaj, N.; Bassi, A.; et al. Identification of Genetic Networks Reveals Complex Associations and Risk Trajectory Linking Mild Cognitive Impairment to Alzheimer’s Disease. Front. Aging Neurosci. 2022, 14, 821789. [Google Scholar] [CrossRef] [PubMed]
- Haghikia, A.; Haghikia, A.; Hellwig, K.; Baraniskin, A.; Holzmann, A.; Décard, B.F.; Thum, T.; Gold, R. Regulated MicroRNAs in the CSF of Patients with Multiple Sclerosis: A Case-Control Study. Neurology 2012, 79, 2166–2170. [Google Scholar] [CrossRef]
- Mohammadipoor-Ghasemabad, L.; Sangtarash, M.H.; Sheibani, V.; Sasan, H.A.; Esmaeili-Mahani, S. Hippocampal MicroRNA-191a-5p Regulates BDNF Expression and Shows Correlation with Cognitive Impairment Induced by Paradoxical Sleep Deprivation. Neuroscience 2019, 414, 49–59. [Google Scholar] [CrossRef]
- Tang, C.Z.; Yang, J.T.; Liu, Q.H.; Wang, Y.R.; Wang, W.S. Up-Regulated MiR-192-5p Expression Rescues Cognitive Impairment and Restores Neural Function in Mice with Depression via the Fbln2-Mediated TGF-Β1 Signaling Pathway. FASEB J. 2019, 33, 606–618. [Google Scholar] [CrossRef]
- Yang, X.L.; Cao, C.Z.; Zhang, Q.X. MiR-195 Alleviates Oxygen-Glucose Deprivation/Reperfusion-Induced Cell Apoptosis via Inhibition of IKKα-Mediated NF-ΚB Pathway. Int. J. Neurosci. 2021, 131, 755–764. [Google Scholar] [CrossRef]
- Cao, J.; Huang, M.; Guo, L.; Zhu, L.; Hou, J.; Zhang, L.; Pero, A.; Ng, S.; El Gaamouch, F.; Elder, G.; et al. MicroRNA-195 Rescues ApoE4-Induced Cognitive Deficits and Lysosomal Defects in Alzheimer’s Disease Pathogenesis. Mol. Psychiatry 2020, 26, 4687–4701. [Google Scholar] [CrossRef]
- Ye, F.; Tian, S.; Hu, H.; Yu, Z. Electroacupuncture Reduces Scopolamine-Induced Amnesia via Mediating the MiR-210/SIN3A and MiR-183/SIN3A Signaling Pathway. Mol. Med. 2020, 26, 107. [Google Scholar] [CrossRef]
- Zou, H.; Ding, Y.; Shi, W.; Xu, X.; Gong, A.; Zhang, Z.; Liu, J. MicroRNA-29c/PTEN Pathway Is Involved in Mice Brain Development and Modulates Neurite Outgrowth in PC12 Cells. Cell. Mol. Neurobiol. 2015, 35, 313–322. [Google Scholar] [CrossRef]
- Moradifard, S.; Hoseinbeyki, M.; Ganji, S.M.; Minuchehr, Z. Analysis of MicroRNA and Gene Expression Profiles in Alzheimer’s Disease: A Meta-Analysis Approach. Sci. Rep. 2018, 8, 4767. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, G.; Tang, X.; Feng, C.; Li, M.; Jiang, X.; Gu, Y.; Yun, Y.; Lu, L.; Feng, X.; et al. MiR-375-3p Mediates Reduced Pineal Function in Hypoxia-Ischemia Brain Damage. Exp. Neurol. 2021, 344, 113814. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Ou, J.; Zhang, Q.; Tang, R.; Wang, J.; Hong, Q.; Guo, X.; Tong, M.; Yang, L.; Chi, X. Effects of Aberrant MiR-384-5p Expression on Learning and Memory in a Rat Model of Attention Deficit Hyperactivity Disorder. Front. Neurol. 2020, 10, 1414. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wei, L.; Zhang, S.; Song, X.; Yang, J.; He, X.; Zheng, X. LncRNA SNHG15 Knockdown Protects Against OGD/R-Induced Neuron Injury by Downregulating TP53INP1 Expression via Binding to MiR-455-3p. Neurochem. Res. 2021, 46, 1019–1030. [Google Scholar] [CrossRef]
- Gao, Z.; Yuan, W.; Yuan, J.; Yuan, K.; Wang, Y. MiR-486-5p Functions as an Oncogene by Targeting PTEN in Non-Small Cell Lung Cancer. Pathol. Res. Pract. 2018, 214, 700–705. [Google Scholar] [CrossRef]
- Faraonio, R.; Salerno, P.; Passaro, F.; Sedia, C.; Iaccio, A.; Bellelli, R.; Nappi, T.C.; Comegna, M.; Romano, S.; Salvatore, G.; et al. A Set of MiRNAs Participates in the Cellular Senescence Program in Human Diploid Fibroblasts. Cell Death Differ. 2012, 19, 713–721. [Google Scholar] [CrossRef]
- Arakawa, Y.; Itoh, S.; Fukazawa, Y.; Ishiguchi, H.; Kohmoto, J.; Hironishi, M.; Ito, H.; Kihira, T. Association between Oxidative Stress and MicroRNA Expression Pattern of ALS Patients in the High-Incidence Area of the Kii Peninsula. Brain Res. 2020, 1746, 147035. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Wang, W.; Yu, S.; Liu, L.; Sun, D.; Li, W.; Jiang, X. Upregulation of Circ_0059961 Suppresses Cholangiocarcinoma Development by Modulating MiR-629-5p/SFRP2 Axis. Pathol. Res. Pract. 2022, 234, 153901. [Google Scholar] [CrossRef]
- Zhao, J.; Zhou, Y.; Guo, M.; Guo, M.; Yue, D.; Yue, D.; Chen, C.; Chen, C.; Liang, G.; Liang, G.; et al. MicroRNA-7: Expression and Function in Brain Physiological and Pathological Processes. Cell Biosci. 2020, 10, 77. [Google Scholar] [CrossRef]
- Xiao, X.; Liu, X.; Jiao, B. Epigenetics: Recent Advances and Its Role in the Treatment of Alzheimer’s Disease. Front. Neurol. 2020, 11, 538301. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).