Comprehensive Analysis of Adverse Events Induced by PARP Inhibitors Using JADER and Time to Onset
Abstract
:1. Introduction
2. Materials and Methods
2.1. JADER and Production of the Data Analysis Table
2.2. Association of PARP Inhibitors with Adverse Events
2.3. Time to Onset Analysis
3. Results
3.1. Signal Detection of Olaparib and Niraparib AEs
3.2. Outcome Analysis after AEs
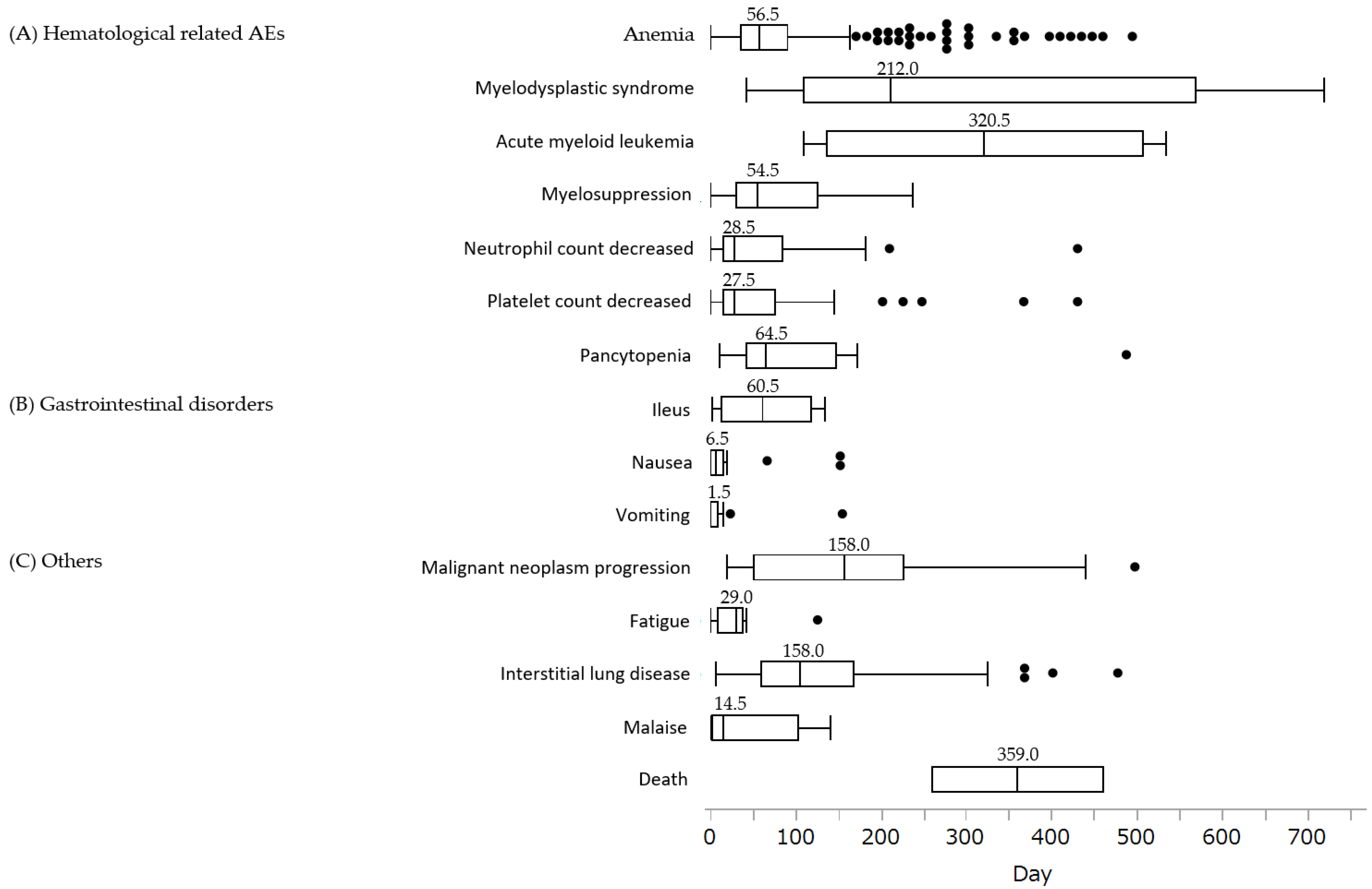
3.3. Time to Onset Analysis and Analysis by Weibull Distribution of Each AE
4. Discussion
4.1. Hematologic Toxicities
4.2. Gastrointestinal Disorders
4.3. Other Adverse Events
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cancer Statistics in Japan. 2022. Available online: https://ganjoho.jp/public/qa_links/report/statistics/2022_en.html (accessed on 6 June 2022).
- da Cunha Colombo Bonadio, R.R.; Fogace, R.N.; Miranda, V.C.; Diz, M.D.P.E. Homologous recombination deficiency in ovarian cancer: A review of its epidemiology and management. Clinics 2018, 73 (Suppl. 1), e450s. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, T.; Aoki, D.; Hattori, K.; Jinushi, M.; Kigawa, J.; Takeshima, N.; Tsuda, H.; Watanabe, Y.; Yoshihara, K.; Sugiyama, T. The first Japanese nationwide multicenter study of BRCA mutation testing in ovarian cancer: CHARacterizing the cross-sectionaL approach to Ovarian cancer geneTic TEsting of BRCA (CHARLOTTE). Int. J. Gynecol. Cancer 2019, 29, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Hirasawa, A.; Imoto, I.; Naruto, T.; Akahane, T.; Yamagami, W.; Nomura, H.; Masuda, K.; Susumu, N.; Tsuda, H.; Aoki, D. Prevalence of pathogenic germline variants detected by multigene sequencing in unselected Japanese patients with ovarian cancer. Oncotarget 2017, 8, 112258–112267. [Google Scholar] [CrossRef]
- Norquist, B.M.; Harrell, M.I.; Brady, M.F.; Walsh, T.; Lee, M.K.; Gulsuner, S.; Bernards, S.S.; Casadei, S.; Yi, Q.; Burger, R.A.; et al. Inherited Mutations in Women With Ovarian Carcinoma. JAMA Oncol. 2016, 2, 482–490. [Google Scholar] [CrossRef] [PubMed]
- BRCA1- and BRCA2-Associated Hereditary Breast and Ovarian Cancer. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1247/ (accessed on 6 June 2022).
- Erkko, H.; Xia, B.; Nikkilä, J.; Schleutker, J.; Syrjäkoski, K.; Mannermaa, A.; Kallioniemi, A.; Pylkäs, K.; Karppinen, S.M.; Rapakko, K.; et al. A recurrent mutation in PALB2 in Finnish cancer families. Nature 2007, 446, 316–319. [Google Scholar] [CrossRef]
- Renwick, A.; Thompson, D.; Seal, S.; Kelly, P.; Chagtai, T.; Ahmed, M.; North, B.; Jayatilake, H.; Barfoot, R.; Spanova, K.; et al. ATM mutations that cause ataxia-telangiectasia are breast cancer susceptibility alleles. Nat. Genet. 2006, 38, 873–875. [Google Scholar] [CrossRef]
- Weischer, M.; Bojesen, S.E.; Ellervik, C.; Tybjaerg-Hansen, A.; Nordestgaard, B.G. CHEK2*1100delC genotyping for clinical assessment of breast cancer risk: Meta-analyses of 26,000 patient cases and 27,000 controls. J. Clin. Oncol. 2008, 26, 542–548. [Google Scholar] [CrossRef]
- Seal, S.; Thompson, D.; Renwick, A.; Elliott, A.; Kelly, P.; Barfoot, R.; Chagtai, T.; Jayatilake, H.; Ahmed, M.; Spanova, K.; et al. Truncating mutations in the Fanconi anemia J gene BRIP1 are low-penetrance breast cancer susceptibility alleles. Nat. Genet. 2006, 38, 1239–1241. [Google Scholar] [CrossRef]
- Meindl, A.; Hellebrand, H.; Wiek, C.; Erven, V.; Wappenschmidt, B.; Niederacher, D.; Freund, M.; Lichtner, P.; Hartmann, L.; Schaal, H.; et al. Germline mutations in breast and ovarian cancer pedigrees establish RAD51C as a human cancer susceptibility gene. Nat. Genet. 2010, 42, 410–414. [Google Scholar] [CrossRef]
- Tew, W.P.; Lacchetti, C.; Ellis, A.; Maxian, K.; Banerjee, S.; Bookman, M.; Jones, M.B.; Lee, J.M.; Lheureux, S.; Liu, J.F.; et al. PARP Inhibitors in the Management of Ovarian Cancer: ASCO Guideline. J. Clin. Oncol. 2020, 38, 3468–3493. [Google Scholar] [CrossRef]
- Sunada, S.; Nakanishi, A.; Miki, Y. Crosstalk of DNA double-strand break repair pathways in poly(ADP-ribose) polymerase inhibitor treatment of breast cancer susceptibility gene 1/2-mutated cancer. Cancer Sci. 2018, 109, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef] [PubMed]
- Pujade-Lauraine, E.; Ledermann, J.A.; Selle, F.; Gebski, V.; Penson, R.T.; Oza, A.M.; Korach, J.; Huzarski, T.; Poveda, A.; Pignata, S.; et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1274–1284. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Pautier, P.; Pignata, S.; Pérol, D.; González-Martín, A.; Berger, R.; Fujiwara, K.; Vergote, I.; Colombo, N.; Mäenpää, J.; et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2416–2428. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.; Im, S.A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Li, W.; Tung, N.; Armstrong, A.; et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 2017, 377, 523–533. [Google Scholar] [CrossRef]
- González-Martín, A.; Pothuri, B.; Vergote, I.; DePont Christensen, R.; Graybill, W.; Mirza, M.R.; McCormick, C.; Lorusso, D.; Hoskins, P.; Freyer, G.; et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2391–2402. [Google Scholar] [CrossRef]
- Mirza, M.R.; Monk, B.J.; Herrstedt, J.; Oza, A.M.; Mahner, S.; Redondo, A.; Fabbro, M.; Ledermann, J.A.; Lorusso, D.; Vergote, I.; et al. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N. Engl. J. Med. 2016, 375, 2154–2164. [Google Scholar] [CrossRef]
- de Bono, J.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D.; et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 382, 2091–2102. [Google Scholar] [CrossRef]
- Golan, T.; Hammel, P.; Reni, M.; Van Cutsem, E.; Macarulla, T.; Hall, M.J.; Park, J.O.; Hochhauser, D.; Arnold, D.; Oh, D.Y.; et al. Maintenance Olaparib for Germline. N. Engl. J. Med. 2019, 381, 317–327. [Google Scholar] [CrossRef]
- Moore, K.N.; Secord, A.A.; Geller, M.A.; Miller, D.S.; Cloven, N.; Fleming, G.F.; Wahner Hendrickson, A.E.; Azodi, M.; DiSilvestro, P.; Oza, A.M.; et al. Niraparib monotherapy for late-line treatment of ovarian cancer (QUADRA): A multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019, 20, 636–648. [Google Scholar] [CrossRef]
- Gallagher, J.R.; Heap, K.J.; Carroll, S.; Travers, K.; Harrow, B.; Westin, S.N. Real-world adverse events with niraparib 200 mg/day maintenance therapy in ovarian cancer: A retrospective study. Future Oncol. 2019, 15, 4197–4206. [Google Scholar] [CrossRef] [PubMed]
- Matulonis, U.A.; Walder, L.; Nøttrup, T.J.; Bessette, P.; Mahner, S.; Gil-Martin, M.; Kalbacher, E.; Ledermann, J.A.; Wenham, R.M.; Woie, K.; et al. Niraparib Maintenance Treatment Improves Time Without Symptoms or Toxicity (TWiST) Versus Routine Surveillance in Recurrent Ovarian Cancer: A TWiST Analysis of the ENGOT-OV16/NOVA Trial. J. Clin. Oncol. 2019, 37, 3183–3191. [Google Scholar] [CrossRef] [PubMed]
- LaFargue, C.J.; Dal Molin, G.Z.; Sood, A.K.; Coleman, R.L. Exploring and comparing adverse events between PARP inhibitors. Lancet Oncol. 2019, 20, e15–e28. [Google Scholar] [CrossRef]
- Tian, X.; Chen, L.; Gai, D.; He, S.; Jiang, X.; Zhang, N. Adverse Event Profiles of PARP Inhibitors: Analysis of Spontaneous Reports Submitted to FAERS. Front. Pharmacol. 2022, 13, 851246. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Zhu, J.; Zhao, W.; Huang, Y.; An, R.; Zheng, H.; Qu, P.; Wang, L.; Zhou, Q.; Wang, D.; et al. Olaparib Maintenance Monotherapy in Asian Patients with Platinum-Sensitive Relapsed Ovarian Cancer: Phase III Trial (L-MOCA). Clin. Cancer Res. 2022, 28, 2278–2285. [Google Scholar] [CrossRef]
- van Puijenbroek, E.P.; Egberts, A.C.; Heerdink, E.R.; Leufkens, H.G. Detecting drug-drug interactions using a database for spontaneous adverse drug reactions: An example with diuretics and non-steroidal anti-inflammatory drugs. Eur. J. Clin. Pharmacol. 2000, 56, 733–738. [Google Scholar] [CrossRef]
- Sauzet, O.; Carvajal, A.; Escudero, A.; Molokhia, M.; Cornelius, V.R. Illustration of the weibull shape parameter signal detection tool using electronic healthcare record data. Drug. Saf. 2013, 36, 995–1006. [Google Scholar] [CrossRef]
- Wang, C.; Li, J. Haematologic toxicities with PARP inhibitors in cancer patients: An up-to-date meta-analysis of 29 randomized controlled trials. J. Clin. Pharm. Ther. 2021, 46, 571–584. [Google Scholar] [CrossRef]
- Morice, P.M.; Chrétien, B.; Da Silva, A.; Dolladille, C.; Alexandre, J. Occurrence of Pancytopenia Among Patients With Cancer Treated With Poly(Adenosine Diphosphate-Ribose) Polymerase Inhibitors: A Pharmacoepidemiologic Study. JAMA Oncol. 2021, 7, 1899–1900. [Google Scholar] [CrossRef]
- Shu, Y.; Ding, Y.; He, X.; Liu, Y.; Wu, P.; Zhang, Q. Hematological toxicities in PARP inhibitors: A real-world study using FDA adverse event reporting system (FAERS) database. Cancer Med. 2022. [CrossRef]
- Stemmer, A.; Shafran, I.; Stemmer, S.M.; Tsoref, D. Comparison of Poly (ADP-ribose) Polymerase Inhibitors (PARPis) as Maintenance Therapy for Platinum-Sensitive Ovarian Cancer: Systematic Review and Network Meta-Analysis. Cancers 2020, 12, 3026. [Google Scholar] [CrossRef] [PubMed]
- Berek, J.S.; Matulonis, U.A.; Peen, U.; Ghatage, P.; Mahner, S.; Redondo, A.; Lesoin, A.; Colombo, N.; Vergote, I.; Rosengarten, O.; et al. Safety and dose modification for patients receiving niraparib. Ann. Oncol. 2018, 29, 1784–1792. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Mirza, M.; Vergote, I.; Li, Y.; Hazard, S.; Clark, R.; Graybill, W.; Pothuri, B.; Monk, B. A prospective evaluation of tolerability of niraparib dosing based upon baseline body weight (wt) and platelet (blplt) count: Blinded pooled interim safety data from the PRIMA Study. Ann. Oncol. 2018, 29, viii335–viii336. [Google Scholar] [CrossRef]
- Morice, P.M.; Leary, A.; Dolladille, C.; Chrétien, B.; Poulain, L.; González-Martín, A.; Moore, K.; O’Reilly, E.M.; Ray-Coquard, I.; Alexandre, J. Myelodysplastic syndrome and acute myeloid leukaemia in patients treated with PARP inhibitors: A safety meta-analysis of randomised controlled trials and a retrospective study of the WHO pharmacovigilance database. Lancet Haematol. 2021, 8, e122–e134. [Google Scholar] [CrossRef]
- Hao, J.; Liu, Y.; Zhang, T.; He, J.; Zhao, H.; An, R.; Xue, Y. Efficacy and safety of PARP inhibitors in the treatment of advanced ovarian cancer: An updated systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Oncol. Hematol. 2021, 157, 103145. [Google Scholar] [CrossRef]
- Fujiwara, K.; Fujiwara, H.; Yoshida, H.; Satoh, T.; Yonemori, K.; Nagao, S.; Matsumoto, T.; Kobayashi, H.; Bourgeois, H.; Harter, P.; et al. Olaparib plus bevacizumab as maintenance therapy in patients with newly diagnosed, advanced ovarian cancer: Japan subset from the PAOLA-1/ENGOT-ov25 trial. J. Gynecol. Oncol. 2021, 32, e82. [Google Scholar] [CrossRef]
- Shenolikar, R.; Durden, E.; Meyer, N.; Lenhart, G.; Moore, K. Incidence of secondary myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) in patients with ovarian or breast cancer in a real-world setting in the United States. Gynecol. Oncol. 2018, 151, 190–195. [Google Scholar] [CrossRef]
- Morton, L.M.; Dores, G.M.; Schonfeld, S.J.; Linet, M.S.; Sigel, B.S.; Lam, C.J.K.; Tucker, M.A.; Curtis, R.E. Association of Chemotherapy for Solid Tumors With Development of Therapy-Related Myelodysplastic Syndrome or Acute Myeloid Leukemia in the Modern Era. JAMA Oncol. 2019, 5, 318–325. [Google Scholar] [CrossRef]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Antiemesis. Available online: https://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf (accessed on 6 June 2022).
- Sun, W.; Li, J.; Zhang, Z.; Su, X. Gastrointestinal events with PARP inhibitors in cancer patients: A meta-analysis of phase II/III randomized controlled trials. J. Clin. Pharm. Ther. 2021, 46, 241–255. [Google Scholar] [CrossRef]
- Moore, K.N.; Mirza, M.R.; Matulonis, U.A. The poly (ADP ribose) polymerase inhibitor niraparib: Management of toxicities. Gynecol. Oncol. 2018, 149, 214–220. [Google Scholar] [CrossRef]
- PLoSker, G.L.; Goa, K.L. Granisetron. A review of its pharmacological properties and therapeutic use as an antiemetic. Drugs 1991, 42, 805–824. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Meng, J.; Wang, G. Risk of selected gastrointestinal toxicities associated with poly (ADP-ribose) polymerase (PARP) inhibitors in the treatment of ovarian cancer: A meta-analysis of published trials. Drug. Des. Devel. Ther. 2018, 12, 3013–3019. [Google Scholar] [CrossRef] [PubMed]
- Satake, R.; Matsumoto, K.; Tanaka, M.; Mukai, R.; Shimada, K.; Yoshida, Y.; Inoue, M.; Hasegawa, S.; Iguchi, K.; Ikesue, H.; et al. Analysis of Drug-Induced Gastrointestinal Obstruction and Perforation Using the Japanese Adverse Drug Event Report Database. Front. Pharmacol. 2021, 12, 692292. [Google Scholar] [CrossRef] [PubMed]
- Ledermann, J.; Harter, P.; Gourley, C.; Friedlander, M.; Vergote, I.; Rustin, G.; Scott, C.; Meier, W.; Shapira-Frommer, R.; Safra, T.; et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N. Engl. J. Med. 2012, 366, 1382–1392. [Google Scholar] [CrossRef]
- Valabrega, G.; Pothuri, B.; Oaknin, A.; Graybill, W.; Sánchez, A.B.; Mccormick, C.; Baurain, J.; Hoskins, P.; Denys, H.; O’Cearbhaill, R.E.; et al. Efficacy and safety of niraparib in older patients (pts) with advanced ovarian cancer (OC): Results from the PRIMA/ENGOT-OV26/GOG-3012 trial. Ann. Oncol. 2020, 31, S551–S589. [Google Scholar] [CrossRef]
- Ma, Z.; Sun, X.; Zhao, Z.; Lu, W.; Guo, Q.; Wang, S.; You, J.; Zhang, Y.; Liu, L. Risk of pneumonitis in cancer patients treated with PARP inhibitors: A meta-analysis of randomized controlled trials and a pharmacovigilance study of the FAERS database. Gynecol. Oncol. 2021, 162, 496–505. [Google Scholar] [CrossRef]

| Olaparib | Niraparib | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Cases (n) | Non-Cases (n) | Rate (%) | ROR (95% CI) | Variable | Cases (n) | Non-Cases (n) | Rate (%) | ROR (95% CI) |
| Malignant neoplasm progression | 242 | 1310 | 15.59 | 73.50 (63.90–84.55) | Ovarian cancer recurrent | 26 | 523 | 4.74 | 1917.13 (1182.47–3108.217) |
| Anemia | 496 | 1056 | 31.96 | 40.50 (36.37–45.09) | Ovarian cancer | 35 | 514 | 6.38 | 500.61 (348.02–720.11) |
| MDS | 30 | 1522 | 1.93 | 11.71 (8.15–16.84) | Disease progression | 57 | 492 | 10.38 | 101.85 (77.17–134.42) |
| Fatigue | 10 | 1542 | 0.64 | 6.46 (3.47–12.06) | Thrombocytopenia | 44 | 505 | 8.01 | 13.60 (9.99–18.52) |
| AML | 12 | 1540 | 0.77 | 6.33 | Ileus | 11 | 538 | 2 | 11.99 |
| (3.58–11.18) | (6.60–21.80) | ||||||||
| Ileus | 14 | 1538 | 0.9 | 5.24 | Condition aggravated | 10 | 539 | 1.82 | 10.11 |
| (3.09–8.88) | (5.40–18.91) | ||||||||
| Myelosuppression | 33 | 1519 | 2.13 | 3.83 | Platelet count decreased | 75 | 474 | 13.66 | 8.90 |
| (2.71–5.40) | (6.98–11.35) | ||||||||
| Nausea | 34 | 1518 | 2.19 | 3.47 | Renal impairment | 41 | 508 | 7.47 | 7.45 |
| (2.47–4.88) | (5.42–10.24) | ||||||||
| Neutrophil count decreased | 63 | 1489 | 4.06 | 2.85 | Anemia | 39 | 510 | 7.1 | 6.46 |
| (2.21–3.67) | (4.66–8.95) | ||||||||
| ILD | 90 | 1462 | 5.8 | 2.19 | Myelosuppression | 15 | 534 | 2.73 | 4.94 |
| (1.77–2.71) | (2.96-8.26) | ||||||||
| Malaise | 15 | 1537 | 0.97 | 2.03 | Neutrophil count decreased | 25 | 524 | 4.55 | 3.21 |
| (1.22–3.38) | (2.15–4.80) | ||||||||
| Pancytopenia | 20 | 1532 | 1.29 | 2.02 | |||||
| (1.30–3.14) | |||||||||
| Death | 14 | 1538 | 0.9 | 1.87 | |||||
| (1.10–3.17) | |||||||||
| Platelet count decreased | 49 | 1503 | 3.16 | 1.83 | |||||
| (1.38–2.43) | |||||||||
| Vomiting | 15 | 1537 | 0.97 | 1.77 | |||||
| (1.06–2.95) | |||||||||
| Neutropenia | 24 | 1528 | 1.55 | 1.38 | |||||
| (0.92–2.06) | |||||||||
| Hemoglobin decreased | 11 | 1541 | 0.71 | 1.35 | |||||
| (0.75–2.44) | |||||||||
| Renal impairment | 17 | 1535 | 1.1 | 1.02 | |||||
| (0.63–1.65) | |||||||||
| Decreased appetite | 11 | 1541 | 0.71 | 0.93 | |||||
| (0.51–1.68) | |||||||||
| Pyrexia | 17 | 1535 | 1.1 | 0.79 | |||||
| (0.49–1.27) | |||||||||
| Febrile neutropenia | 12 | 1540 | 0.77 | 0.74 | |||||
| (0.42–1.31) | |||||||||
| White blood cell count decreased | 14 | 1538 | 0.9 | 0.67 | |||||
| (0.40–1.14) |
| Adverse Events | Case (n) | Post-Event Outcome | |||||
|---|---|---|---|---|---|---|---|
| Recovered | Remission | Not recovered | With Sequelae | Death | Unclear | ||
| (A) Hematological-related AEs | |||||||
| Anemia | 496 | 223 (45.0%) | 93 (18.8%) | 46 (9.2%) | 0 (0%) | 1 (0.2%) | 133 (26.8%) |
| MDS | 30 | 0 (0%) | 1 (3.3%) | 14 (46.7%) | 0 (0%) | 8 (26.7%) | 7 (23.3%) |
| AML | 12 | 0 (0%) | 0 (0%) | 3 (25.0%) | 0 (0%) | 6 (50.0%) | 3 (25.0%) |
| Myelosuppression | 33 | 12 (36.3%) | 2 (6.1%) | 2 (6.1%) | 0 (0%) | 0 (0%) | 17 (51.5%) |
| Neutrophil count decreased | 63 | 23 (36.5%) | 8 (12.7%) | 3 (4.8%) | 0 (0%) | 0 (0%) | 29 (46.0%) |
| Platelet count decreased | 49 | 26 (53.1%) | 8 (16.3%) | 3 (6.1%) | 0 (0%) | 2 (4.1 %) | 10 (20.4%) |
| Pancytopenia | 20 | 8 (40.0%) | 4 (20.0%) | 1 (5.0%) | 0 (0%) | 0 (0%) | 7 (35.0%) |
| (B) Gastrointestinal disorders | |||||||
| Ileus | 14 | 2 (14.3%) | 1 (7.1%) | 1 (7.1%) | 0 (0%) | 0 (0%) | 10 (71.5%) |
| Nausea | 34 | 14 (41.2%) | 6 (17.6%) | 7 (20.6%) | 0 (0%) | 0 (0%) | 7 (20.6%) |
| Vomiting | 15 | 6 (40.0%) | 4 (26.7%) | 2 (13.3%) | 0 (0%) | 0 (0%) | 3 (20.0%) |
| (C) Others | |||||||
| Malignant neoplasm progression | 242 | 0 (0%) | 0 (0%) | 1 (0.4%) | 0 (0%) | 32 (13.2%) | 209 (86.4%) |
| Fatigue | 5 | 4 (80.0%) | 0 (0%) | 1 (20.0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| ILD | 90 | 37 (41.1%) | 31 (34.5%) | 2 (2.2%) | 2 (2.2%) | 0 (0%) | 18 (20.0%) |
| Malaise | 15 | 7 (46.7%) | 3 (20.0%) | 2 (13.3%) | 0 (0%) | 0 (0%) | 3 (20.0%) |
| Death | 14 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 14 (100.0%) | 0 (0%) |
| Adverse Events | Case (n) | Post-Event Outcome | |||||
|---|---|---|---|---|---|---|---|
| Recovered | Remission | Not Recovered | With Sequelae | Death | Unclear | ||
| (A) Hematological-related AEs | |||||||
| Thrombocytopenia | 44 | 20 (45.4%) | 7 (15.9%) | 8 (18.2%) | 0 (0%) | 0 (0%) | 9 (20.5%) |
| Platelet count decreased | 75 | 36 (48.0%) | 13 (17.3%) | 17 (22.7%) | 0 (0%) | 0 (0%) | 9 (12.0%) |
| Anemia | 39 | 14 (35.9%) | 10 (25.6%) | 8 (20.5%) | 0 (0%) | 0 (0%) | 7 (18.0%) |
| Myelosuppression | 15 | 2 (13.3%) | 1 (6.7%) | 2 (13.3%) | 0 (0%) | 0 (0%) | 10 (66.7%) |
| Neutrophil count decreased | 25 | 12 (48.0%) | 5 (20.0%) | 6 (24.0%) | 0 (0%) | 0 (0%) | 2 (8.0%) |
| (B) Gastrointestinal disorders | |||||||
| Ileus | 11 | 3 (27.3%) | 1 (9.1%) | 4 (36.3%) | 0 (0%) | 0 (0%) | 3 (27.3%) |
| (C) Others | |||||||
| Ovarian cancer recurrent | 26 | 1 (3.8%) | 1 (3.8%) | 7 (27.0%) | 0 (0%) | 2 (7.7%) | 15 (57.7%) |
| Ovarian cancer | 35 | 1 (2.8%) | 1 (2.8%) | 15 (42.9%) | 0 (0%) | 3 (8.6%) | 15 (42.9%) |
| Disease progression | 57 | 2 (3.5%) | 3 (3.5%) | 21 (36.8%) | 0 (0%) | 4 (7.0%) | 28 (49.2%) |
| Condition aggravated | 10 | 0 (0%) | 0 (0%) | 4 (40.0%) | 0 (0%) | 2 (20.0%) | 4 (40.0%) |
| Renal impairment | 41 | 8 (19.5%) | 3 (7.3%) | 13 (31.7%) | 0 (0%) | 0 (0.0%) | 17 (41.5%) |
| Adverse Events | Case (n) | Scale Parameter | Shape Parameter |
|---|---|---|---|
| α (95% CI) | β (95% CI) | ||
| (A) Hematological-related AEs | |||
| Anemia | 327 | 84.54 (76.42–93.39) | 1.15 (1.06–1.24) |
| MDS | 18 | 361.28 (241.26–527.09) | 1.33 (0.88–1.88) |
| AML | 4 | 363.24 (181.38–708.13) | 2.06 (0.77–4.21) |
| Myelosuppression | 15 | 74.67 (40.29–134.06) | 0.96 (0.60–1.40) |
| Neutrophil count decreased | 31 | 55.16 (33.81–88.03) | 0.80 (0.60–1.03) |
| Platelet count decreased | 41 | 60.93 (40.87–89.39) | 0.85 (0.67–1.04) |
| Pancytopenia | 14 | 118.11 (68.90–196.54) | 1.13 (0.74–1.59) |
| (B) Gastrointestinal disorders | |||
| Ileus | 5 | 65.97 (21.34–193.61) | 1.08 (0.45–2.09) |
| Nausea | 27 | 9.60 (4.39–20.21) | 0.54 (0.41–0.70) |
| Vomiting | 14 | 6.68 (2.04–20.49) | 0.51 (0.34–0.72) |
| (C) Others | |||
| Malignant neoplasm progression | 24 | 187.71 (134.30–257.64) | 1.35 (0.97–1.81) |
| Fatigue | 8 | 34.74 (14.52–79.09) | 0.98 (0.53–1.57) |
| ILD | 67 | 151.25 (126.37–179.90) | 1.46 (1.21–1.73) |
| Malaise | 13 | 35.78 (13.60–88.19) | 0.67 (0.41–1.01) |
| Death | 2 | 397.34 (210.89–772.73) | 4.17 (0.91–11.26) |
| Adverse Events | Case (n) | Scale Parameter | Shape Parameter |
|---|---|---|---|
| α (95% CI) | β (95% CI) | ||
| (A) Hematological-related AEs | |||
| Thrombocytopenia | 34 | 46.55 (32.96–64.74) | 1.08 (0.82–1.37) |
| Platelet count decreased | 65 | 53.20 (43.52–64.62) | 1.31 (1.07–1.57) |
| Anemia | 30 | 49.45 (35.05–68.73) | 1.16 (0.86–1.50) |
| Myelosuppression | 8 | 57.31 (30.39–103.86) | 1.34 (0.74–2.11) |
| Neutrophil count decreased | 23 | 47.37 (32.47–66.99) | 1.24 (0.87–1.67) |
| (B) Gastrointestinal disorders | |||
| Ileus | 10 | 53.39 (36.08–77.07) | 1.90 (1.12–2.88) |
| (C) Others | |||
| Ovarian cancer recurrent | 20 | 41.94 (30.03–57.51) | 1.50 (1.05–2.01) |
| Ovarian cancer | 28 | 67.74 (47.49–95.05) | 1.17 (0.85–1.53) |
| Disease progression | 43 | 55.77 (42.50–72.42) | 1.21 (0.95–1.49) |
| Condition aggravated | 8 | 50.81 (29.50–84.69) | 1.56 (0.87–2.44) |
| Renal impairment | 32 | 46.34 (31.42–67.17) | 0.99 (0.75–1.27) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamaoka, K.; Fujiwara, M.; Uchida, M.; Uesawa, Y.; Muroi, N.; Shimizu, T. Comprehensive Analysis of Adverse Events Induced by PARP Inhibitors Using JADER and Time to Onset. Life 2022, 12, 1355. https://doi.org/10.3390/life12091355
Yamaoka K, Fujiwara M, Uchida M, Uesawa Y, Muroi N, Shimizu T. Comprehensive Analysis of Adverse Events Induced by PARP Inhibitors Using JADER and Time to Onset. Life. 2022; 12(9):1355. https://doi.org/10.3390/life12091355
Chicago/Turabian StyleYamaoka, Kenta, Masaki Fujiwara, Mayako Uchida, Yoshihiro Uesawa, Nobuyuki Muroi, and Tadashi Shimizu. 2022. "Comprehensive Analysis of Adverse Events Induced by PARP Inhibitors Using JADER and Time to Onset" Life 12, no. 9: 1355. https://doi.org/10.3390/life12091355
APA StyleYamaoka, K., Fujiwara, M., Uchida, M., Uesawa, Y., Muroi, N., & Shimizu, T. (2022). Comprehensive Analysis of Adverse Events Induced by PARP Inhibitors Using JADER and Time to Onset. Life, 12(9), 1355. https://doi.org/10.3390/life12091355







