Effects of Obesity and Diabesity on Ventricular Muscle Structure and Function in the Zucker Rat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Isolation of Ventricular Myocytes
2.3. Simultaneous Measurement of Ventricular Myocyte Shortening and Intracellular Ca2+
2.4. Myofilament Sensitivity to Ca2+
2.5. Protein Assessment
2.6. Ultrastructural Assessment
2.7. Statistical Evaluation
3. Results
3.1. General Characteristics
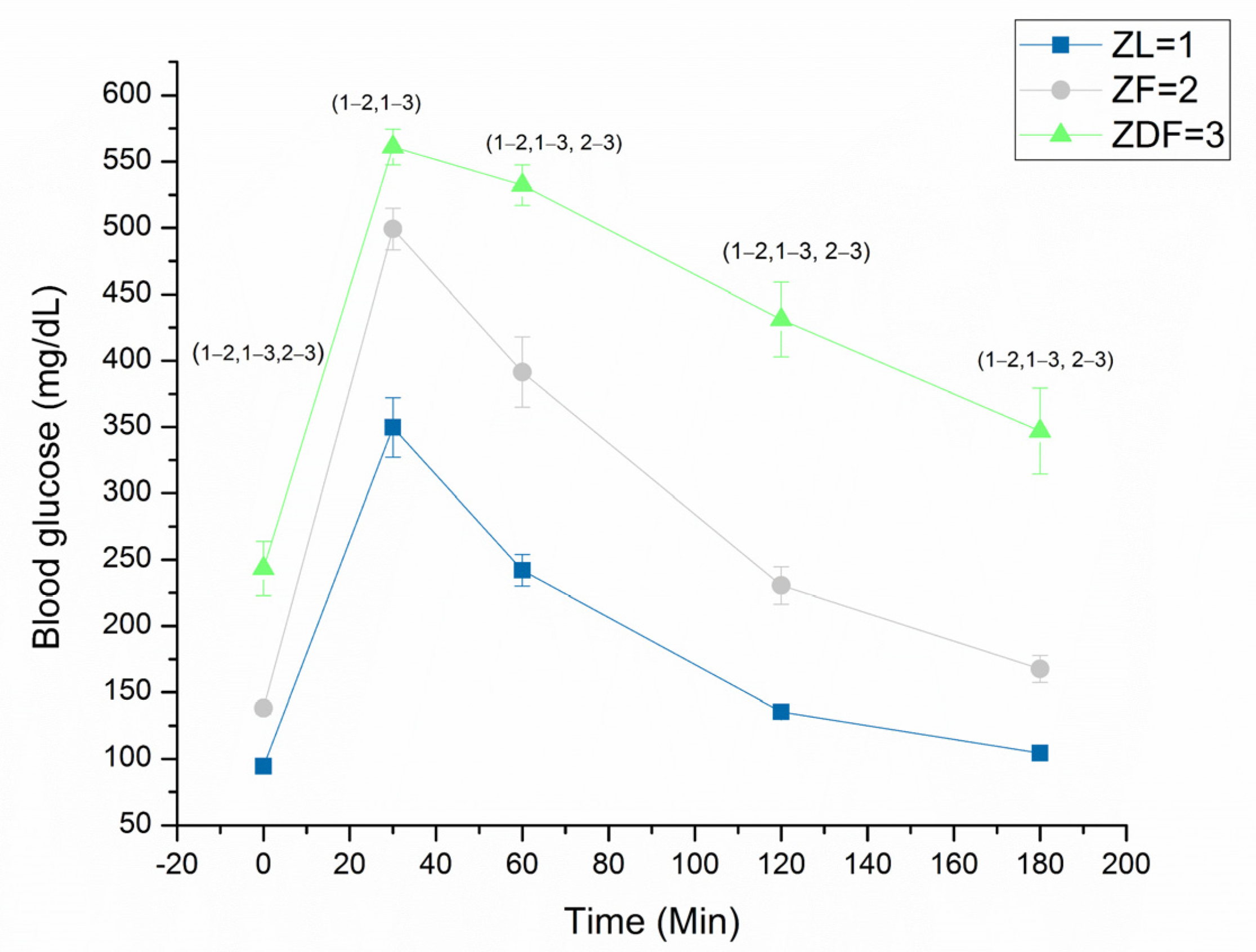
3.2. Glucose Tolerance Test
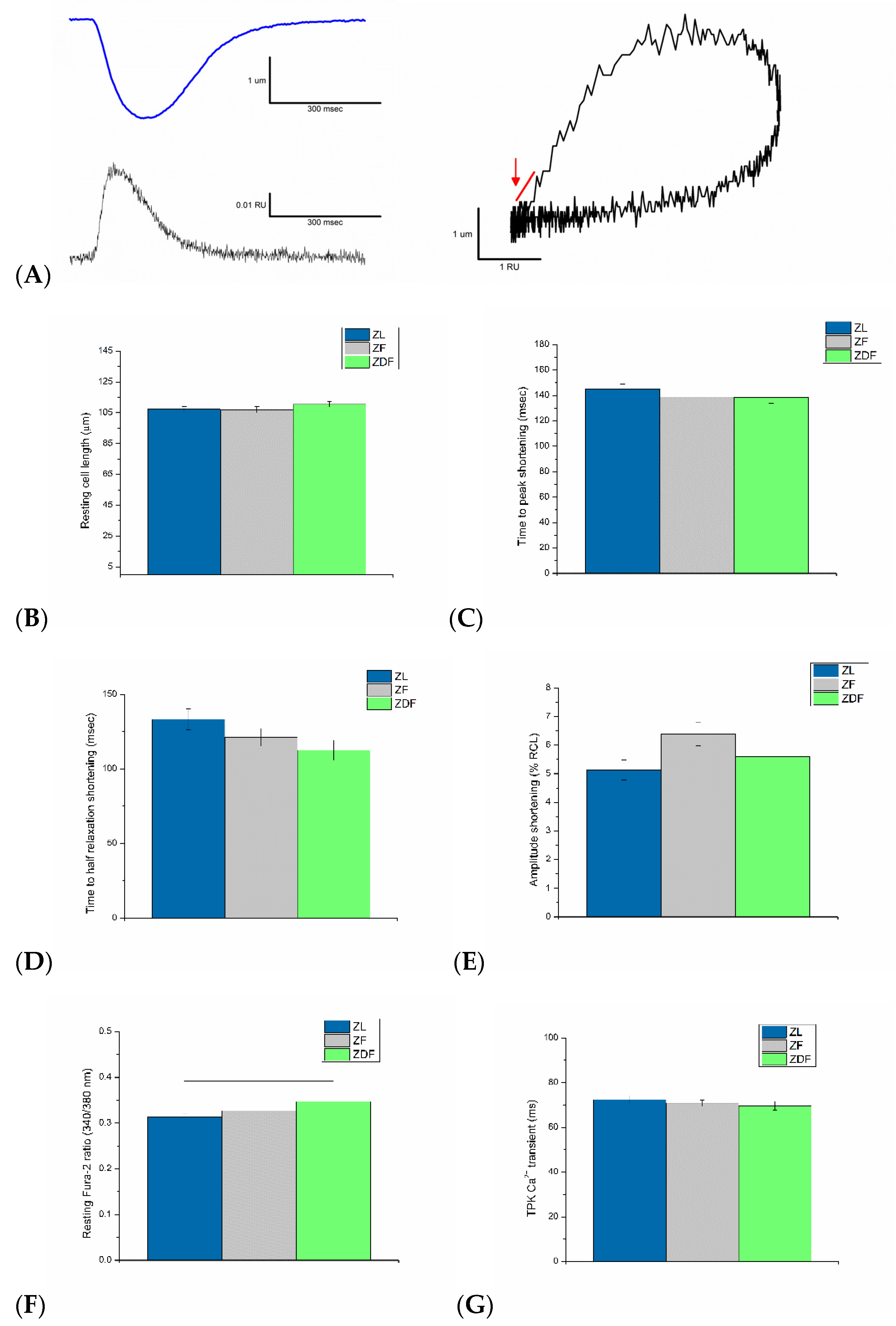
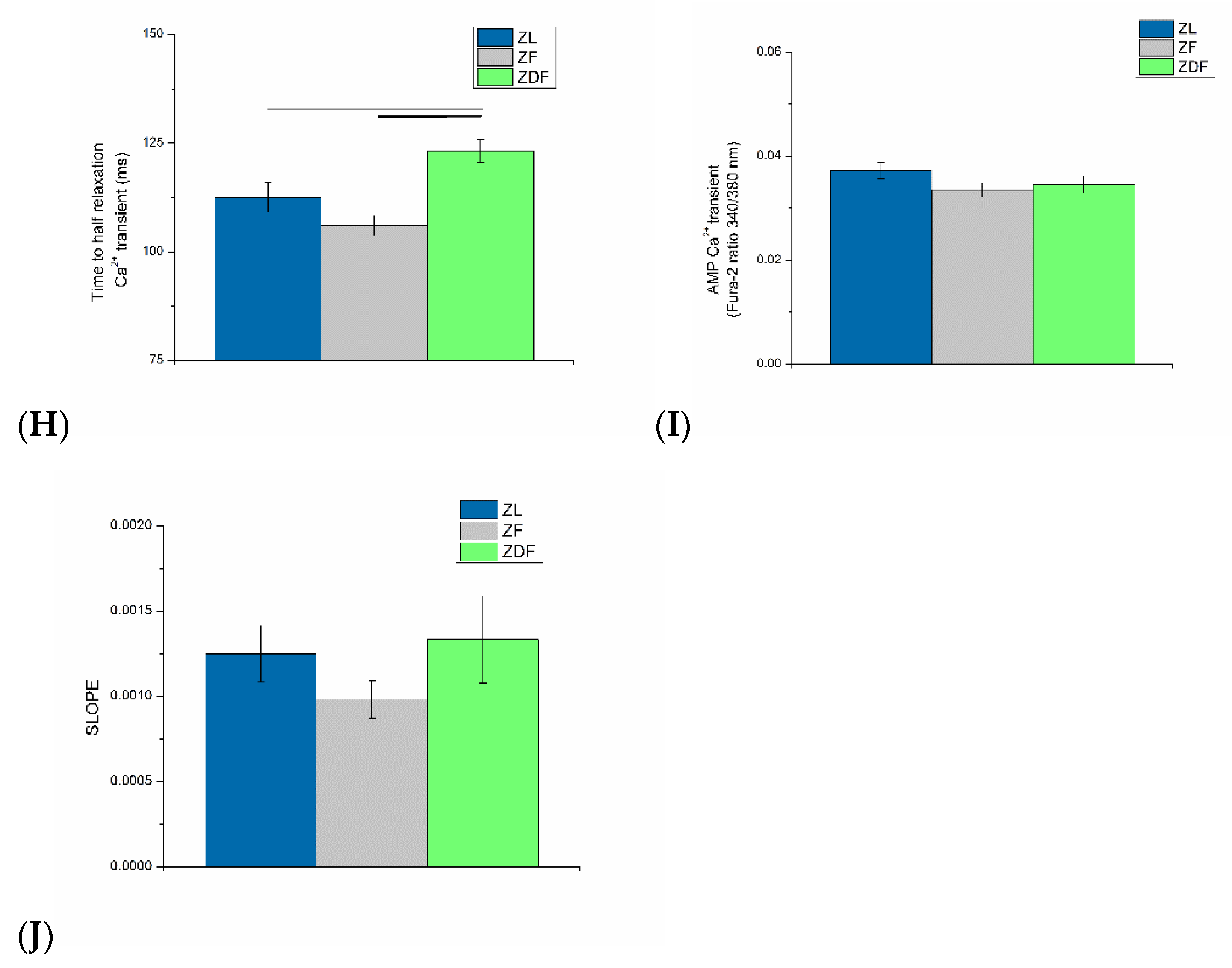
3.3. Ventricular Myocyte Shortening and Intracellular Ca2+
3.4. Myofilament Sensitivity to Ca2+
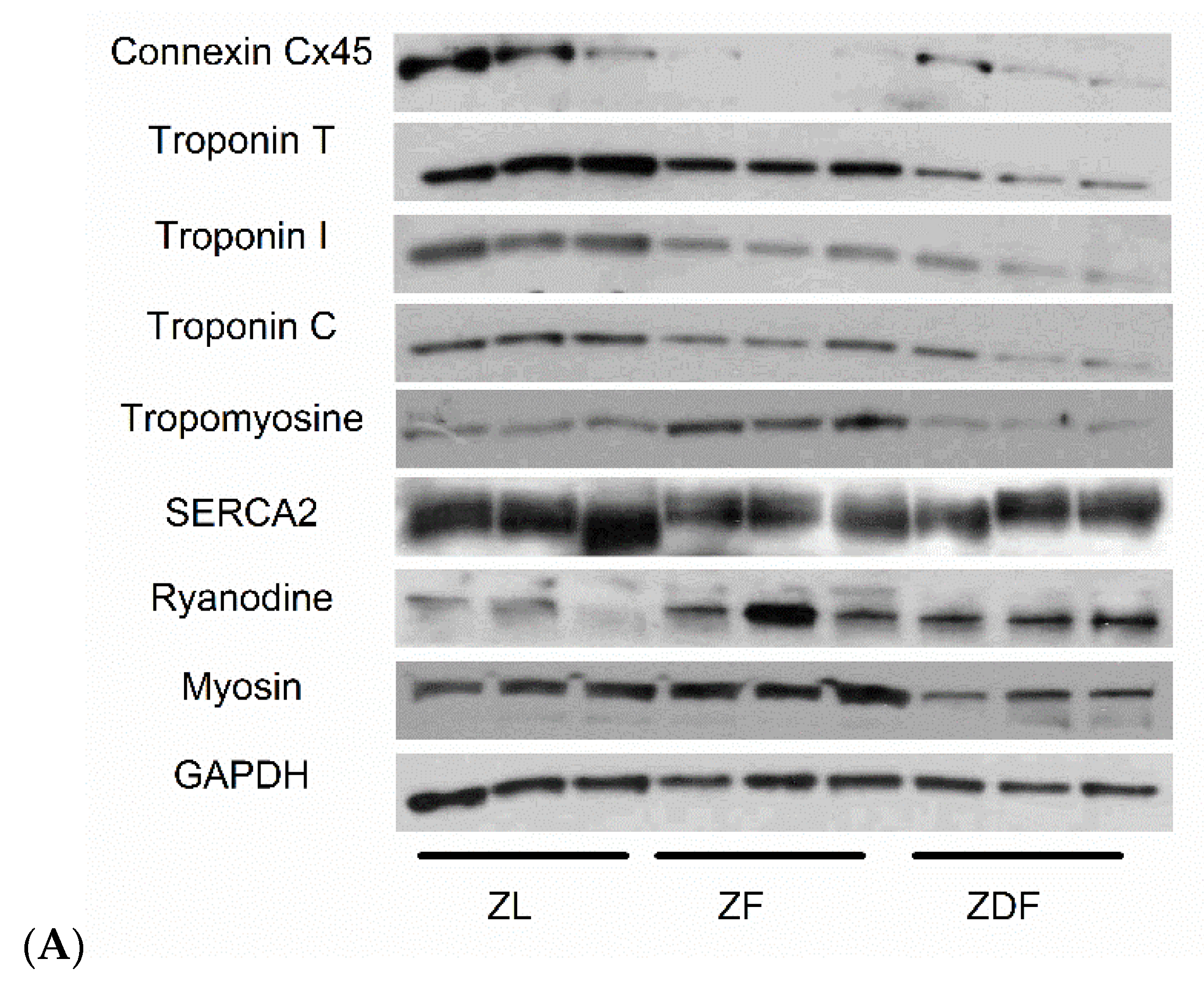
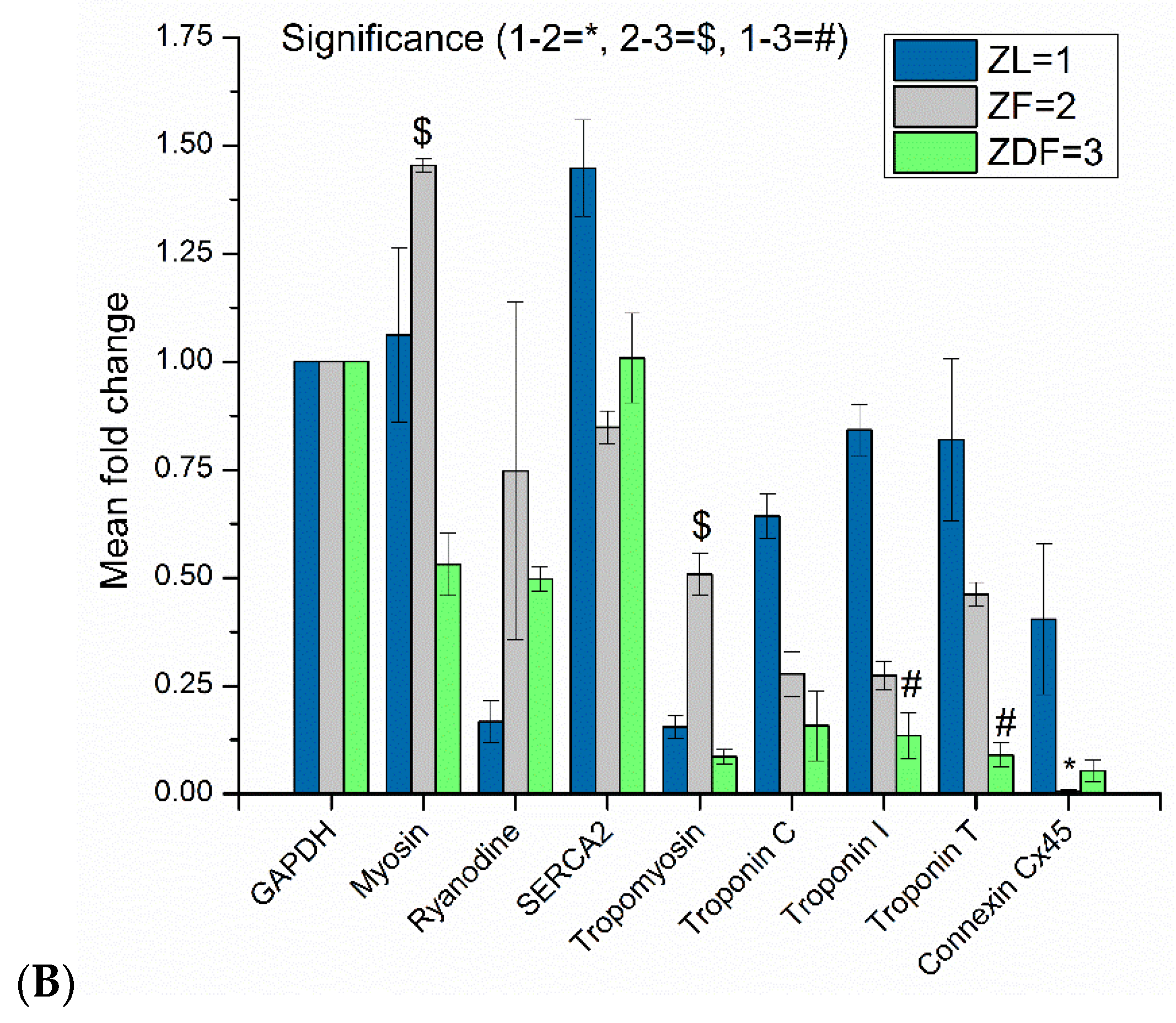
3.5. Protein Assessment
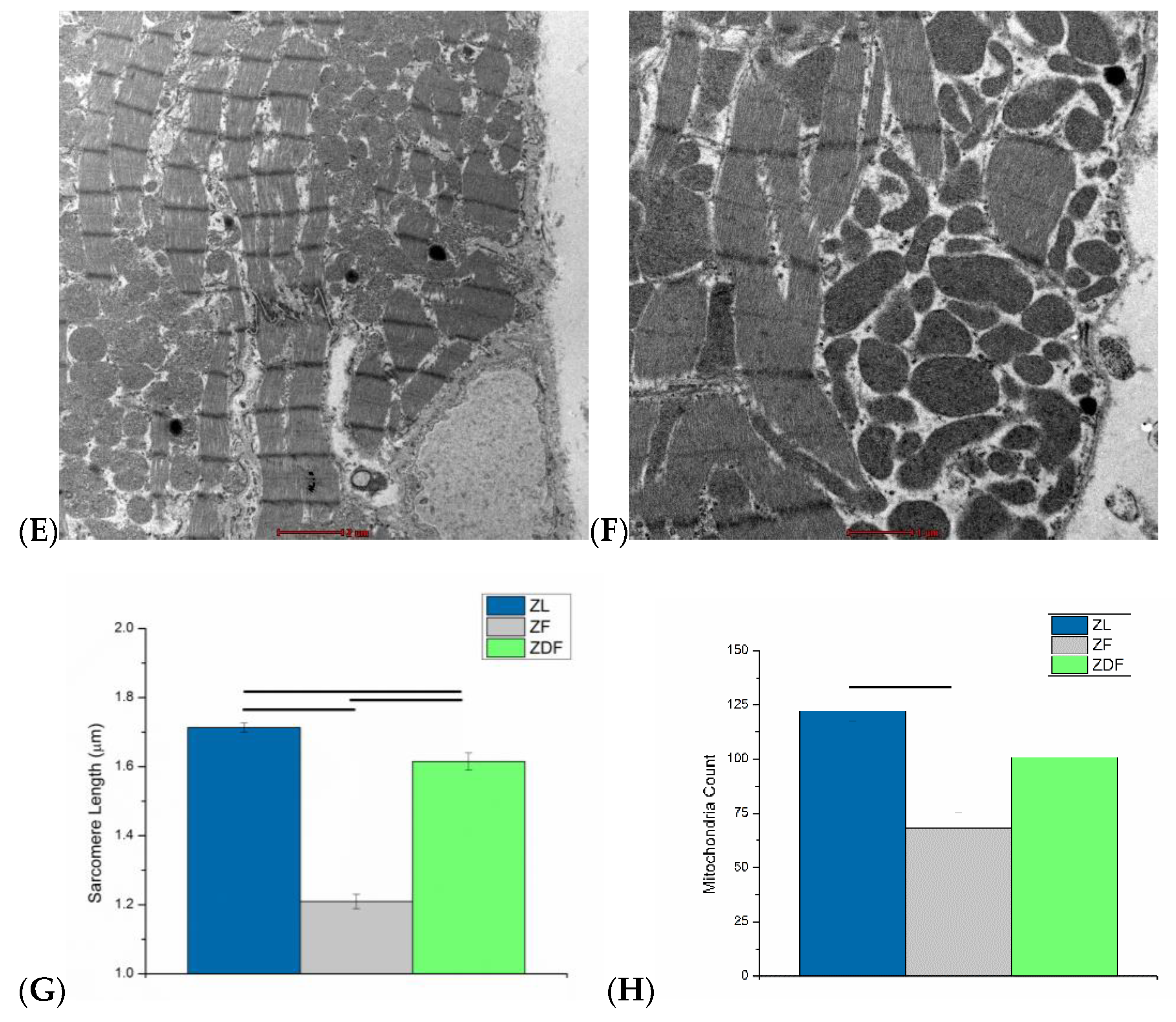
3.6. Ultrastructural Assessment
4. Discussion
5. Conclusions
5.1. Study Limitations and Future Direction
5.2. Clinical Impilcations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Obesity and Overweight. World Health Organization Fact Sheets. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 14 February 2022).
- World Health Organization; World Heart Federation. World Stroke Organization Global Atlas on Cardiovascular Disease Prevention and Control; WHO: Geneva, Switzerland, 2011; p. 164. [Google Scholar]
- Weyer, C.; Funahashi, T.; Tanaka, S.; Hotta, K.; Matsuzawa, Y.; Pratley, R.E.; Tataranni, P.A. Hypoadiponectinemia in Obesity and Type 2 Diabetes: Close Association with Insulin Resistance and Hyperinsulinemia. J. Clin. Endocrinol. Metab. 2001, 86, 1930–1935. [Google Scholar] [CrossRef]
- Zimmet, P.; Alberti, K.G.M.M.; Shaw, J. Global and Societal Implications of the Diabetes Epidemic. Nature 2001, 414, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, N.; Hoshino, M.; Hidaka, S.; Yoshida, E.; Beppu, M.; Hoshikawa, R.; Sudo, K.; Kawada, A.; Takagi, S.; Seino, S. A Novel Rat Model of Type 2 Diabetes: The Zucker Fatty Diabetes Mellitus ZFDM Rat. J. Diabetes Res. 2013, 2013, 103731–103740. [Google Scholar] [CrossRef] [PubMed]
- Takaya, K.; Ogawa, Y.; Isse, N.; Okazaki, T.; Satoh, N.; Masuzaki, H.; Mori, K.; Tamura, N.; Hosoda, K.; Nakao, K. Molecular Cloning of Rat Leptin Receptor Isoform Complementary DNAs-Identification of a Missense Mutation in Zucker Fatty (Fa/Fa) Rats. Biochem. Biophys. Res. Commun. 1996, 225, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, S.; Terentyev, D. Proarrhythmic Remodeling of Calcium Homeostasis in Cardiac Disease; Implications for Diabetes and Obesity. Front. Physiol. 2018, 9, 1517. [Google Scholar] [CrossRef] [PubMed]
- Howarth, F.C.; Norstedt, G.; Boldyriev, O.I.; Qureshi, M.A.; Mohamed, O.; Parekh, K.; Venkataraman, B.; Subramanya, S.; Shmygol, A.; Al Kury, L.T. Effects of Prolactin on Ventricular Myocyte Shortening and Calcium Transport in the Streptozotocin-Induced Diabetic Rat. Heliyon 2020, 6, e03797. [Google Scholar] [CrossRef]
- Smail, M.; Al Kury, L.; Qureshi, M.A.; Shmygol, A.; Oz, M.; Singh, J.; Howarth, F.C. Cell Shortening and Calcium Dynamics in Epicardial and Endocardial Myocytes from the Left Ventricle of Goto-Kakizaki Type 2 Diabetic Rats. Exp. Physiol. 2018, 103, 502–511. [Google Scholar] [CrossRef]
- Howarth, F.C.; Qureshi, M.A.; Hassan, Z.; Al Kury, L.T.; Isaev, D.; Parekh, K.; Yammahi, S.R.K.D.K.D.; Oz, M.; Adrian, T.E.; Adeghate, E. Changing Pattern of Gene Expression Is Associated with Ventricular Myocyte Dysfunction and Altered Mechanisms of Ca2+signalling in Young Type 2 Zucker Diabetic Fatty Rat Heart. Exp. Physiol. 2011, 96, 325–337. [Google Scholar] [CrossRef]
- Howarth, F.C.; Qureshi, M.A.; Hassan, Z.; Isaev, D.; Parekh, K.; John, A.; Oz, M.; Raza, H.; Adeghate, E.; Adrian, T.E. Contractility of Ventricular Myocytes Is Well Preserved despite Altered Mechanisms of Ca2+ Transport and a Changing Pattern of MRNA in Aged Type 2 Zucker Diabetic Fatty Rat Heart. Mol. Cell. Biochem. 2012, 361, 267–280. [Google Scholar] [CrossRef]
- Spurgeon, H.A.; duBell, W.H.; Stern, M.D.; Sollott, S.J.; Ziman, B.D.; Silverman, H.S.; Capogrossi, M.C.; Talo, A.; Lakatta, E.G. Cytosolic Calcium and Myofilaments in Single Rat Cardiac Myocytes Achieve a Dynamic Equilibrium during Twitch Relaxation. J. Physiol. 1992, 447, 83–102. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671. [Google Scholar] [CrossRef] [PubMed]
- Fahim, M.A.; Tariq, S.; Adeghate, E. Vitamin E Modifies the Ultrastructure of Testis and Epididymis in Mice Exposed to Lead Intoxication. Ann. Anat.—Anat. Anzeiger 2013, 195, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Daniels, A.; Linz, D.; Van Bilsen, M.; Rütten, H.; Sadowski, T.; Ruf, S.; Juretschke, H.P.; Neumann-Haefelin, C.; Munts, C.; Van Der Vusse, G.J.; et al. Long-Term Severe Diabetes Only Leads to Mild Cardiac Diastolic Dysfunction in Zucker Diabetic Fatty Rats. Eur. J. Heart Fail. 2012, 14, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Chohnan, S.; Matsuno, S.; Shimizu, K.; Tokutake, Y.; Kohari, D.; Toyoda, A. Coenzyme A and Its Thioester Pools in Obese Zucker and Zucker Diabetic Fatty Rats. Nutrients 2020, 12, 417. [Google Scholar] [CrossRef]
- Clark, J.B.; Palmer, C.J.; Shaw, W.N. The Diabetic Zucker Fatty Rat. Proc. Soc. Exp. Biol. Med. 1983, 173, 68–75. [Google Scholar] [CrossRef]
- Sárközy, M.; Zvara, Á.; Gyémánt, N.; Fekete, V.; Kocsis, G.F.; Pipis, J.; Szucs, G.; Csonka, C.; Puskás, L.G.; Ferdinandy, P.; et al. Metabolic Syndrome Influences Cardiac Gene Expression Pattern at the Transcript Level in Male ZDF Rats. Cardiovasc. Diabetol. 2013, 12, 16. [Google Scholar] [CrossRef]
- Harvey, R.A.; Ferrier, D. Lippincott’s Illustrated Reviews: Biochemistry. In Lippincott Williams & Wilkins; Harvey, R.A., Ferrier, D., Eds.; Wolters Kluwer Health; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011; pp. 307–356. ISBN 978-1-60831-412-6. [Google Scholar]
- Posner, B.I. Insulin Signalling: The Inside Story. Can. J. Diabetes 2017, 41, 108–113. [Google Scholar] [CrossRef]
- Daniels, L.J.; Wallace, R.S.; Nicholson, O.M.; Wilson, G.A.; McDonald, F.J.; Jones, P.P.; Baldi, J.C.; Lamberts, R.R.; Erickson, J.R. Inhibition of Calcium/Calmodulin-Dependent Kinase II Restores Contraction and Relaxation in Isolated Cardiac Muscle from Type 2 Diabetic Rats. Cardiovasc. Diabetol. 2018, 17, 89–104. [Google Scholar] [CrossRef]
- Winkle, A.J.; Nassal, D.M.; Shaheen, R.; Thomas, E.; Mohta, S.; Gratz, D.; Weinberg, S.H.; Hund, T.J. Emerging Therapeutic Targets for Cardiac Hypertrophy. Expert Opin. Ther. Targets 2022, 26, 29–40. [Google Scholar] [CrossRef]
- Guo, T.; Zhang, T.; Mestril, R.; Bers, D.M. Ca2+/Calmodulin-Dependent Protein Kinase II Phosphorylation of Ryanodine Receptor Does Affect Calcium Sparks in Mouse Ventricular Myocytes. Circ. Res. 2006, 99, 398–406. [Google Scholar] [CrossRef]
- Maier, L.S. CaMKIIδ Overexpression in Hypertrophy and Heart Failure: Cellular Consequences for Excitation-Contraction Coupling. Brazilian J. Med. Biol. Res. 2005, 38, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Wehrens, X.H.T.; Lehnart, S.E.; Reiken, S.R.; Marks, A.R. Ca2+/Calmodulin-Dependent Protein Kinase II Phosphorylation Regulates the Cardiac Ryanodine Receptor. Circ. Res. 2004, 94, e61–e70. [Google Scholar] [CrossRef] [PubMed]
- Fulop, N.; Mason, M.M.; Dutta, K.; Wang, P.; Davidoff, A.J.; Marchase, R.B.; Chatham, J.C.; Fülöp, N.; Mason, M.M.; Dutta, K.; et al. Impact of Type 2 Diabetes and Aging on Cardiomyocyte Function and O-Linked N-Acetylglucosamine Levels in the Heart. Am. J. Physiol.—Cell Physiol. 2007, 292, C1370–C1378. [Google Scholar] [CrossRef]
- Fabiato, A. Time and Calcium Dependence of Activation and Inactivation of Calcium-Induced Release of Calcium from the Sarcoplasmic Reticulum of a Skinned Canine Cardiac Purkinje Cell. J. Gen. Physiol. 1985, 85, 247–289. [Google Scholar] [CrossRef]
- Belke, D.D.; Swanson, E.A.; Dillmann, W.H. Decreased Sarcoplasmic Reticulum Activity and Contractility in Diabetic Db/Db Mouse Heart. Diabetes 2004, 53, 3201–3208. [Google Scholar] [CrossRef] [PubMed]
- Stølen, T.O.; Høydal, M.A.; Kemi, O.J.; Catalucci, D.; Ceci, M.; Aasum, E.; Larsen, T.; Rolim, N.; Condorelli, G.; Smith, G.L.; et al. Interval Training Normalizes Cardiomyocyte Function, Diastolic Ca2+ Control, and SR Ca2+ Release Synchronicity In a Mouse Model of Diabetic Cardiomyopathy. Circ. Res. 2009, 105, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.H.; Rozanski, G.J.; Patel, K.P.; Bidasee, K.R. Dyssynchronous (Non-Uniform) Ca2+ Release in Myocytes from Streptozotocin-Induced Diabetic Rats. J. Mol. Cell. Cardiol. 2007, 42, 234–246. [Google Scholar] [CrossRef]
- Choi, K.M.; Zhong, Y.; Hoit, B.D.; Grupp, I.L.; Hahn, H.; Dilly, K.W.; Guatimosim, S.; Jonathan Lederer, W.; Matlib, M.A. Defective Intracellular Ca2+ Signaling Contributes to Cardiomyopathy in Type 1 Diabetic Rats. Am. J. Physiol. Hear. Circ. Physiol. 2002, 283, H1398–H1408. [Google Scholar] [CrossRef]
- Pereira, L.; Matthes, J.; Schuster, I.; Valdivia, H.H.; Herzig, S.; Richard, S.; Gómez, A.M. Mechanisms of [Ca2+]i Transient Decrease in Cardiomyopathy of Db/Db Type 2 Diabetic Mice. Diabetes 2006, 55, 608–615. [Google Scholar] [CrossRef]
- Zhou, B.Q.; Hu, S.J.; Wang, G.B. The Analysis of Ultrastructure and Gene Expression of Sarco/Endoplasmic Reticulum Calcium Handling Proteins in Alloxan-Induced Diabetic Rat Myocardium. Acta Cardiol. 2006, 61, 21–27. [Google Scholar] [CrossRef]
- Reuter, H.; Grönke, S.; Adam, C.; Ribati, M.; Brabender, J.; Zobel, C.; Frank, K.F.; Wippermann, J.; Schwinger, R.H.G.; Brixius, K.; et al. Sarcoplasmic Ca2+ Release Is Prolonged in Nonfailing Myocardium of Diabetic Patients. Mol. Cell. Biochem. 2008, 308, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Pavlović, D.; McLatchie, L.M.; Shattock, M.J. The Rate of Loss of T-Tubules in Cultured Adult Ventricular Myocytes Is Species Dependent. Exp. Physiol. 2010, 95, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Ferrantini, C.; Coppini, R.; Sacconi, L.; Tosi, B.; Zhang, M.L.; Wang, G.L.; De Vries, E.; Hoppenbrouwers, E.; Pavone, F.; Cerbai, E.; et al. Impact of Detubulation on Force and Kinetics of Cardiac Muscle Contraction. J. Gen. Physiol. 2014, 143, 783–797. [Google Scholar] [CrossRef] [PubMed]
- Silverthorn, D.U.; Ober, W.C.; Garrison, C.W.; Silverthorn, A.C.; Johnson, B.R. Human Physiology: An Integrated Approach, 5th ed.; Pearson Education Inc/Benjamin Cummings: San Francisco, CA, USA, 2007; ISBN 0-321-39624-3. [Google Scholar]
- Solaro, R.J.; Rarick, H.M. Troponin and Tropomyosin: Proteins That Switch on and Tune in the Activity of Cardiac Myofilaments. Circ. Res. 1998, 83, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Howarth, F.C.; Qureshi, M.A. Myofilament Sensitivity to Ca2+ in Ventricular Myocytes from the Goto-Kakizaki Diabetic Rat. Mol. Cell. Biochem. 2008, 315, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Dillmann, W.H. Diabetes Mellitus Induces Changes in Cardiac Myosin of the Rat. Diabetes 1980, 29, 579–582. [Google Scholar] [CrossRef] [PubMed]
- Bidasee, K.R.; Nallani, K.; Henry, B.; Dincer, U.D.; Besch, H.R. Chronic Diabetes Alters Function and Expression of Ryanodine Receptor Calcium-Release Channels in Rat Hearts. Mol. Cell. Biochem. 2003, 249, 113–123. [Google Scholar] [CrossRef]
- Bidasee, K.R.; Nallani, K.; Yu, Y.; Cocklin, R.R.; Zhang, Y.; Wang, M.; Dincer, U.D.; Besch, H.R. Chronic Diabetes Increases Advanced Glycation End Products on Cardiac Ryanodine Receptors/Calcium-Release Channels. Diabetes 2003, 52, 1825–1836. [Google Scholar] [CrossRef]
- Relling, D.P.; Esberg, L.B.; Fang, C.X.; Johnson, W.T.; Murphy, E.J.; Carlson, E.C.; Saari, J.T.; Ren, J. High-Fat Diet-Induced Juvenile Obesity Leads to Cardiomyocyte Dysfunction and Upregulation of Foxo3a Transcription Factor Independent of Lipotoxicity and Apoptosis. J. Hypertens. 2006, 24, 549–561. [Google Scholar] [CrossRef]
- Lima-Leopoldo, A.P.; Sugizaki, M.M.; Leopoldo, A.S.; Carvalho, R.F.; Nogueira, C.R.; Nascimento, A.F.; Martinez, P.F.; Luvizotto, R.A.M.; Padovani, C.R.; Cicogna, A.C. Obesity Induces Upregulation of Genes Involved in Myocardial Ca2+ Handling. Brazilian J. Med. Biol. Res. 2008, 41, 615–620. [Google Scholar] [CrossRef]
- Leopoldo, A.S.; Lima-Leopoldo, A.P.; Sugizaki, M.M.; do Nascimento, A.F.; de Campos, D.H.S.; de Azevedo Melo Luvizotto, R.; Castardeli, E.; Alves, C.A.B.; Brum, P.C.; Cicogna, A.C. Involvement of L-Type Calcium Channel and Serca2a in Myocardial Dysfunction Induced by Obesity. J. Cell. Physiol. 2011, 226, 2934–2942. [Google Scholar] [CrossRef] [PubMed]
- Bottinelli, R.; Coviello, D.A.; Redwood, C.S.; Pellegrino, M.A.; Maron, B.J.; Spirito, P.; Watkins, H.; Reggiani, C. A Mutant Tropomyosin That Causes Hypertrophic Cardiomyopathy Is Expressed in Vivo and Associated with an Increased Calcium Sensitivity. Circ. Res. 1998, 82, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Sequeira, V.; Wijnker, P.J.M.; Nijenkamp, L.L.A.M.; Kuster, D.W.D.; Najafi, A.; Witjas-Paalberends, E.R.; Regan, J.A.; Boontje, N.; Ten Cate, F.J.; Germans, T.; et al. Perturbed Length-Dependent Activation in Human Hypertrophic Cardiomyopathy with Missense Sarcomeric Gene Mutations. Circ. Res. 2013, 112, 1491–1505. [Google Scholar] [CrossRef] [PubMed]
- Mirza, M.; Marston, S.; Willott, R.; Ashley, C.; Mogensen, J.; McKenna, W.; Robinson, P.; Redwood, C.; Watkins, H. Dilated Cardiomyopathy Mutations in Three Thin Filament Regulatory Proteins Result in a Common Functional Phenotype. J. Biol. Chem. 2005, 280, 28498–28506. [Google Scholar] [CrossRef]
- Sakakibara, M.; Hirashiki, A.; Cheng, X.W.; Bando, Y.; Ohshima, K.; Okumura, T.; Funahashi, H.; Ohshima, S.; Murohara, T. Association of Diabetes Mellitus with Myocardial Collagen Accumulation and Relaxation Impairment in Patients with Dilated Cardiomyopathy. Diabetes Res. Clin. Pract. 2011, 92, 348–355. [Google Scholar] [CrossRef]
- Teunissen, B.E.J.; Bierhuizen, M.F.A. Transcriptional Control of Myocardial Connexins. Cardiovasc. Res. 2004, 62, 246–255. [Google Scholar] [CrossRef]
- Sultan, A.; Jacobson, M.; Adeghate, E.; Oulhaj, A.; Shafiullah, M.; Qureshi, A.; Howarth, F.C. Effects of Obesity and Diabesity on Heart Rhythm in the Zucker Rat. Clin. Exp. Pharmacol. Physiol. 2021, 48, 735–747. [Google Scholar] [CrossRef]
- Howarth, F.C.; Nowotny, N.; Zilahi, E.; El Haj, M.A.; Lei, M. Altered Expression of Gap Junction Connexin Proteins May Partly Underlie Heart Rhythm Disturbances in the Streptozotocin-Induced Diabetic Rat Heart. Mol. Cell. Biochem. 2007, 305, 145–151. [Google Scholar] [CrossRef]
- Okruhlicova, L.; Tribulova, N.; Weismann, P.; Sotnikova, R. Ultrastructure and Histochemistry of Rat Myocardial Capillary Endothelial Cells in Response to Diabetes and Hypertension. Cell Res. 2005, 15, 532–538. [Google Scholar] [CrossRef]
- Taegtmeyer, H.; McNulty, P.; Young, M.E. Adaptation and Maladaptation of the Heart in Diabetes: Part I: General Concepts. Circulation 2002, 105, 1727–1733. [Google Scholar] [CrossRef]
- From, A.M.; Scott, C.G.; Chen, H.H. The Development of Heart Failure in Patients With Diabetes Mellitus and Pre-Clinical Diastolic Dysfunction. A Population-Based Study. J. Am. Coll. Cardiol. 2010, 55, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Amaral, N.; Okonko, D.O. Metabolic Abnormalities of the Heart in Type II Diabetes. Diabetes Vasc. Dis. Res. 2015, 12, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; DeMazumder, D.; Sidor, A.; Brian Foster, D.; O’Rourke, B. Mitochondrial ROS Drive Sudden Cardiac Death and Chronic Proteome Remodeling in Heart Failure. Circ. Res. 2018, 123, 356–371. [Google Scholar] [CrossRef] [PubMed]

| ZL | ZF | ZDF | |
|---|---|---|---|
| Body weight (g) | 429.67 ± 9.43 (18) [11.61] | 715.67 ± 13.81 (21) [44.52] * | 601.86 ± 32.63 (21) [32.67] * |
| Heart weight (g) | 1.7 ± 0.07 (18) [16.83] | 2.2 ± 0.06 (21) [40.07] * | 2.1 ± 0.08 (21) [32.64] * |
| Non-fasting blood Glucose (mg/dL) | 118.47 ± 4.18 (17) [14.21] | 136.67 ± 4.38 (21) [23.98] | 345.10 ± 25.16 (21) [48.81] *# |
| Heart weight/body weight ratio (mg/g) | 2.54 ± 0.09 (18) [17.56] | 3.26 ± 0.11 (21) [43.67] * | 2.84 ± 0.06 (21) [28.43] # |
| Plasma Insulin (µg/L) | 2.09 ± 0.57 (10) [8.25] | 12.70 ± 1.15 (9) [23.0] * | 2.14 ± 0.29 (8) [11.06] # |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sultan, A.; Adeghate, E.; Emerald, B.S.; Qureshi, M.A.; Minhas, S.T.; Howarth, F.C. Effects of Obesity and Diabesity on Ventricular Muscle Structure and Function in the Zucker Rat. Life 2022, 12, 1221. https://doi.org/10.3390/life12081221
Sultan A, Adeghate E, Emerald BS, Qureshi MA, Minhas ST, Howarth FC. Effects of Obesity and Diabesity on Ventricular Muscle Structure and Function in the Zucker Rat. Life. 2022; 12(8):1221. https://doi.org/10.3390/life12081221
Chicago/Turabian StyleSultan, Ahmed, Ernest Adeghate, Bright Starling Emerald, Muhammad A. Qureshi, Saeed Tariq Minhas, and Frank Christopher Howarth. 2022. "Effects of Obesity and Diabesity on Ventricular Muscle Structure and Function in the Zucker Rat" Life 12, no. 8: 1221. https://doi.org/10.3390/life12081221
APA StyleSultan, A., Adeghate, E., Emerald, B. S., Qureshi, M. A., Minhas, S. T., & Howarth, F. C. (2022). Effects of Obesity and Diabesity on Ventricular Muscle Structure and Function in the Zucker Rat. Life, 12(8), 1221. https://doi.org/10.3390/life12081221







