Skeletal-Muscle-Specific Overexpression of Chrono Leads to Disruption of Glucose Metabolism and Exercise Capacity
Abstract
1. Introduction
2. Method
2.1. Animals
2.2. Endurance Exercise Capacity Test
2.3. Blood Lactate
2.4. Glucose Tolerance Test (GTT)
2.5. Nonfasted Blood Glucose and Insulin
2.6. Muscle Glycogen Content and Activity of Glycogen Synthase (Gys) and Hexokinase (Hk)
2.7. Real-Time Quantitative PCR (RT-qPCR) Analysis
2.8. Western Blot
2.9. Co-Immunoprecipitation (Co-IP) Assay
2.10. Data Processing and Statistical Methods
3. Results
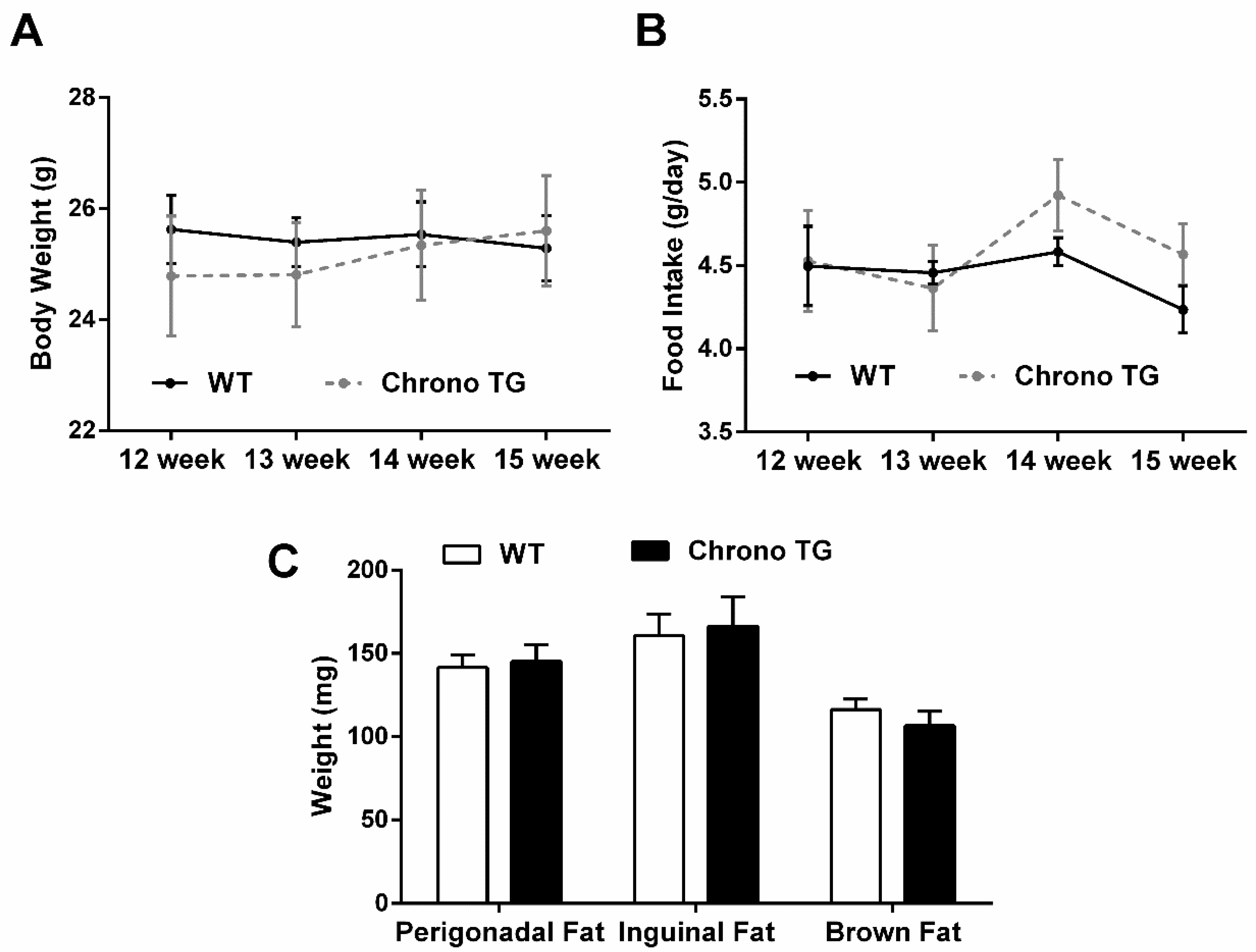
3.1. Food Intake, Body Weight, and Adipose Tissue Weight
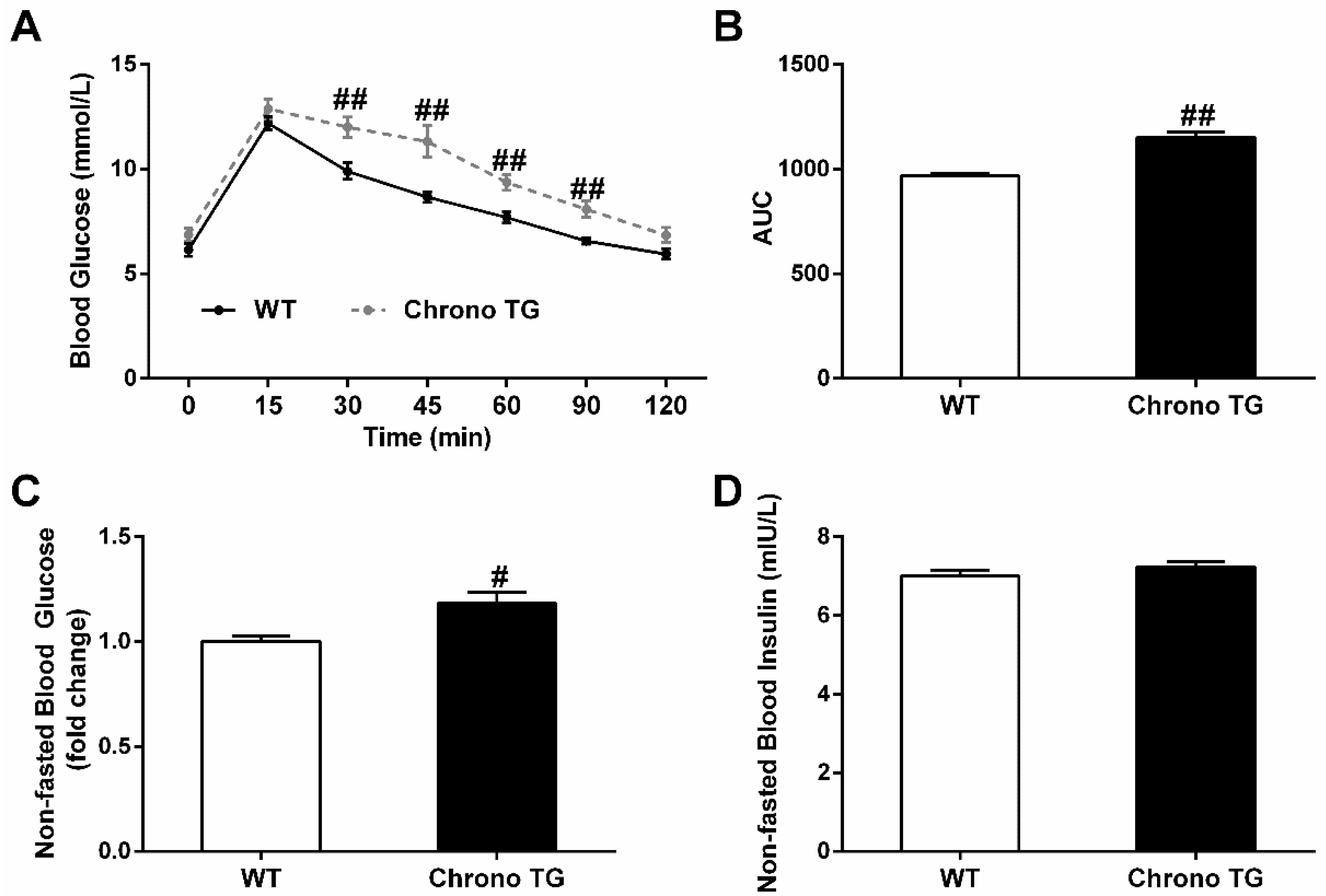
3.2. GTT, Nonfasted Blood Glucose, and Insulin
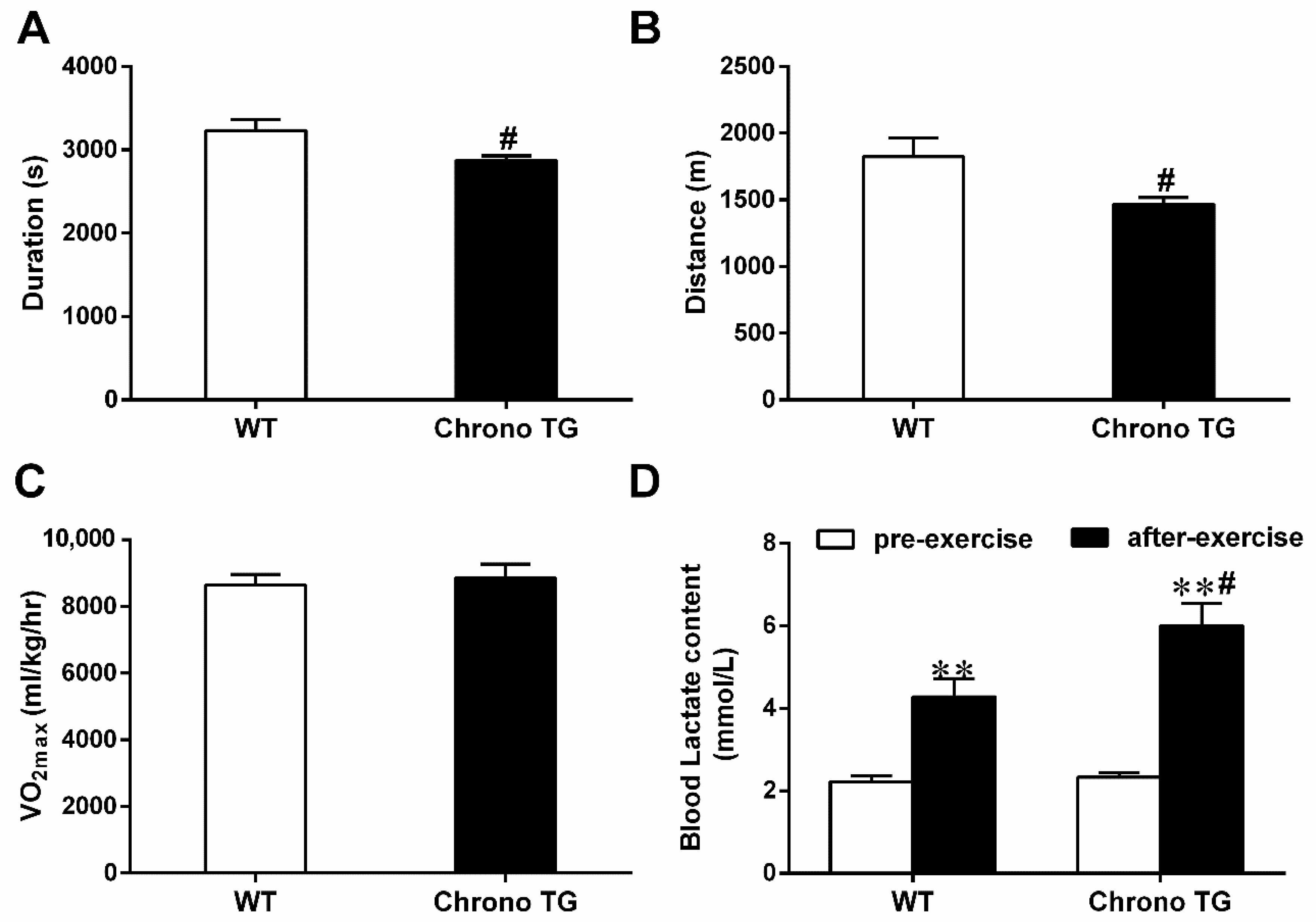
3.3. Exercise Capacity and Blood Lactate Content
3.4. The mRNA Expression of Chrono, Tbc1d1, Glut4, Hk2, Pfkm, Pdp1, and Pdk4; and the Hk Activity and the Amount of Chrono Bound to Bmal1
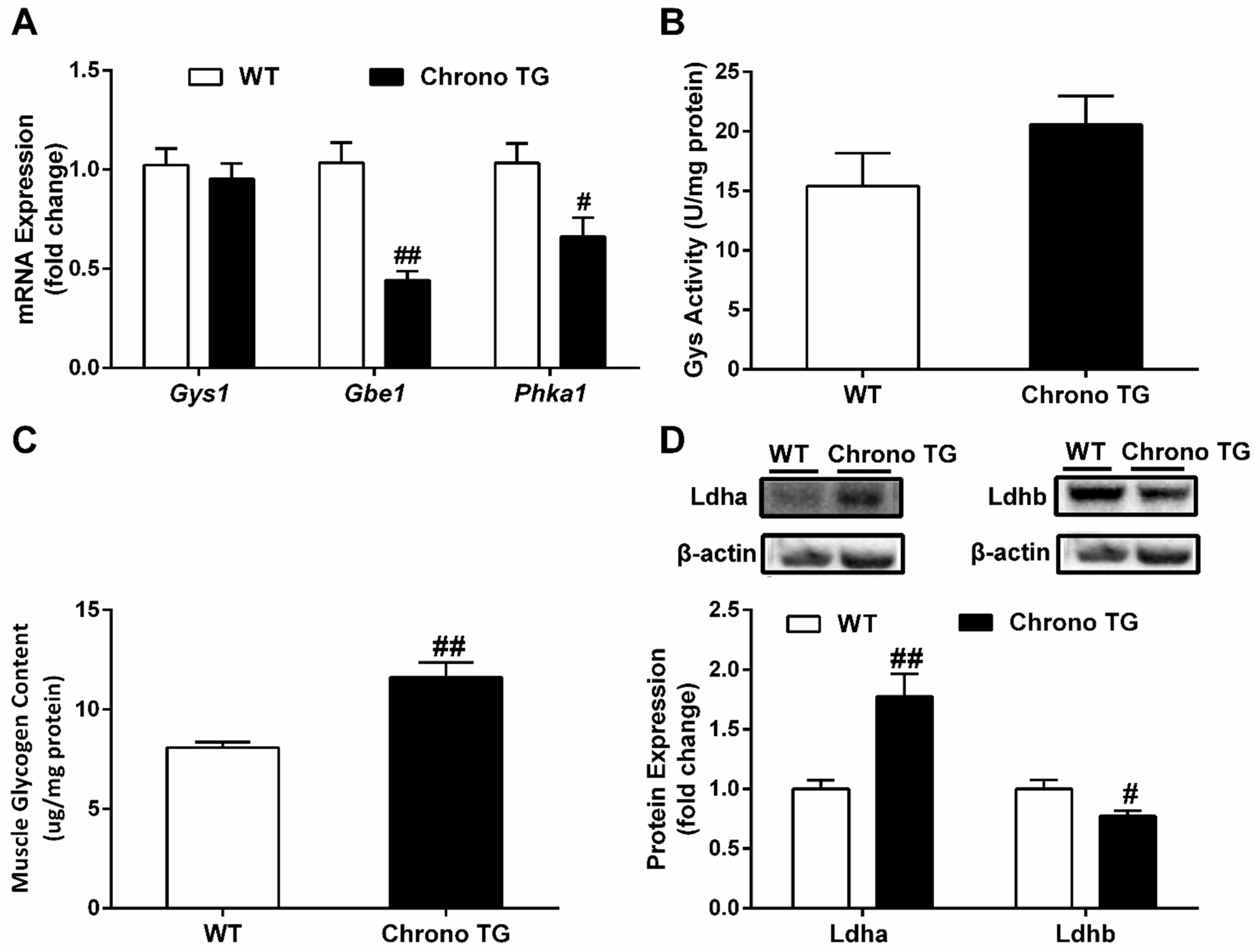
3.5. The mRNA Expression of Gys1, Gbe1, and Phka1; Activity of Gys; Glycogen Content; as well as Protein Expression of Ldha and Ldhb
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frontera, W.R.; Ochala, J. Skeletal muscle: A brief review of structure and function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Tripathy, D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 2009, 32 (Suppl. S2), S157–S163. [Google Scholar] [CrossRef] [PubMed]
- Wefers, J.; van Moorsel, D.; Hansen, J.; Connell, N.J.; Havekes, B.; Hoeks, J.; Lichtenbelt, W.D.V.M.; Duez, H.; Phielix, E.; Kalsbeek, A.; et al. Circadian misalignment induces fatty acid metabolism gene profiles and compromises insulin sensitivity in human skeletal muscle. Proc. Natl. Acad. Sci. USA 2018, 115, 7789–7794. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, B.M.; Altıntaş, A.; Smith, J.A.B.; Sardon-Puig, L.; Zhang, X.; Basse, A.L.; Laker, R.C.; Gao, H.; Liu, Z.; Dollet, L.; et al. Disrupted circadian oscillations in type 2 diabetes are linked to altered rhythmic mitochondrial metabolism in skeletal muscle. Sci. Adv. 2021, 7, eabi9654. [Google Scholar] [CrossRef] [PubMed]
- Eilers, W.; Chambers, D.; Cleasby, M.; Foster, K. Local myostatin inhibition improves skeletal muscle glucose uptake in insulin-resistant high-fat diet-fed mice. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E163–E174. [Google Scholar] [CrossRef] [PubMed]
- Pataky, M.W.; Wang, H.; Yu, C.S.; Arias, E.B.; Ploutz-Snyder, R.J.; Zheng, X.; Cartee, G.D. High-Fat Diet-Induced Insulin Resistance in Single Skeletal Muscle Fibers is Fiber Type Selective. Sci. Rep. 2017, 7, 13642. [Google Scholar] [CrossRef]
- Souto Padron de Figueiredo, A.; Salmon, A.B.; Bruno, F.; Jimenez, F.; Martinez, H.G.; Halade, G.V.; Ahuja, S.S.; Clark, R.A.; DeFronzo, R.A.; Abboud, H.E.; et al. Nox2 mediates skeletal muscle insulin resistance induced by a high fat diet. J. Biol. Chem. 2015, 290, 13427–13439. [Google Scholar] [CrossRef]
- Kwon, O.S.; Tanner, R.E.; Barrows, K.M.; Runtsch, M.; Symons, J.D.; Jalili, T.; Bikman, B.T.; McClain, D.A.; O’Connell, R.M.; Drummond, M.J. MyD88 regulates physical inactivity-induced skeletal muscle inflammation, ceramide biosynthesis signaling, and glucose intolerance. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E11–E21. [Google Scholar] [CrossRef]
- Mohawk, J.A.; Green, C.B.; Takahashi, J.S. Central and peripheral circadian clocks in mammals. Annu. Rev. Neurosci. 2012, 35, 445–462. [Google Scholar] [CrossRef]
- Dibner, C.; Schibler, U.; Albrecht, U. The mammalian circadian timing system: Organization and coordination of central and peripheral clocks. Annu. Rev. Physiol. 2010, 72, 517–549. [Google Scholar] [CrossRef]
- Takahashi, J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2017, 18, 164–179. [Google Scholar] [CrossRef] [PubMed]
- Hodge, B.A.; Wen, Y.; Riley, L.A.; Zhang, X.; England, J.H.; Harfmann, B.D.; Schroder, E.A.; Esser, K.A. The endogenous molecular clock orchestrates the temporal separation of substrate metabolism in skeletal muscle. Skelet. Muscle 2015, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhou, B.; Yan, M.; Huang, R.; Wang, Y.; He, Z.; Yang, Y.; Dai, C.; Wang, Y.; Zhang, F.; et al. CLOCK and BMAL1 Regulate Muscle Insulin Sensitivity via SIRT1 in Male Mice. Endocrinology 2016, 157, 2259–2269. [Google Scholar] [CrossRef] [PubMed]
- Harfmann, B.D.; Schroder, E.A.; Kachman, M.T.; Hodge, B.A.; Zhang, X.; Esser, K.A. Muscle-specific loss of Bmal1 leads to disrupted tissue glucose metabolism and systemic glucose homeostasis. Skelet. Muscle 2016, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Goriki, A.; Hatanaka, F.; Myung, J.; Kim, J.K.; Yoritaka, T.; Tanoue, S.; Abe, T.; Kiyonari, H.; Fujimoto, K.; Kato, Y.; et al. A novel protein, CHRONO, functions as a core component of the mammalian circadian clock. PLoS Biol. 2014, 12, e1001839. [Google Scholar] [CrossRef] [PubMed]
- Anafi, R.C.; Lee, Y.; Sato, T.K.; Venkataraman, A.; Ramanathan, C.; Kavakli, I.H.; Hughes, M.E.; Baggs, J.E.; Growe, J.; Liu, A.C.; et al. Machine learning helps identify CHRONO as a circadian clock component. PLoS Biol. 2014, 12, e1001840. [Google Scholar] [CrossRef]
- Yang, Y.; Li, N.; Qiu, J.; Ge, H.; Qin, X. Identification of the Repressive Domain of the Negative Circadian Clock Component CHRONO. Int. J. Mol. Sci. 2020, 21, 2469. [Google Scholar] [CrossRef]
- Burch, N.; Arnold, A.S.; Item, F.; Summermatter, S.; Brochmann Santana Santos, G.; Christe, M.; Boutellier, U.; Toigo, M.; Handschin, C. Electric pulse stimulation of cultured murine muscle cells reproduces gene expression changes of trained mouse muscle. PLoS ONE 2010, 5, e10970. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Klip, A.; McGraw, T.E.; James, D.E. Thirty sweet years of GLUT4. J. Biol. Chem. 2019, 294, 11369–11381. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Morino, T.; Ishii, T.; Kanzaki, M. Cooperative actions of Tbc1d1 and AS160/Tbc1d4 in GLUT4-trafficking activities. J. Biol. Chem. 2019, 294, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, J.; Paglialunga, S.; Smith, B.K.; Miotto, P.M.; Simnett, G.; Robson, H.L.; Jain, S.S.; Herbst, E.A.F.; Desjardins, E.M.; Dyck, D.J.; et al. Ablating the protein TBC1D1 impairs contraction-induced sarcolemmal glucose transporter 4 redistribution but not insulin-mediated responses in rats. J. Biol. Chem. 2017, 292, 16653–16664. [Google Scholar] [CrossRef] [PubMed]
- Zisman, A.; Peroni, O.D.; Abel, E.D.; Michael, M.D.; Mauvais-Jarvis, F.; Lowell, B.B.; Wojtaszewski, J.F.; Hirshman, M.F.; Virkamaki, A.; Goodyear, L.J.; et al. Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance. Nat. Med. 2000, 6, 924–928. [Google Scholar] [CrossRef] [PubMed]
- Dokas, J.; Chadt, A.; Nolden, T.; Himmelbauer, H.; Zierath, J.R.; Joost, H.G.; Al-Hasani, H. Conventional knockout of Tbc1d1 in mice impairs insulin- and AICAR-stimulated glucose uptake in skeletal muscle. Endocrinology 2013, 154, 3502–3514. [Google Scholar] [CrossRef]
- Roberts, D.J.; Miyamoto, S. Hexokinase II integrates energy metabolism and cellular protection: Akting on mitochondria and TORCing to autophagy. Cell Death Differ. 2015, 22, 248–257. [Google Scholar] [CrossRef]
- Winther, S.; Isidor, M.S.; Basse, A.L.; Skjoldborg, N.; Cheung, A.; Quistorff, B.; Hansen, J.B. Restricting glycolysis impairs brown adipocyte glucose and oxygen consumption. Am. J. Physiol. Endocrinol. Metab. 2018, 314, E214–E223. [Google Scholar] [CrossRef]
- Ristow, M.; Vorgerd, M.; Möhlig, M.; Schatz, H.; Pfeiffer, A. Deficiency of phosphofructo-1-kinase/muscle subtype in humans impairs insulin secretion and causes insulin resistance. J. Clin. Investig. 1997, 100, 2833–2841. [Google Scholar] [CrossRef]
- Tanner, L.B.; Goglia, A.G.; Wei, M.H.; Sehgal, T.; Parsons, L.R.; Park, J.O.; White, E.; Toettcher, J.E.; Rabinowitz, J.D. Four Key Steps Control Glycolytic Flux in Mammalian Cells. Cell Syst. 2018, 7, 49–62.e8. [Google Scholar] [CrossRef]
- Ng, F.; Tang, B.L. Pyruvate dehydrogenase complex (PDC) export from the mitochondrial matrix. Mol. Membr. Biol. 2014, 31, 207–210. [Google Scholar] [CrossRef]
- Huang, B.; Gudi, R.; Wu, P.; Harris, R.A.; Hamilton, J.; Popov, K.M. Isoenzymes of pyruvate dehydrogenase phosphatase. DNA-derived amino acid sequences, expression, and regulation. J. Biol. Chem. 1998, 273, 17680–17688. [Google Scholar] [CrossRef]
- Huang, B.; Wu, P.; Popov, K.M.; Harris, R.A. Starvation and diabetes reduce the amount of pyruvate dehydrogenase phosphatase in rat heart and kidney. Diabetes 2003, 52, 1371–1376. [Google Scholar] [CrossRef]
- Holness, M.J.; Bulmer, K.; Smith, N.D.; Sugden, M.C. Investigation of potential mechanisms regulating protein expression of hepatic pyruvate dehydrogenase kinase isoforms 2 and 4 by fatty acids and thyroid hormone. Biochem. J. 2003, 369, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Mori, J.; Alrob, O.A.; Wagg, C.S.; Harris, R.A.; Lopaschuk, G.D.; Oudit, G.Y. ANG II causes insulin resistance and induces cardiac metabolic switch and inefficiency: A critical role of PDK4. Am. J. Physiol.-Heart Circ. Physiol. 2013, 304, H1103–H1113. [Google Scholar] [CrossRef] [PubMed]
- Iijima, H.; Iwano, R.; Tanaka, Y.; Muroya, K.; Fukuda, T.; Sugie, H.; Kurosawa, K.; Adachi, M. Analysis of GBE1 mutations via protein expression studies in glycogen storage disease type IV: A report on a non-progressive form with a literature review. Mol. Genet. Metab. Rep. 2018, 17, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Jeyasingham, M.D.; Artigues, A.; Nadeau, O.W.; Carlson, G.M. Structural evidence for co-evolution of the regulation of contraction and energy production in skeletal muscle. J. Mol. Biol. 2008, 377, 623–629. [Google Scholar] [CrossRef][Green Version]
- Davidson, J.J.; Ozçelik, T.; Hamacher, C.; Willems, P.J.; Francke, U.; Kilimann, M.W. cDNA cloning of a liver isoform of the phosphorylase kinase alpha subunit and mapping of the gene to Xp22.2–p22.1, the region of human X-linked liver glycogenosis. Proc. Natl. Acad. Sci. USA 1992, 89, 2096–2100. [Google Scholar] [CrossRef]
- Uruno, A.; Yagishita, Y.; Katsuoka, F.; Kitajima, Y.; Nunomiya, A.; Nagatomi, R.; Pi, J.; Biswal, S.S.; Yamamoto, M. Nrf2-Mediated Regulation of Skeletal Muscle Glycogen Metabolism. Mol. Cell. Biol. 2016, 36, 1655–1672. [Google Scholar] [CrossRef]
- Preisler, N.; Orngreen, M.C.; Echaniz-Laguna, A.; Laforet, P.; Lonsdorfer-Wolf, E.; Doutreleau, S.; Geny, B.; Akman, H.O.; Dimauro, S.; Vissing, J. Muscle phosphorylase kinase deficiency: A neutral metabolic variant or a disease? Neurology 2012, 78, 265–268. [Google Scholar] [CrossRef]
- Said, S.M.; Murphree, M.I.; Mounajjed, T.; El-Youssef, M.; Zhang, L. A novel GBE1 gene variant in a child with glycogen storage disease type IV. Hum. Pathol. 2016, 54, 152–156. [Google Scholar] [CrossRef]
- Ward, T.L.; Valberg, S.J.; Adelson, D.L.; Abbey, C.A.; Binns, M.M.; Mickelson, J.R. Glycogen branching enzyme (GBE1) mutation causing equine glycogen storage disease IV. Mamm. Genome 2004, 15, 570–577. [Google Scholar] [CrossRef]
- Wehner, M.; Clemens, P.R.; Engel, A.G.; Kilimann, M.W. Human muscle glycogenosis due to phosphorylase kinase deficiency associated with a nonsense mutation in the muscle isoform of the alpha subunit. Hum. Mol. Genet. 1994, 3, 1983–1987. [Google Scholar] [CrossRef] [PubMed]
- Kaslow, H.R.; Lesikar, D.D. Isozymes of glycogen synthase. FEBS Lett. 1984, 172, 294–298. [Google Scholar] [CrossRef]
- McCue, M.E.; Valberg, S.J.; Miller, M.B.; Wade, C.; DiMauro, S.; Akman, H.O.; Mickelson, J.R. Glycogen synthase (GYS1) mutation causes a novel skeletal muscle glycogenosis. Genomics 2008, 91, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Xirouchaki, C.E.; Mangiafico, S.P.; Bate, K.; Ruan, Z.; Huang, A.M.; Tedjosiswoyo, B.W.; Lamont, B.; Pong, W.; Favaloro, J.; Blair, A.R.; et al. Impaired glucose metabolism and exercise capacity with muscle-specific glycogen synthase 1 (gys1) deletion in adult mice. Mol. Metab. 2016, 5, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Preisler, N.; Haller, R.G.; Vissing, J. Exercise in muscle glycogen storage diseases. J. Inherit. Metab. Dis. 2015, 38, 551–563. [Google Scholar] [CrossRef]
- García, M.; Pujol, A.; Ruzo, A.; Riu, E.; Ruberte, J.; Arbós, A.; Serafín, A.; Albella, B.; Felíu, J.E.; Bosch, F. Phosphofructo-1-kinase deficiency leads to a severe cardiac and hematological disorder in addition to skeletal muscle glycogenosis. PLoS Genet. 2009, 5, e1000615. [Google Scholar] [CrossRef]
- Dawson, D.M.; Goodfriend, T.L.; Kaplan, N.O. Lactic dehydrogenases: Functions of the two types rates of synthesis of the two major forms can be correlated with metabolic differentiation. Science 1964, 143, 929–933. [Google Scholar] [CrossRef]
- Dyar, K.A.; Ciciliot, S.; Wright, L.E.; Biensø, R.S.; Tagliazucchi, G.M.; Patel, V.R.; Forcato, M.; Paz, M.I.; Gudiksen, A.; Solagna, F.; et al. Muscle insulin sensitivity and glucose metabolism are controlled by the intrinsic muscle clock. Mol. Metab. 2014, 3, 29–41. [Google Scholar] [CrossRef]
- Dreos, R.; Ambrosini, G.; Périer, R.C.; Bucher, P. The Eukaryotic Promoter Database: Expansion of EPDnew and new promoter analysis tools. Nucleic Acids Res. 2015, 43, D92–D96. [Google Scholar] [CrossRef]

| Gene Name | Forward | Reverse |
|---|---|---|
| Gys1 | 5′-GAACGCAGTGCTTTTCGAGG-3′ | 5′-CCAGATAGTAGTTGTCACCCCAT-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, S.; Yan, L.; Zhu, R.; Wei, H.; Wang, J.; Zheng, L.; Zhang, Y. Skeletal-Muscle-Specific Overexpression of Chrono Leads to Disruption of Glucose Metabolism and Exercise Capacity. Life 2022, 12, 1233. https://doi.org/10.3390/life12081233
He S, Yan L, Zhu R, Wei H, Wang J, Zheng L, Zhang Y. Skeletal-Muscle-Specific Overexpression of Chrono Leads to Disruption of Glucose Metabolism and Exercise Capacity. Life. 2022; 12(8):1233. https://doi.org/10.3390/life12081233
Chicago/Turabian StyleHe, Shiyi, Lu Yan, Rongxin Zhu, Hao Wei, Jianxiong Wang, Lan Zheng, and Ying Zhang. 2022. "Skeletal-Muscle-Specific Overexpression of Chrono Leads to Disruption of Glucose Metabolism and Exercise Capacity" Life 12, no. 8: 1233. https://doi.org/10.3390/life12081233
APA StyleHe, S., Yan, L., Zhu, R., Wei, H., Wang, J., Zheng, L., & Zhang, Y. (2022). Skeletal-Muscle-Specific Overexpression of Chrono Leads to Disruption of Glucose Metabolism and Exercise Capacity. Life, 12(8), 1233. https://doi.org/10.3390/life12081233





