Treatment of Metastatic Melanoma at First Diagnosis: Review of the Literature
Abstract
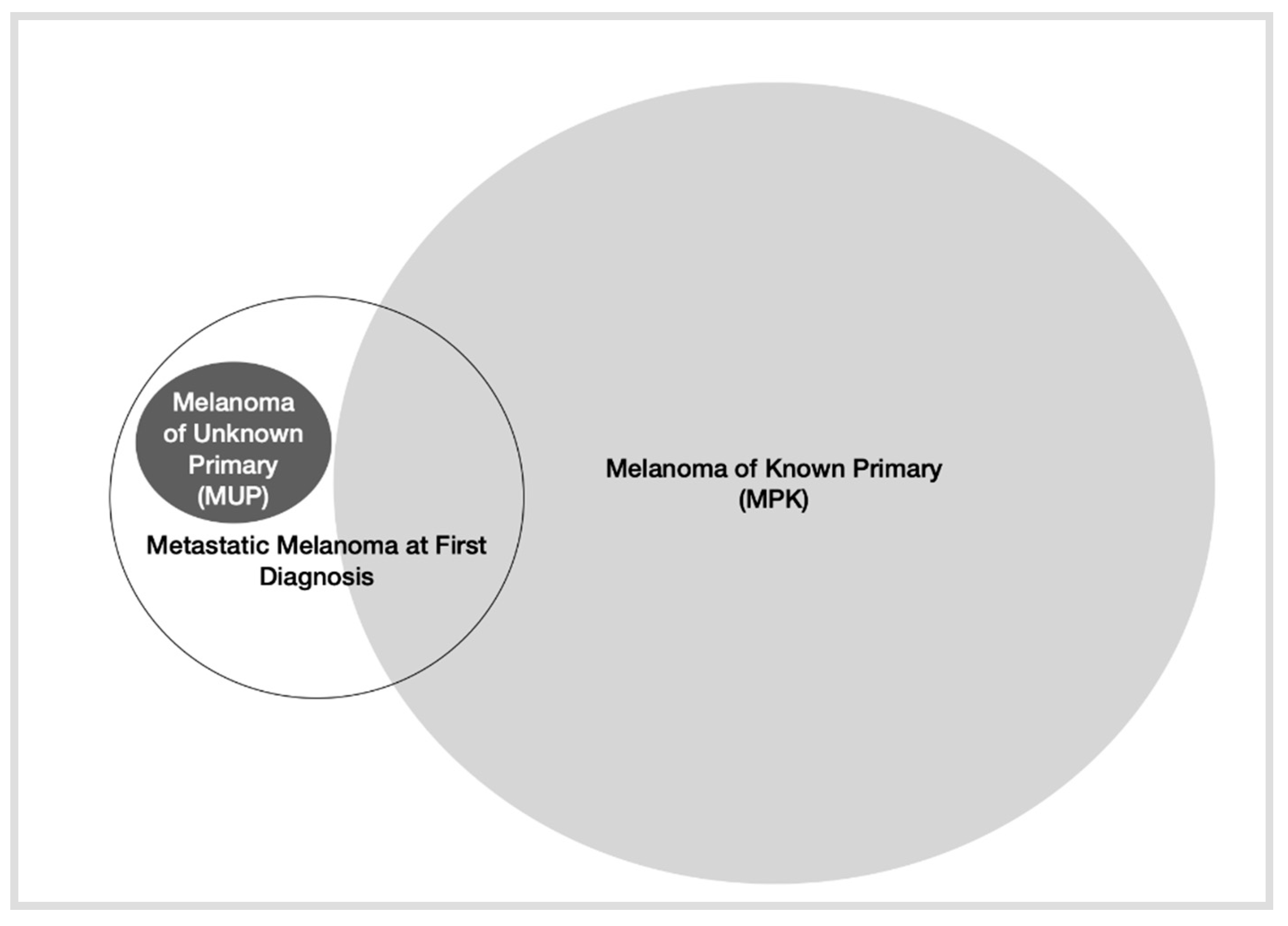
1. Introduction
2. Epidemiology of MM at First Diagnosis
3. Molecular Characterization of MM at First Diagnosis
4. Representation of MM at First Diagnosis in Pivotal Studies
4.1. Targeted Therapy
4.2. Immunotherapy
4.3. Chemotherapy
5. Representation of MM at First Diagnosis in Real-World Data
6. Limitations
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Cancer Institute. Melanoma of the Skin-Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/melan.html (accessed on 19 July 2022).
- Leeneman, B.; Schreuder, K.; de Groot, C.A.U.; van Akkooi, A.C.; Haanen, J.B.; Wakkee, M.; Franken, M.G.; Louwman, M.W. Stage-specific trends in incidence and survival of cutaneous melanoma in the Netherlands (2003–2018): A nationwide population-based study. Eur. J. Cancer 2021, 154, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Conforti, C.; Zalaudek, I. Epidemiology and Risk Factors of Melanoma: A Review. Dermatol. Pract. Concept. 2021, 11 (Suppl. 1), e2021161S. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, A.M.M.; Chiarion-Sileni, V.; Grob, J.; Dummer, R.; Wolchok, J.D.; Schmidt, H.; Hamid, O.; Robert, C.; Ascierto, P.A.; Richards, J.M.; et al. Prolonged Survival in Stage III Melanoma with Ipilimumab Adjuvant Therapy. N. Engl. J. Med. 2016, 375, 1845–1855, Correction in N. Engl. J. Med. 2018, 379, 2185. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.; Mandalà, M.; Del Vecchio, M.; Gogas, H.J.; Arance, A.M.; Cowey, C.L.; Dalle, S.; Schenker, M.; Chiarion-Sileni, V.; Marquez-Rodas, I.; et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N. Engl. J. Med. 2017, 377, 1824–1835. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, A.M.M.; Blank, C.U.; Mandalà, M.; Long, G.V.; Atkinson, V.; Dalle, S.; Haydon, A.; Lichinitser, M.; Khattak, A.; Carlino, M.S.; et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N. Engl. J. Med. 2018, 378, 1789–1801. [Google Scholar] [CrossRef]
- Long, G.V.; Hauschild, A.; Santinami, M.; Atkinson, V.; Mandalà, M.; Sileni, V.C.; Larkin, J.; Nyakas, M.; Dutriaux, C.; Haydon, A.; et al. Adjuvant Dabrafenib plus Trametinib in Stage IIIBRAF-Mutated Melanoma. N. Engl. J. Med. 2017, 377, 1813–1823. [Google Scholar] [CrossRef]
- Luke, J.J.; Rutkowski, P.; Queirolo, P.; Del Vecchio, M.; Mackiewicz, J.; Chiarion-Sileni, V.; Merino, L.D.L.C.; Khattak, M.A.; Schadendorf, D.; Long, G.V.; et al. Pembrolizumab versus placebo as adjuvant therapy in completely resected stage IIB or IIC melanoma (KEYNOTE-716): A randomised, double-blind, phase 3 trial. Lancet 2022, 399, 1718–1729. [Google Scholar] [CrossRef]
- Lawrence, M.S.; Stojanov, P.; Polak, P.; Kryukov, G.V.; Cibulskis, K.; Sivachenko, A.; Carter, S.L.; Stewart, C.; Mermel, C.H.; Roberts, S.A.; et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 2013, 499, 214–218. [Google Scholar] [CrossRef]
- Atkins, M.B.; Lee, S.J.; Chmielowski, B.; Ribas, A.; Tarhini, A.A.; Truong, T.; Davar, D.; O’Rourke, M.A.; Curti, B.D.; Brell, J.M.; et al. DREAMseq (Doublet, Randomized Evaluation in Advanced Melanoma Sequencing): A phase III trial—ECOG-ACRIN EA6134. J. Clin. Oncol. 2021, 39 (Suppl. 36), 356154. [Google Scholar] [CrossRef]
- American Cancer Society. Cancer Facts & Figures. 2022. Available online: https://www.cancer.org/cancer/melanoma-skin-cancer/about/key-statistics.html (accessed on 19 July 2022).
- Balch, C.M.; Buzaid, A.C.; Soong, S.-J.; Atkins, M.B.; Cascinelli, N.; Coit, D.G.; Fleming, I.D.; Gershenwald, J.E.; Houghton, A.; Kirkwood, J.M.; et al. Final Version of the American Joint Committee on Cancer Staging System for Cutaneous Melanoma. J. Clin. Oncol. 2001, 19, 3635–3648. [Google Scholar] [CrossRef]
- Diepgen, T.L.; Mahler, V. The epidemiology of skin cancer. Br. J. Dermatol. 2002, 146 (Suppl. 61), 1–6. [Google Scholar] [CrossRef] [PubMed]
- Agarwala, S.S. Current systemic therapy for metastatic melanoma. Expert Rev. Anticancer Ther. 2009, 9, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Korn, E.L.; Liu, P.-Y.; Lee, S.J.; Chapman, J.-A.W.; Niedzwiecki, D.; Suman, V.J.; Moon, J.; Sondak, V.K.; Atkins, M.B.; Eisenhauer, E.A.; et al. Meta-Analysis of Phase II Cooperative Group Trials in Metastatic Stage IV Melanoma to Determine Progression-Free and Overall Survival Benchmarks for Future Phase II Trials. J. Clin. Oncol. 2008, 26, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Uhara, H.; Kiyohara, Y.; Uehara, J.; Fujisawa, Y.; Takenouchi, T.; Otsuka, M.; Uchi, H.; Fukushima, S.; Minami, H.; Hatsumichi, M.; et al. Five-year survival with nivolumab in previously untreated Japanese patients with advanced or recurrent malignant melanoma. J. Dermatol. 2021, 48, 592–599. [Google Scholar] [CrossRef]
- Hill, M.V.; Vidri, R.J.; Deng, M.; Handorf, E.; Olszanski, A.J.; Farma, J.M. Real-world frequency of BRAF testing and utilization of therapies in patients with advanced melanoma. Melanoma Res. 2022, 32, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Reyn, B.; Van Eycken, E.; Louwman, M.; Henau, K.; Schreuder, K.; Brochez, L.; Garmyn, M.; Kukutsch, N. Incidence and survival of cutaneous melanoma in Belgium and the Netherlands from 2004 to 2016: Striking differences and similarities of two neighbouring countries. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1528–1535. [Google Scholar] [CrossRef]
- Dasgupta, T.; Bowden, L.; Berg, J.W. Malignant melanoma of unknown primary origin. Surg. Gynecol. Obstet. 1963, 117, 341–345. [Google Scholar]
- Kamposioras, K.; Pentheroudakis, G.; Pectasides, D.; Pavlidis, N. Malignant melanoma of unknown primary site. To make the long story short. A systematic review of the literature. Crit. Rev. Oncol. Hematol. 2011, 78, 112–126. [Google Scholar] [CrossRef]
- Scott, J.F.; Conic, R.Z.; Thompson, C.L.; Gerstenblith, M.R.; Bordeaux, J.S. Stage IV melanoma of unknown primary: A population-based study in the United States from 1973 to 2014. J. Am. Acad. Dermatol. 2018, 79, 258–265.e4. [Google Scholar] [CrossRef]
- Boussios, S.; Rassy, E.; Samartzis, E.; Moschetta, M.; Sheriff, M.; Pérez-Fidalgo, J.A.; Pavlidis, N. Melanoma of unknown primary: New perspectives for an old story. Crit. Rev. Oncol. Hematol. 2020, 158, 103208. [Google Scholar] [CrossRef]
- Tos, T.; Klyver, H.; Drzewiecki, K.T. Extensive screening for primary tumor is redundant in melanoma of unknown primary. J. Surg. Oncol. 2011, 104, 724–727. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.M.; Choi, Y.Y.; Kim, D.S.; Lee, J.H.; Jang, H.S.; Lee, J.H.; Kim, H.; Oh, B.H.; Roh, M.R.; Nam, K.A.; et al. Metastatic melanomas of unknown primary show better prognosis than those of known primary: A systematic review and meta-analysis of observational studies. J. Am. Acad. Dermatol. 2015, 72, 59–70. [Google Scholar] [CrossRef]
- Song, Y.; Karakousis, G.C. Melanoma of unknown primary. J. Surg. Oncol. 2018, 119, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, G.; Argenziano, G. The WHO 2018 Classification of Cutaneous Melanocytic Neoplasms: Suggestions from Routine Practice. Front. Oncol. 2021, 11, 675296. [Google Scholar] [CrossRef] [PubMed]
- Teixido, C.; Castillo, P.; Martinez-Vila, C.; Arance, A.; Alos, L. Molecular Markers and Targets in Melanoma. Cells 2021, 10, 2320. [Google Scholar] [CrossRef]
- Katz, K.A.; Jonasch, E.; Hodi, F.S.; Soiffer, R.; Kwitkiwski, K.; Sober, A.J.; Haluska, F.G. Melanoma of unknown primary: Experience at Massachusetts General Hospital and Dana-Farber Cancer Institute. Melanoma Res. 2005, 15, 77–82. [Google Scholar] [CrossRef]
- Larkin, J.; Ascierto, P.A.; Dréno, B.; Atkinson, V.; Liszkay, G.; Maio, M.; Mandalà, M.; Demidov, L.; Stroyakovskiy, D.; Thomas, L.; et al. Combined Vemurafenib and Cobimetinib in BRAF-Mutated Melanoma. N. Engl. J. Med. 2014, 371, 1867–1876. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Dréno, B.; Larkin, J.; Ribas, A.; Liszkay, G.; Maio, M.; Mandalà, M.; Demidov, L.; Stroyakovskiy, D.; Thomas, L.; et al. 5-Year Outcomes with Cobimetinib plus Vemurafenib in BRAFV600 Mutation–Positive Advanced Melanoma: Extended Follow-up of the coBRIM Study. Clin. Cancer Res. 2021, 27, 5225–5235. [Google Scholar] [CrossRef]
- Robert, C.; Karaszewska, B.; Schachter, J.; Rutkowski, P.; Mackiewicz, A.; Stroiakovski, D.; Lichinitser, M.; Dummer, R.; Grange, F.; Mortier, L.; et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N. Engl. J. Med. 2015, 372, 30–39. [Google Scholar] [CrossRef]
- Long, G.V.; Stroyakovskiy, D.; Gogas, H.; Levchenko, E.; de Braud, F.; Larkin, J.; Garbe, C.; Jouary, T.; Hauschild, A.; Grob, J.-J.; et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: A multicentre, double-blind, phase 3 randomised controlled trial. Lancet 2015, 386, 444–451. [Google Scholar] [CrossRef]
- Robert, C.; Grob, J.J.; Stroyakovskiy, D.; Karaszewska, B.; Hauschild, A.; Levchenko, E.; Chiarion Sileni, V.; Schachter, J.; Garbe, C.; Bondarenko, I.; et al. Five-Year Outcomes with Dabrafenib plus Trametinib in Metastatic Melanoma. N. Engl. J. Med. 2019, 381, 626–636. [Google Scholar] [CrossRef]
- Dummer, R.; Ascierto, P.A.; Gogas, H.J.; Arance, A.; Mandala, M.; Liszkay, G.; Garbe, C.; Schadendorf, D.; Krajsova, I.; Gutzmer, R.; et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2018, 19, 603–615. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Dummer, R.; Gogas, H.J.; Flaherty, K.T.; Arance, A.; Mandala, M.; Liszkay, G.; Garbe, C.; Schadendorf, D.; Krajsova, I.; et al. Update on tolerability and overall survival in COLUMBUS: Landmark analysis of a randomised phase 3 trial of encorafenib plus binimetinib vs vemurafenib or encorafenib in patients with BRAF V600–mutant melanoma. Eur. J. Cancer 2020, 126, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF gene in human cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Dummer, R.; Long, G.V.; Robert, C.; Tawbi, H.A.; Flaherty, K.T.; Ascierto, P.A.; Nathan, P.D.; Rutkowski, P.; Leonov, O.; Dutriaux, C.; et al. Randomized Phase III Trial Evaluating Spartalizumab Plus Dabrafenib and Trametinib for BRAF V600–Mutant Unresectable or Metastatic Melanoma. J. Clin. Oncol. 2022, 40, 1428–1438. [Google Scholar] [CrossRef]
- Gutzmer, R.; Stroyakovskiy, D.; Gogas, H.; Robert, C.; Lewis, K.; Protsenko, S.; Pereira, R.P.; Eigentler, T.; Rutkowski, P.; Demidov, L.; et al. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAFV600 mutation-positive melanoma (IMspire150): Primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2020, 395, 1835–1844, Published correction appears in Lancet 2020, 396, 466. [Google Scholar] [CrossRef]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in Previously Untreated Melanoma without BRAF Mutation. N. Engl. J. Med. 2015, 372, 320–330. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Long, G.V.; Robert, C.; Brady, B.; Dutriaux, C.; Di Giacomo, A.M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; et al. Survival Outcomes in Patients with Previously Untreated BRAF Wild-Type Advanced Melanoma Treated with Nivolumab Therapy: Three-Year Follow-up of a Randomized Phase 3 Trial. JAMA Oncol. 2019, 5, 187–194, Correction in JAMA Oncol. 2019, 5, 271. [Google Scholar] [CrossRef]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Di Giacomo, A.M.; Mortier, L.; Rutkowski, P.; Hassel, J.C.; McNeil, C.M.; Kalinka, E.A.; et al. Five-Year Outcomes with Nivolumab in Patients with Wild-Type BRAF Advanced Melanoma. J. Clin. Oncol. 2020, 38, 3937–3946. [Google Scholar] [CrossRef]
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef]
- Robert, C.; Ribas, A.; Schachter, J.; Arance, A.; Grob, J.-J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.M.; Lotem, M.; et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): Post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019, 20, 1239–1251. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.-J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2017, 377, 1345–1356, Correction in N. Engl. J. Med. 2018, 379, 2185. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Long-Term Outcomes with Nivolumab Plus Ipilimumab or Nivolumab Alone Versus Ipilimumab in Patients with Advanced Melanoma. J. Clin. Oncol. 2022, 40, 127–137. [Google Scholar] [CrossRef]
- Lebbé, C.; Meyer, N.; Mortier, L.; Marquez-Rodas, I.; Robert, C.; Rutkowski, P.; Menzies, A.M.; Eigentler, T.; Ascierto, P.A.; Smylie, M.; et al. Evaluation of Two Dosing Regimens for Nivolumab in Combination with Ipilimumab in Patients with Advanced Melanoma: Results from the Phase IIIb/IV CheckMate 511 Trial. J. Clin. Oncol. 2019, 37, 867–875. [Google Scholar] [CrossRef]
- Ribas, A.; Kefford, R.; Marshall, M.A.; Punt, C.J.A.; Haanen, J.B.; Marmol, M.; Garbe, C.; Gogas, H.; Schachter, J.; Linette, G.; et al. Phase III Randomized Clinical Trial Comparing Tremelimumab with Standard-of-Care Chemotherapy in Patients with Advanced Melanoma. J. Clin. Oncol. 2013, 31, 616–622. [Google Scholar] [CrossRef]
- Sherrill, B.; Wang, J.; Kotapati, S.; Chin, K. Q-TWiST analysis comparing ipilimumab/dacarbazine vs placebo/dacarbazine for patients with stage III/IV melanoma. Br. J. Cancer 2013, 109, 8–13. [Google Scholar] [CrossRef][Green Version]
- Arance, A.M.; de la Cruz-Merino, L.; Petrella, T.M.; Jamal, R.; Ny, L.; Carneiro, A.; Berrocal, A.; Marquez-Rodas, I.; Spreafico, A.; Atkinson, V.; et al. Lenvatinib (len) plus pembrolizumab (pembro) for patients (pts) with advanced melanoma and confirmed progression on a PD-1 or PD-L1 inhibitor: Updated findings of LEAP-004. J. Clin. Oncol. 2021, 39, 9504. [Google Scholar] [CrossRef]
- Diab, A.; Tykodi, S.S.; Daniels, G.A.; Maio, M.; Curti, B.D.; Lewis, K.D.; Jang, S.; Kalinka, E.; Puzanov, I.; Spira, A.I.; et al. Bempegaldesleukin Plus Nivolumab in First-Line Metastatic Melanoma. J. Clin. Oncol. 2021, 39, 2914–2925. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Gutiérrez, E.C.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef]
- O’Day, S.J.; Eggermont, A.M.; Chiarion-Sileni, V.; Kefford, R.; Grob, J.J.; Mortier, L.; Robert, C.; Schachter, J.; Testori, A.; Mackiewicz, J.; et al. Final Results of Phase III SYMMETRY Study: Randomized, Double-Blind Trial of Elesclomol Plus Paclitaxel Versus Paclitaxel Alone as Treatment for Chemotherapy-Naive Patients with Advanced Melanoma. J. Clin. Oncol. 2013, 31, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Kefford, R.F.; Clingan, P.R.; Brady, B.; Ballmer, A.; Morganti, A.; Hersey, P. A randomized, double-blind, placebo-controlled study of high-dose bosentan in patients with stage IV metastatic melanoma receiving first-line dacarbazine chemotherapy. Mol. Cancer 2010, 9, 69. [Google Scholar] [CrossRef]
- Utter, K.; Goldman, C.; Weiss, S.A.; Shapiro, R.L.; Berman, R.; Wilson, M.A.; Pavlick, A.C.; Osman, I. Treatment Outcomes for Metastatic Melanoma of Unknown Primary in the New Era: A Single-Institution Study and Review of the Literature. Oncology 2017, 93, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Gambichler, T.; Chatzipantazi, M.; Schröter, U.; Stockfleth, E.; Gedik, C. Patients with melanoma of unknown primary show better outcome under immune checkpoint inhibitor therapy than patients with known primary: Preliminary results. OncoImmunology 2019, 8, e1677139. [Google Scholar] [CrossRef] [PubMed]
- Del Fiore, P.; Rastrelli, M.; Dall’Olmo, L.; Cavallin, F.; Cappellesso, R.; Vecchiato, A.; Buja, A.; Spina, R.; Parisi, A.; Mazzarotto, R.; et al. Melanoma of Unknown Primary: Evaluation of the Characteristics, Treatment Strategies, Prognostic Factors in a Monocentric Retrospective Study. Front. Oncol. 2021, 11, 627527, Published correction appears in Front. Oncol. 2021, 11, 686051. [Google Scholar] [CrossRef] [PubMed]
- Lebbe, C.; Lorigan, P.; Ascierto, P.; Testori, A.; Bédane, C.; Middleton, M.; van Baardewijk, M.; Konto, C.; Dueymes, A.; Maio, M. Treatment patterns and outcomes among patients diagnosed with unresectable stage III or IV melanoma in Europe: A retrospective, longitudinal survey (MELODY study). Eur. J. Cancer 2012, 48, 3205–3214. [Google Scholar] [CrossRef] [PubMed]
- Bedane, C.; Leccia, M.-T.; Sassolas, B.; Bregman, B.; Lebbé, C. Treatment patterns and outcomes in patients with advanced melanoma in France. Curr. Med Res. Opin. 2013, 29, 1297–1305. [Google Scholar] [CrossRef]
- Ernst, D.; Petrella, T.; Joshua, A.; Hamou, A.; Thabane, M.; Vantyghem, S.; Gwadry-Sridhar, F. Burden of Illness for Metastatic Melanoma in Canada, 2011–2013. Curr. Oncol. 2016, 23, e563–e570. [Google Scholar] [CrossRef]
- Zhou, C.; Louwman, M.; Wakkee, M.; van der Veldt, A.; Grünhagen, D.; Verhoef, C.; Mooyaart, A.; Nijsten, T.; Hollestein, L. Primary Melanoma Characteristics of Metastatic Disease: A Nationwide Cancer Registry Study. Cancers 2021, 13, 4431. [Google Scholar] [CrossRef]
- Marquez-Rodas, I.; Guerrero, M.B.; Couselo, E.M.; Manzano, J.; Herrero, G.C.; Soria, A.; Cerezuela, P.; Hernandez, T.P.; Berrocal, A.; Tarruella, M.M.; et al. 1056P Survival of patients with advanced melanoma according to first-line treatment and key prognostic factors: Real-world data from GEM1801 study. Ann. Oncol. 2021, 32, S881–S882. [Google Scholar] [CrossRef]
| Article | Ernst et al. [16] | Hill et al. [17] | ||
|---|---|---|---|---|
| Staging a | Stage IV at Initial Diagnosis | All | Stage IV at Initial Diagnosis | All |
| All patients (n%) | 78 (9.6) | 810 (100) | 1191 (26.7) | 4459 |
| Stage | Stage IV | Stage 0-IV | Stage IV | III-IV/Recurrence |
| Mean age (years) | 60.69 | 58.74 | 66 | 64.4 |
| Sex [n (%)] | ||||
| Men | 45 (9.3) | 485 (59.9) | - | - |
| Women | 33 (10.2) | 325(40.1) | - | - |
| ECOG (%) | ||||
| 0–1 | 665 (55.8) | 2619 (58.7) | ||
| 2 | 96 (8.1) | 248 (5.5) | ||
| 3–4 | 44 (3.7) | 91 (2.0) | ||
| Mean time to recurrence (years) | 3.84 ± 5.19 | 4.94 ± 6.69 | ||
| Mutation type [n (%)] | ||||
| BRAF | 11 (21.6) | 51 (6.3) | 444 (37.3) | 1314 (42) |
| CKIT | 1 (16.7) | 6 (0.7) | - | - |
| NRAS | 0 | 1 (0.1) | - | - |
| MEK | 0 | 0 | - | - |
| GNAO | 0 | 0 | - | - |
| GNA11 | 0 | 0 | - | - |
| Metastatic [n (%)] | ||||
| Yes | 11 (21.6) | 354 (44.0) | ||
| No | 1 (16.7) | 454 (56.1) | ||
| Resectable [n (%)] | ||||
| Yes | 0 | 704 (86.9) | ||
| No | 78 (73.6) | 106 (13.1) | ||
| Developed metastatic during course of disease | ||||
| No | 78 (100) | 454 (56.0) | ||
| Yes | 0 | 346 (42.7) | ||
| Median Overall Survival [months (95% CI)] | ||||
| Initially stage 0–II | 111.3 | |||
| Initially stage III | (95.6–131) | |||
| Initially stage IV | 76.3 (59.3–93.3) | |||
| 59.9 (38.2–81.7) | ||||
| First Line Treatment (%) | ||||
| Immunotherapy | 665 (73.1) | 1652 (73.5) | ||
| BRAF inhibitor | 186 (20.4) | 393 (17.5) | ||
| Clinical trial | 28 (3.1) | 106 (4.7) | ||
| Chemotherapy | 19 (2.1) | 51 (2.3) | ||
| Interferon | 5 (0.5) | 14 (0.6) | ||
| IL-2 | 1 (0.1) | 4 (0.2) | ||
| Clinical Trial | COBRIM [29,30] | COMBI-V/D [31,32,33] | COLUMBUS [34,35] |
|---|---|---|---|
| Experimental Drug | Cobi + Vem | Dabra-Trame | Enco-Bini |
| Control Drug | Pbo + Vem | Vemu/Dabra | Vemu |
| Stage at randomization a,b (%) | |||
| III | 9 | 2 | 4.7 |
| IV (M1c) | 91 | 98 | 95.3 |
| Stage IV at first diagnosis a,b | NR | NR | NR |
| mPFS a | 12.6 | 11.1 | 14.9 |
| mOS a | 22.5 | 25.9 | 33.6 |
| mOS by stage a,b: III M1a M1b M1c | NR 54.8 19.4 18.9 | NR HR III-M1b vs. M1c 0.76 (0.58–1) | NR |
| MUP patients | |||
| Included | Yes | Yes | Yes |
| Outcomes | NR | NR | NR |
| Clinical Trial | CHECKMATE-066 [39,40,41] | KEYNOTE-006 [42,43] | CHECKMATE-067 [44,45] |
|---|---|---|---|
| Experimental Drug | Nivo | Pem | Nivo-Ipi |
| Control Drug | DTIC | Ipi | Ipi/Nivo |
| Stage at randomization a,b (%) | |||
| III | NR | 3.1 | |
| IV (M1c) | 61% M1c | 96.9 (66.2) | (58.9) |
| Stage IV at first diagnosis a,b | NR c | NR | NR |
| mPFS a | 5.1 | 8.4 | 11.5 |
| mOS a | 37.3 | 31.1 | 60.0 |
| mOS by stage a,b: III M1a M1b M1c | NR | NR | NR |
| MUP patients | |||
| Included | Yes | Yes | Yes |
| Outcomes | NR | NR | NR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berciano-Guerrero, M.-A.; Guardamagna, M.; Perez-Ruiz, E.; Jurado, J.-M.; Barragán, I.; Rueda-Dominguez, A. Treatment of Metastatic Melanoma at First Diagnosis: Review of the Literature. Life 2022, 12, 1302. https://doi.org/10.3390/life12091302
Berciano-Guerrero M-A, Guardamagna M, Perez-Ruiz E, Jurado J-M, Barragán I, Rueda-Dominguez A. Treatment of Metastatic Melanoma at First Diagnosis: Review of the Literature. Life. 2022; 12(9):1302. https://doi.org/10.3390/life12091302
Chicago/Turabian StyleBerciano-Guerrero, Miguel-Angel, Mora Guardamagna, Elisabeth Perez-Ruiz, Jose-Miguel Jurado, Isabel Barragán, and Antonio Rueda-Dominguez. 2022. "Treatment of Metastatic Melanoma at First Diagnosis: Review of the Literature" Life 12, no. 9: 1302. https://doi.org/10.3390/life12091302
APA StyleBerciano-Guerrero, M.-A., Guardamagna, M., Perez-Ruiz, E., Jurado, J.-M., Barragán, I., & Rueda-Dominguez, A. (2022). Treatment of Metastatic Melanoma at First Diagnosis: Review of the Literature. Life, 12(9), 1302. https://doi.org/10.3390/life12091302







