Atlas of Nervous System Vascular Malformations: A Systematic Review
Abstract
:1. Introduction
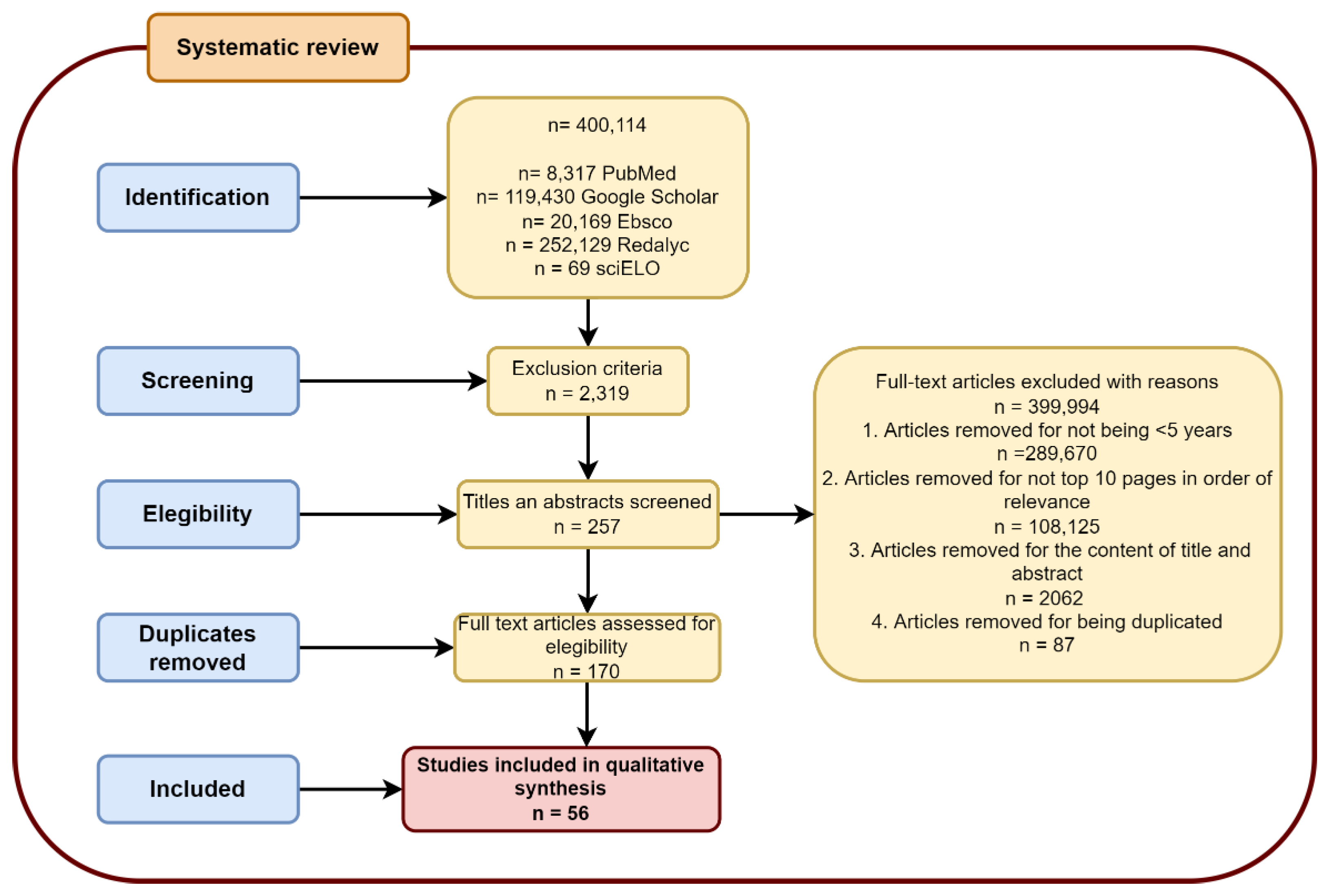
2. Materials and Methods
2.1. Search Strategies
2.2. Study Selection
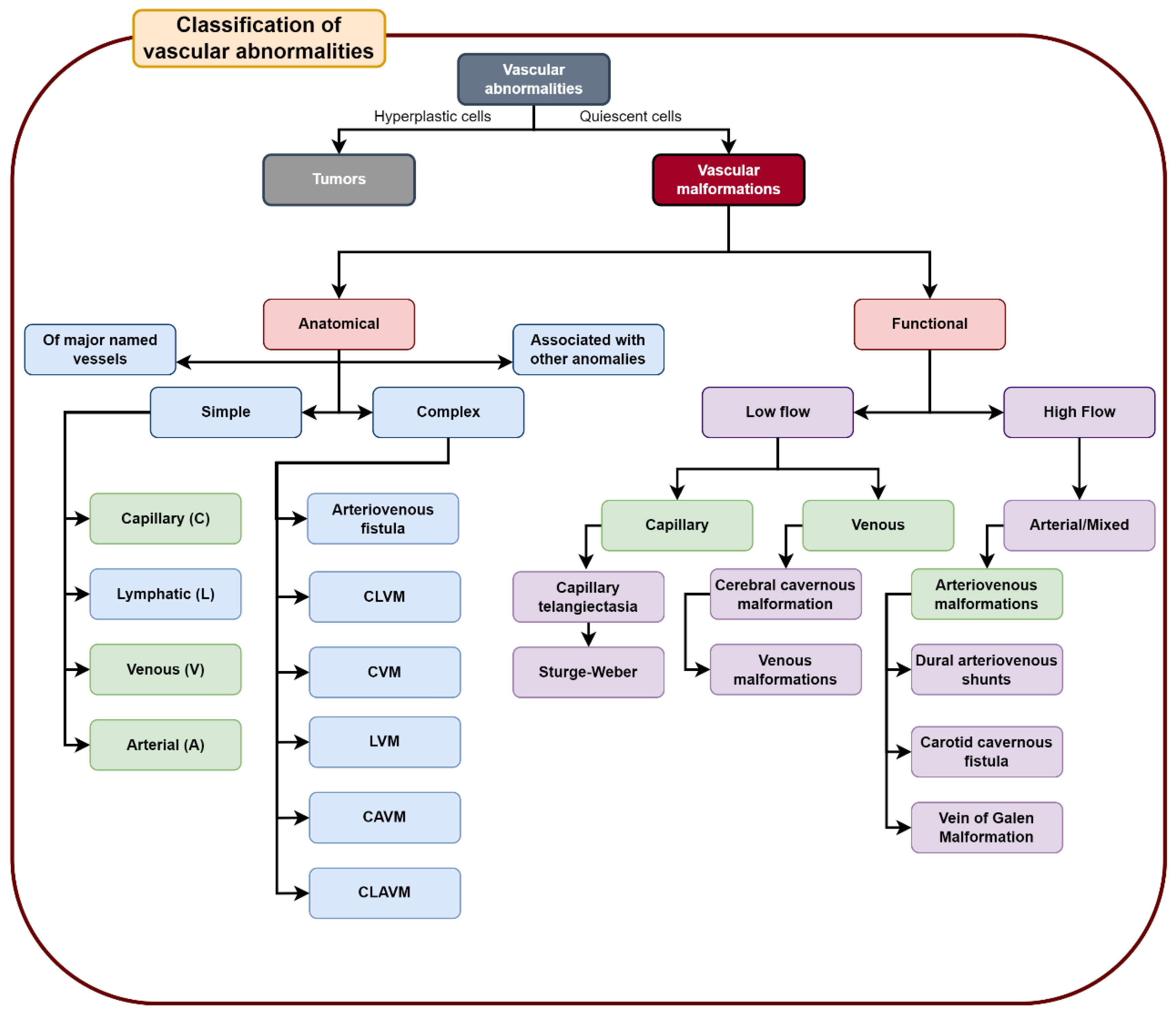
3. Results
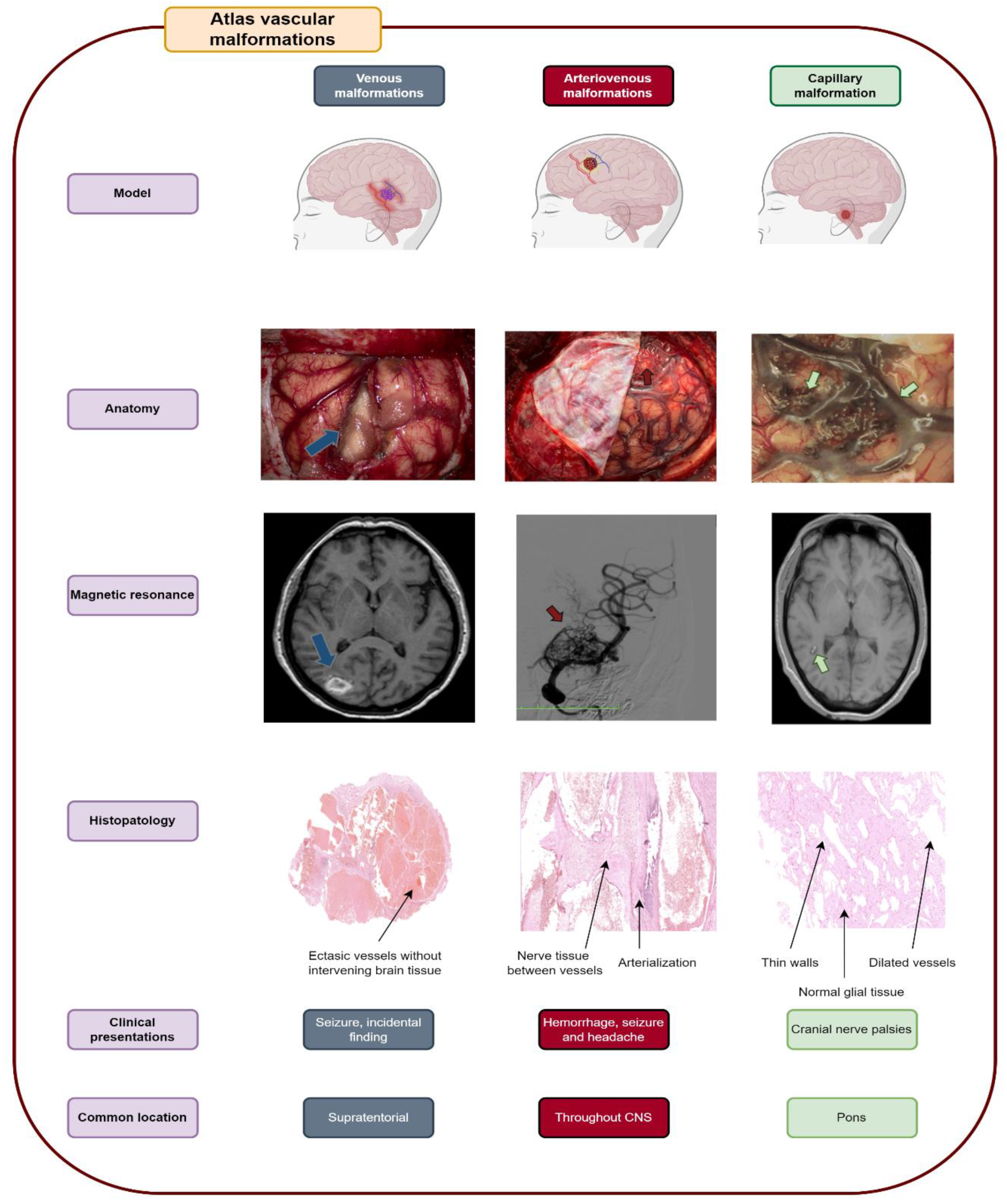
3.1. Venous Malformations
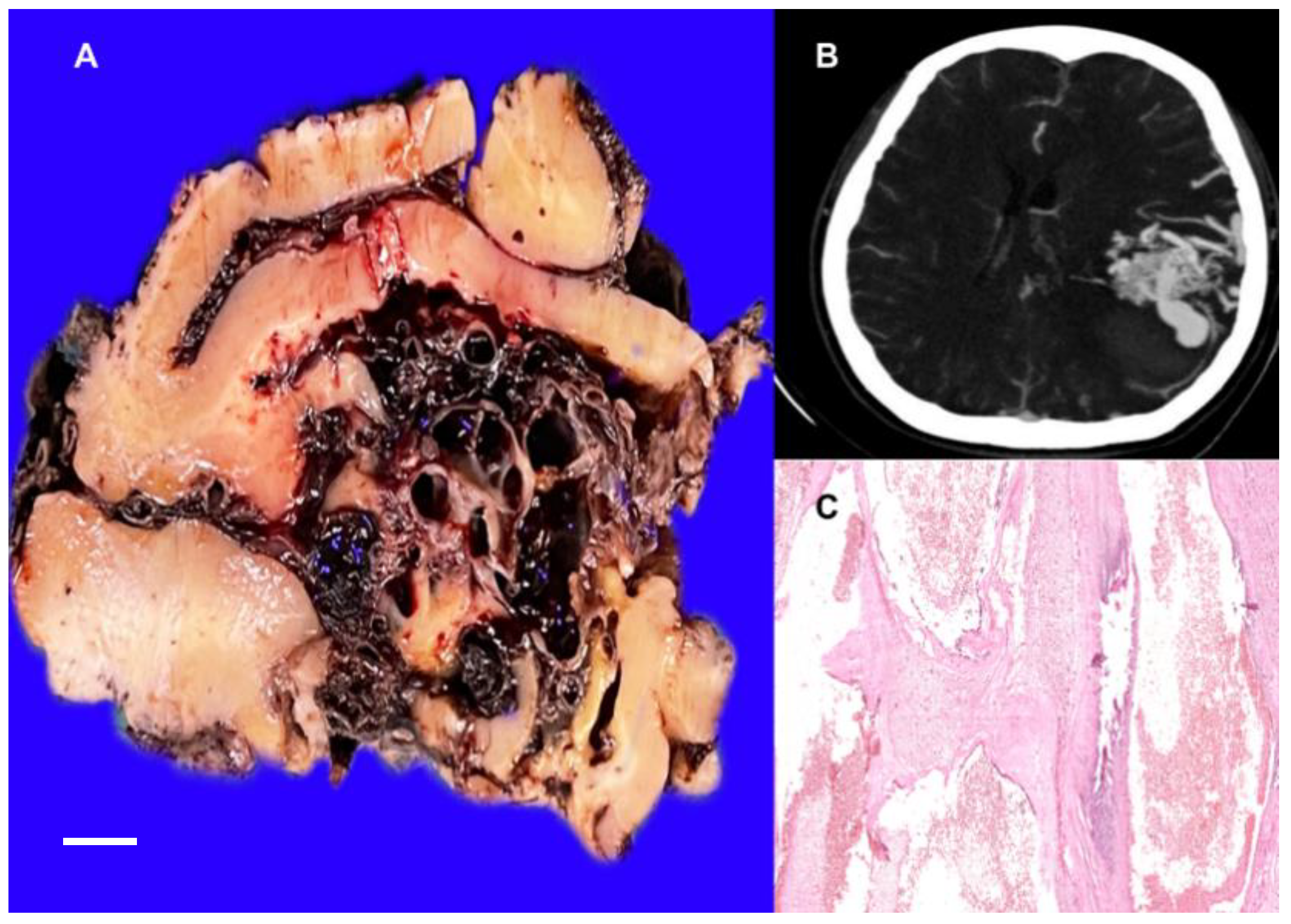
3.1.1. Cerebral Venous Malformations
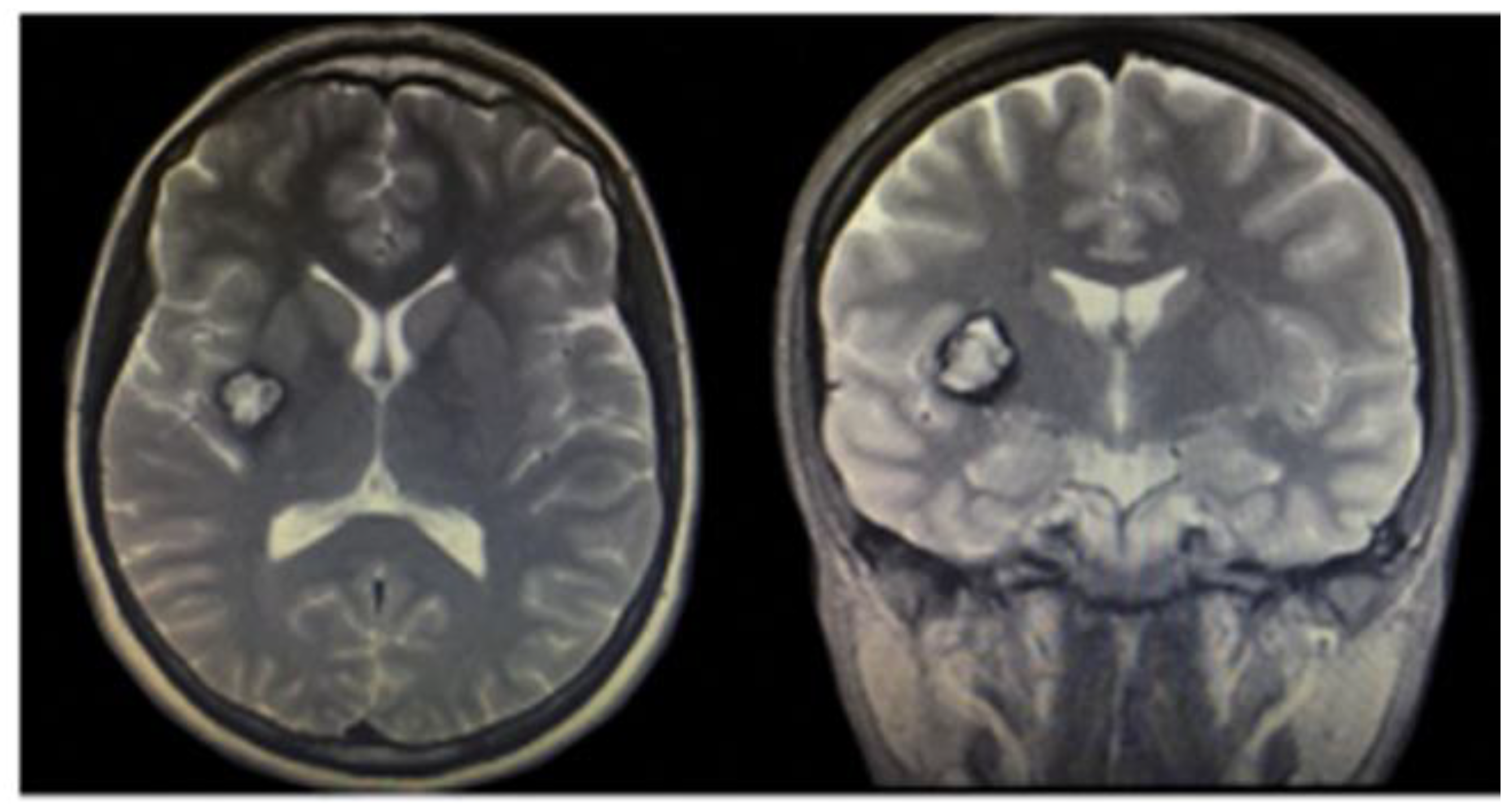
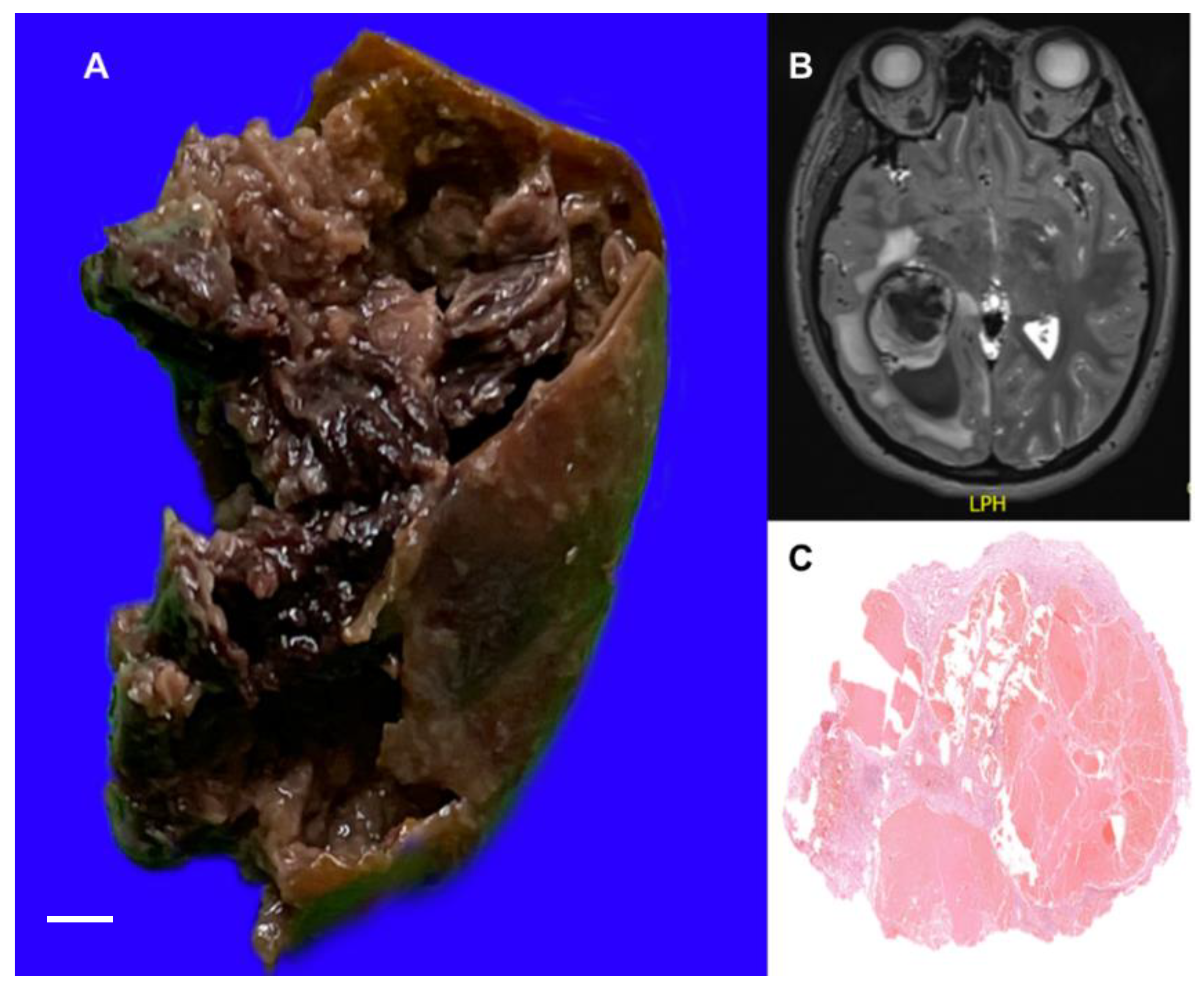
3.1.2. Brain Cavernous Angiomas
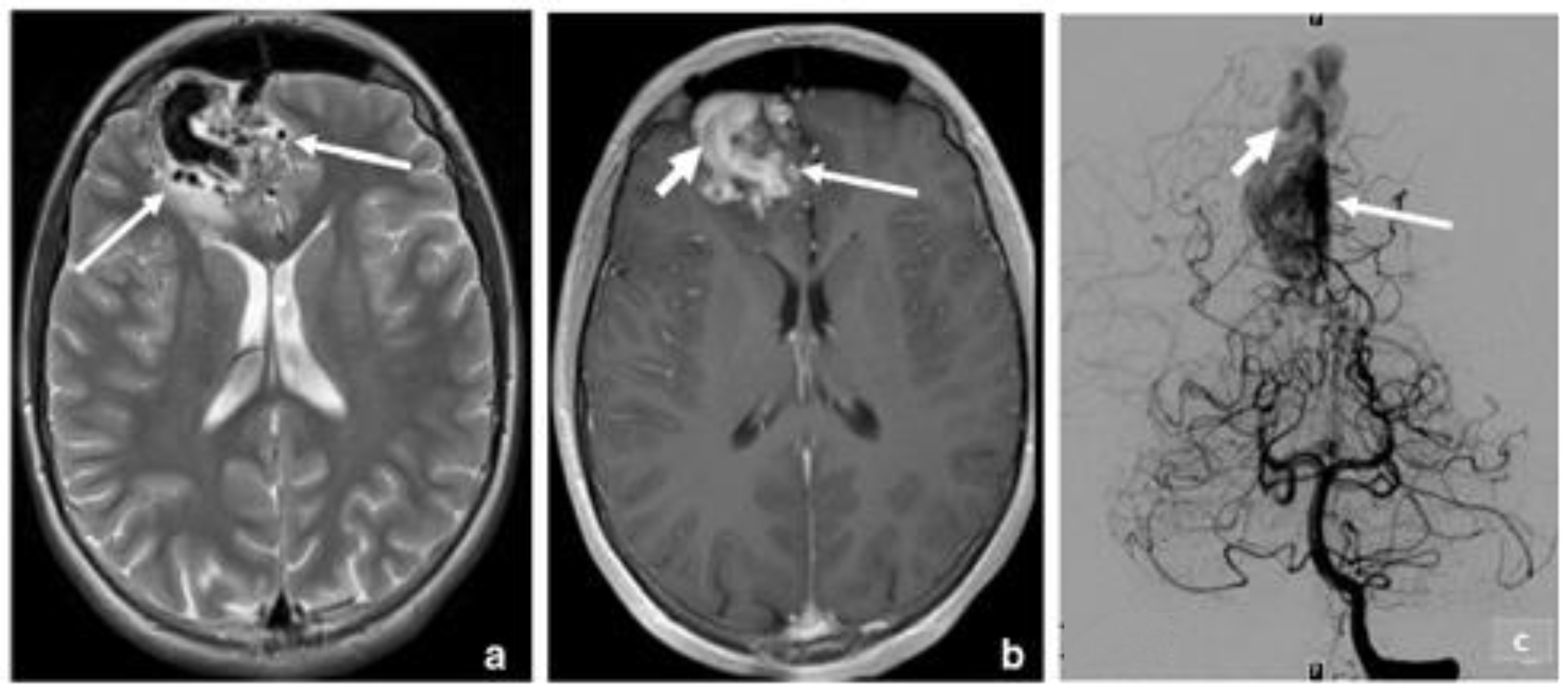
3.2. Arteriovenous Malformation (AV Angioma, Cirsoid Angioma)
3.3. Capillary Malformations
3.3.1. Cerebral Capillary Telangiectasia
3.3.2. Capillary-Venous Angioma (Sturge–Weber)
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Micali, G.; Verzì, A.E.; Musumeci, M.L.; Lacarrubba, F. Vascular Anomalies. In Atlas of Pediatric Dermatoscopy; Micali, G., Lacarrubba, F., Stinco, G., Argenziano, G., Neri, I., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 235–241. ISBN 978-3-319-71167-6. [Google Scholar]
- Sadick, M.; Wohlgemuth, W.A.; Huelse, R.; Lange, B.; Henzler, T.; Schoenberg, S.O.; Sadick, H. Interdisciplinary Management of Head and Neck Vascular Anomalies: Clinical Presentation, Diagnostic Findings and Minimalinvasive Therapies. Eur. J. Radiol. Open 2017, 4, 63–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taghinia, A.H.; Upton, J. Vascular Anomalies. J. Hand. Surg. Am. 2018, 43, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Narsinh, K.H.; Gautam, A.; Baker, A.; Cooke, D.L.; Dowd, C.F. Vascular Anomalies: Classification and Management. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2021; Volume 176. [Google Scholar]
- Borst, A.J.; Nakano, T.A.; Blei, F.; Adams, D.M.; Duis, J. A Primer on a Comprehensive Genetic Approach to Vascular Anomalies. Front. Pediatr. 2020, 8, 579591. [Google Scholar] [CrossRef]
- Ahlawat, S.; Fayad, L.M.; Durand, D.J.; Puttgen, K.; Tekes, A. International Society for the Study of Vascular Anomalies Available ISSVA Classification of Vascular Anomalies. Curr. Probl. Diagn. Radiol. 2018, 48, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Esposito, F.; Ferrara, D.; Di Serafino, M.; Diplomatico, M.; Vezzali, N.; Giugliano, A.M.; Colafati, G.S.; Zeccolini, M.; Tomà, P. Classification and Ultrasound Findings of Vascular Anomalies in Pediatric Age: The Essential. J. Ultrasound 2019, 22, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Bertino, F.; Trofimova, A.V.; Gilyard, S.N.; Hawkins, C.M. Vascular Anomalies of the Head and Neck: Diagnosis and Treatment. Pediatr. Radiol. 2021, 51, 1162–1184. [Google Scholar] [CrossRef]
- Ricci, K.W. Advances in the Medical Management of Vascular Anomalies. Semin. Interv. Radiol. 2017, 34, 239–249. [Google Scholar] [CrossRef]
- Adams, D.M. Practical Genetic and Biologic Therapeutic Considerations in Vascular Anomalies. Tech. Vasc. Interv. Radiol. 2019, 22, 100629. [Google Scholar] [CrossRef]
- Pahl, K.S.; Kim, K.; Sams, C.; Alvarez, H.; Smith, S.V.; Blatt, J. Inconsistency in Classifying Vascular Anomalies: What’s in a Name? Pediatr. Blood Cancer 2018, 65, e26836. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Zuberi, S.M.; Wirrell, E.; Yozawitz, E.; Wilmshurst, J.M.; Specchio, N.; Riney, K.; Pressler, R.; Auvin, S.; Samia, P.; Hirsch, E.; et al. ILAE Classification and Definition of Epilepsy Syndromes with Onset in Neonates and Infants: Position Statement by the ILAE Task Force on Nosology and Definitions. Epilepsia 2022, 63, 1349–1397. [Google Scholar] [CrossRef] [PubMed]
- Eberson, S.N.; Desai, S.B.; Metry, D. A Basic Introduction to Pediatric Vascular Anomalies. Semin. Interv. Radiol 2019, 36, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Brahmbhatt, A.N.; Skalski, K.A.; Bhatt, A.A. Vascular Lesions of the Head and Neck: An Update on Classification and Imaging Review. Insights Imaging 2020, 11, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mylonas, S.; Brunkwall, S.; Brunkwall, J. Vaskuläre Anomalien. Teil II: Vaskuläre Malformationen. Der. Chirurg. 2018, 89, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.; Fiani, B.; Hadi, H.; Arshad, M.; Cathel, A.; Naeem, M.; Parsons, M.S.; Farooqui, M.; Bucklin, A.A.; Leone, M.J.; et al. Cerebral Vascular Malformations and Their Imaging Modalities. Neurol. Sci. 2020, 41, 2407–2421. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Hwang, S.K.; Chung, H.Y. The Molecular Pathophysiology of Vascular Anomalies: Genomic Research. Arch. Plast Surg. 2020, 47, 203–208. [Google Scholar] [CrossRef]
- Padia, R.; Bly, R.; Bull, C.; Geddis, A.E.; Perkins, J. Medical Management of Vascular Anomalies. Curr. Treat. Options Pediatr. 2018, 4, 221–236. [Google Scholar] [CrossRef]
- Ebrahimzadeh, K.; Tavassol, H.H.; Mousavinejad, S.A.; Ansari, M.; Kazemi, R.; Bahrami-Motlagh, H.; Jalili Khoshnoud, R.; Sharifi, G.; Samadian, M.; Rezaei, O. The Sensorineural Hearing Loss Related to a Rare Infratentorial Developmental Venous Angioma: A Case Report and Review of Literature. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2021. [Google Scholar] [CrossRef]
- Akers, A.; Al-Shahi Salman, R.; Awad, I.A.; Dahlem, K.; Flemming, K.; Hart, B.; Kim, H.; Jusue-Torres, I.; Kondziolka, D.; Lee, C.; et al. Synopsis of Guidelines for the Clinical Management of Cerebral Cavernous Malformations: Consensus Recommendations Based on Systematic Literature Review by the Angioma Alliance Scientific Advisory Board Clinical Experts Panel. Neurosurgery 2017, 80, 665–680. [Google Scholar] [CrossRef] [Green Version]
- Polster, S.P.; Cao, Y.; Carroll, T.; Flemming, K.; Girard, R.; Hanley, D.; Hobson, N.; Kim, H.; Koenig, J.; Koskimäki, J.; et al. Trial Readiness in Cavernous Angiomas with Symptomatic Hemorrhage (CASH). Neurosurgery 2019, 84, 954–964. [Google Scholar] [CrossRef]
- Girard, R.; Li, Y.; Stadnik, A.; Shenkar, R.; Hobson, N.; Romanos, S.; Srinath, A.; Moore, T.; Lightle, R.; Shkoukani, A.; et al. A Roadmap for Developing Plasma Diagnostic and Prognostic Biomarkers of Cerebral Cavernous Angioma with Symptomatic Hemorrhage (CASH). Neurosurgery 2021, 88, 686–697. [Google Scholar] [CrossRef]
- Bokhari, M.R.; Al-Dhahir, M.A. Brain Cavernous Angiomas. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Awad, I.A.; Polster, S.P. Cavernous Angiomas: Deconstructing a Neurosurgical Disease: JNSPG 75th Anniversary Invited Review Article. J. Neurosurg. 2019, 131, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Snellings, D.A.; Hong, C.C.; Ren, A.A.; Lopez-Ramirez, M.A.; Girard, R.; Srinath, A.; Marchuk, D.A.; Ginsberg, M.H.; Awad, I.A.; Kahn, M.L. Cerebral Cavernous Malformation: From Mechanism to Therapy. Circ. Res. 2021, 129, 195–215. [Google Scholar] [CrossRef] [PubMed]
- McKerracher, L.; Shenkar, R.; Abbinanti, M.; Cao, Y.; Peiper, A.; Liao, J.K.; Lightle, R.; Moore, T.; Hobson, N.; Gallione, C.; et al. A Brain-Targeted Orally Available ROCK2 Inhibitor Benefits Mild and Aggressive Cavernous Angioma Disease. Transl. Stroke Res. 2020, 11, 365–376. [Google Scholar] [CrossRef]
- Tang, A.T.; Choi, J.P.; Kotzin, J.J.; Yang, Y.; Hong, C.C.; Hobson, N.; Girard, R.; Zeineddine, H.A.; Lightle, R.; Moore, T.; et al. Endothelial TLR4 and the Microbiome Drive Cerebral Cavernous Malformations. Nature 2017, 545, 305–310. [Google Scholar] [CrossRef] [Green Version]
- Polster, S.P.; Sharma, A.; Tanes, C.; Tang, A.T.; Mericko, P.; Cao, Y.; Carrión-Penagos, J.; Girard, R.; Koskimäki, J.; Zhang, D.; et al. Permissive Microbiome Characterizes Human Subjects with a Neurovascular Disease Cavernous Angioma. Nat. Commun. 2020, 11, 2659. [Google Scholar] [CrossRef] [PubMed]
- Koskimäki, J.; Zhang, D.; Carrión-Penagos, J.; Girard, R.; Piedad, K.; Polster, S.P.; Lyne, S.; Stadnik, A.; Awad, I.A. Symptomatic Brain Hemorrhages from Cavernous Angioma After Botulinum Toxin Injections, a Role of TLR/MEKK3 Mechanism? Case Report and Review of the Literature. World Neurosurg. 2020, 136, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Hassani, F.D.; Karekezi, C.; El Abbadi, N. Rare Case of Giant Pediatric Cavernous Angioma of the Temporal Lobe: A Case Report and Review of the Literature. Surg. Neurol. Int. 2020, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Kitaura, H.; Hiraishi, T.; Itoh, Y.; Oishi, M.; Fujii, Y.; Fukuda, M.; Kakita, A. Reactive Astrocytes Contribute to Epileptogenesis in Patients with Cavernous Angioma. Epilepsy Res. 2021, 176, 106732. [Google Scholar] [CrossRef]
- Zanello, M.; Goodden, J.R.; Colle, H.; Wager, M.; De Witt Hamer, P.C.; Smits, A.; Bello, L.; Tate, M.; Spena, G.; Bresson, D.; et al. Predictors of Epileptic Seizures and Ability to Work in Supratentorial Cavernous Angioma Located within Eloquent Brain Areas. Neurosurgery 2019, 85, E702–E713. [Google Scholar] [CrossRef] [Green Version]
- Wei, S.; Li, Y.; Polster, S.P.; Weber, C.R.; Awad, I.A.; Shen, L. Cerebral Cavernous Malformation Proteins in Barrier Maintenance and Regulation. Int. J. Mol. Sci. 2020, 21, 675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenblum, J.S.; Nazari, M.; Al-Khalili, Y.; Potigailo, V.; Veznedaroglu, E. Unilateral symptomatic hypertrophic olivary degeneration secondary to Midline brainstem cavernous angioma: A case report and review of the literature. World Neurosurg. 2018, 110, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Marcati, E.; Ferrari, E.; Fava, E.; Talamonti, G.; D’Aliberti, G.A. Clinical considerations on a right operculo-insular cavernous angioma: An illustrative case. Acta Neurochir. 2021, 163, 2755–2759. [Google Scholar] [CrossRef] [PubMed]
- Ruschel, L.G.; Brock, R.S.; Teles Gomes, M.D.; Vellutini, E.D.; de Oliveira, M.F. Improvement of hemichorea following surgical resection of a putaminal cavernous angioma: Case report and review of literature. World Neurosurg. 2020, 138, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Umemura, T.; Nishizawa, S.; Miyachi, H.; Yamamoto, J. Removal of double cavernous angioma of the frontal lobe using a three-dimensional printed model: A case report. J. UOEH. 2020, 42, 217–222. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, M.; Yin, H.; Huang, M.; Zhu, L.; Liang, X.; Deng, Y.; Hu, S.; Zhang, X.; Liu, J. Cavernous angioma of the cerebellopontine angle presenting as hemifacial spasm. Neurol India. 2018, 66, 1826–1828. [Google Scholar]
- Mokin, M.; Agazzi, S.; Dawson, L.; Primiani, C.T. Neuroimaging of Cavernous Malformations. Curr. Pain Headache Rep. 2017, 21, 47. [Google Scholar] [CrossRef]
- Paiva, A.; de Alcântara, E.; Araujo, J.; Veiga, J. Propranolol as Treatment for Cavernous Angioma Malformation—A Prospective Study and a Critical Review. Arq. Bras. Neurocir. Braz. Neurosurg. 2018, 37, A0748. [Google Scholar]
- Faye, M.; Diallo, M.; Sghiouar, M.; Ndiaye Sy, E.C.; Borius, P.Y.; Régis, J.-M. Stereotactic Radiosurgery for Thalamus Arteriovenous Malformations. J. Radiosurg. SBRT 2020, 6, 269–275. [Google Scholar] [PubMed]
- Healy, V.; O’Halloran, P.J.; Husien, M.B.; Bolger, C.; Farrell, M. Intermixed Arteriovenous Malformation and Hemangioblastoma: Case Report and Literature Review. CNS Oncol. 2020, 9, CNS66. [Google Scholar] [CrossRef] [PubMed]
- Sabayan, B.; Lineback, C.; Viswanathan, A.; Leslie-Mazwi, T.M.; Shaibani, A. Central Nervous System Vascular Malformations: A Clinical Review. Ann. Clin. Transl. Neurol. 2021, 8, 504–522. [Google Scholar] [CrossRef] [PubMed]
- Goyal, P.; Mangla, R.; Gupta, S.; Malhotra, A.; Almast, J.; Sapire, J.; Kolar, B. Pediatric Congenital Cerebrovascular Anomalies: Congenital Cerebrovascular Anomalies. J. Neuroimaging 2019, 29, 165–181. [Google Scholar] [CrossRef] [PubMed]
- De Benedictis, A.; Pietrobattista, A.; Talamanca, L.F.; Monti, L.; Paolantonio, G.; Natali, G.L.; Bua, A.; Savioli, A.; Alessandra Marasi, E.; Randi, F.; et al. “De Novo” Brain Arteriovenous Malformation in a Child with Congenital Porto-Systemic Shunt and Multisystemic Angiomas. Clin. Neurol. Neurosurg. 2022, 217, 107236. [Google Scholar] [CrossRef]
- Nguyen, H.-L.; Boon, L.M.; Vikkula, M. Genetics of Vascular Anomalies. Semin. Pediatric Surg. 2020, 29, 150967. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues de Oliveira, L.F.; de Castro-Afonso, L.H.; de Freitas, R.K.; Colli, B.O.; Abud, D.G. De Novo Intracranial Arteriovenous Malformation—Case Report and Literature Review. World Neurosurg. 2020, 138, 349–351. [Google Scholar] [CrossRef]
- Guizado Infante, V.M.; Mejía Maggi, N.C.; Carrera Silva, D.M. Malformación Arteriovenosa Cerebelosa. Caso Clínico. Eugenio Espejo 2019, 13, 71–78. [Google Scholar]
- Kassiri, J.; Rajapakse, T.; Wheatley, M.; Sinclair, D.B. Neurovascular Lesions in Pediatric Epilepsy. J. Child. Neurol. 2019, 34, 549–555. [Google Scholar] [CrossRef]
- Rinaldo, L.; Lanzino, G.; Flemming, K.D.; Krings, T.; Brinjikji, W. Symptomatic Developmental Venous Anomalies. Acta Neurochir. 2020, 162, 1115–1125. [Google Scholar] [CrossRef]
- Feutren, T.; Huertas, A.; Salleron, J.; Anxionnat, R.; Bracard, S.; Klein, O.; Peiffert, D.; Bernier-Chastagner, V. Modern Robot-Assisted Radiosurgery of Cerebral Angiomas—Own Experiences, System Comparisons, and Comprehensive Literature Overview. Neurosurg. Rev. 2018, 41, 787–797. [Google Scholar] [CrossRef]
- Hart, B.L.; Mabray, M.C.; Morrison, L.; Whitehead, K.J.; Kim, H. Systemic and CNS Manifestations of Inherited Cerebrovascular Malformations. Clin. Imaging 2021, 75, 55–66. [Google Scholar] [CrossRef]
- Wang, A.T.; Pillai, P.; Guran, E.; Carter, H.; Minasian, T.; Lenart, J.; Vandse, R. Anesthetic Management of Awake Craniotomy for Resection of the Language and Motor Cortex Vascular Malformations. World Neurosurg. 2020, 143, e136–e148. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Alfayate, R.; Martínez-Moreno, N.; Rosati, S.D.; Moreu-Gamazo, M.; Pérez-García, C.; Martínez-Alvarez, R. Klippel-Trenaunay-Weber Syndrome Associated with Multiple Cerebral Arteriovenous Malformations: Usefulness of Gamma Knife Stereotactic Radiosurgery in This Syndrome. World Neurosurg. 2020, 141, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Kern, P. Pathophysiology of Telangiectasias of the Lower Legs and Its Therapeutic Implication: A Systematic Review. Phlebology 2018, 33, 225–233. [Google Scholar] [CrossRef]
- McKinney, A.M. Slow-Flow, Asymptomatic Vascular Malformations: Brain Capillary Telangiectasias and Developmental Venous Anomalies. In Atlas of Normal Imaging Variations of the Brain, Skull, and Craniocervical Vasculature; Springer International Publishing: Cham, Switzerland, 2017; pp. 487–521. ISBN 978-3-319-39789-4. [Google Scholar]
- Larson, A.S.; Flemming, K.D.; Lanzino, G.; Brinjikji, W. Brain Capillary Telangiectasias: From Normal Variants to Disease. Acta Neurochir. 2020, 162, 1101–1113. [Google Scholar] [CrossRef] [PubMed]
- Braileanu, M.; Wicks, J.M.; Saindane, A.M. Appearance of an Unusual Ring Enhancing Brain Capillary Telangiectasia on 3.0T MRI with Dynamic Susceptibility Contrast Perfusion. Radiol. Case Rep. 2020, 15, 1331–1334. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, Y.; Tong, F.; Huang, W.; Tinzing, L.; Le Grange, J.M.; Wang, F.; Zhou, Y. Sudden Death from an Epileptic Seizure Due to Capillary Telangiectasias in the Hippocampus. Forensic. Sci. Med. Pathol. 2019, 15, 243–248. [Google Scholar] [CrossRef]
- Luz, A.R.; de Souza Pereira da Silva, M.C.; de Moura Vergara, R.; Mourão, M.S.F. Lower Limb Ulcers. In Vascular Diseases for the Non-Specialist; Navarro, T.P., Dardik, A., Junqueira, D., Cisneros, L., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 203–220. ISBN 978-3-319-46057-4. [Google Scholar]
- Sebold, A.J.; Day, A.M.; Ewen, J.; Adamek, J.; Byars, A.; Cohen, B.; Kossoff, E.H.; Mizuno, T.; Ryan, M.; Sievers, J.; et al. Sirolimus Treatment in Sturge-Weber Syndrome. Pediatric Neurol. 2021, 115, 29–40. [Google Scholar] [CrossRef]
- Hassanpour, K.; Nourinia, R.; Gerami, E.; Mahmoudi, G.; Esfandiari, H. Ocular Manifestations of the Sturge–Weber Syndrome. J. Ophthalmic Vis. Res. 2021, 16, 415. [Google Scholar] [CrossRef]
- Bianchi, F.; Auricchio, A.M.; Battaglia, D.I.; Chieffo, D.R.P.; Massimi, L. Sturge-Weber Syndrome: An Update on the Relevant Issues for Neurosurgeons. Childs Nerv. Syst. 2020, 36, 2553–2570. [Google Scholar] [CrossRef]
- Zallmann, M.; Leventer, R.J.; Mackay, M.T.; Ditchfield, M.; Bekhor, P.S.; Su, J.C. Screening for Sturge-Weber Syndrome: A State-of-the-Art Review. Pediatr. Dermatol. 2018, 35, 30–42. [Google Scholar] [CrossRef] [Green Version]
- Fjær, R.; Marciniak, K.; Sundnes, O.; Hjorthaug, H.; Sheng, Y.; Hammarström, C.; Sitek, J.C.; Vigeland, M.D.; Backe, P.H.; Øye, A.-M.; et al. A Novel Somatic Mutation in GNB2 Provides New Insights to the Pathogenesis of Sturge–Weber Syndrome. Hum. Mol. Genet. 2021, 30, 1919–1931. [Google Scholar] [CrossRef]
- Kasinathan, A.; Saini, A.G.; Vyas, S.; Singhi, P. Angiodysplastic Sturge Weber Syndrome. BMJ Case Rep. 2018, 2018, bcr-2017-222869. [Google Scholar]
- Day, A.M.; McCulloch, C.E.; Hammill, A.M.; Juhász, C.; Lo, W.D.; Pinto, A.L.; Miles, D.K.; Fisher, B.J.; Ball, K.L.; Wilfong, A.A.; et al. Physical and Family History Variables Associated With Neurological and Cognitive Development in Sturge-Weber Syndrome. Pediatric Neurol. 2019, 96, 30–36. [Google Scholar] [CrossRef]
- Maraña Pérez, A.I.; Ruiz-Falcó Rojas, M.L.; Puertas Martín, V.; Domínguez Carral, J.; Carreras Sáez, I.; Duat Rodríguez, A.; Sánchez González, V. Análisis Del Síndrome de Sturge-Weber: Estudio Retrospectivo de Múltiples Variables Asociadas. Neurología 2017, 32, 363–370. [Google Scholar] [CrossRef]
- Mohammadipanah, F.; Salimi, F. Potential Biological Targets for Bioassay Development in Drug Discovery of Sturge-Weber Syndrome. Chem. Biol. Drug Des. 2017, 91, 359–369. [Google Scholar] [CrossRef]
- Castillo-Rangel, C.; Salinas--Velázquez, O.; Gomez-Ibarra, A.; Becerra-Escobedo, G.; Pérez Pérez, V.H.; Marín-Márquez, G. Report of an epicranial arteriovenous malformation. Cesk Slov. Neurol. N 2021, 84, 488–490. [Google Scholar]
- Kim, J.; Jeong, J.; Behen, M.E.; Pilli, V.K.; Luat, A.; Chugani, H.T.; Juhász, C. Metabolic Correlates of Cognitive Function in Children with Unilateral Sturge–Weber Syndrome: Evidence for Regional Functional Reorganization and Crowding. Hum. Brain Mapp. 2018, 39, 1596–1606. [Google Scholar] [CrossRef] [Green Version]
- Dekeuleneer, V.; Seront, E.; Van Damme, A.; Boon, L.M.; Vikkula, M. Theranostic Advances in Vascular Malformations. J. Investig. Dermatol. 2020, 140, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Polster, S.P.; Stadnik, A.; Akers, A.L.; Cao, Y.; Christoforidis, G.A.; Fam, M.D.; Flemming, K.D.; Girard, R.; Hobson, N.; Koenig, J.I.; et al. Atorvastatin Treatment of Cavernous Angiomas with Symptomatic Hemorrhage Exploratory Proof of Concept (AT CASH EPOC) Trial. Neurosurgery 2019, 85, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, J.; Malinverno, M.; Globisch, M.A.; Maderna, C.; Corada, M.; Orsenigo, F.; Conze, L.L.; Rorsman, C.; Sundell, V.; Arce, M.; et al. Propranolol Reduces the Development of Lesions and Rescues Barrier Function in Cerebral Cavernous Malformations: A Preclinical Study. Stroke 2021, 52, 1418–1427. [Google Scholar] [CrossRef] [PubMed]
- The Treat-CCM Investigators; Lanfranconi, S.; Scola, E.; Bertani, G.A.; Zarino, B.; Pallini, R.; d’Alessandris, G.; Mazzon, E.; Marino, S.; Carriero, M.R.; et al. Propranolol for Familial Cerebral Cavernous Malformation (Treat_CCM): Study Protocol for a Randomized Controlled Pilot Trial. Trials 2020, 21, 401. [Google Scholar] [CrossRef]
- Higueros, E.; Roe, E.; Granell, E.; Baselga, E. Síndrome de Sturge-Weber: Revisión. Actas Dermo-Sifiliográficas 2017, 108, 407–417. [Google Scholar] [CrossRef]
- Bashir, U.; Shah, S.; Jeph, S.; O’Keeffe, M.; Khosa, F. Magnetic Resonance (MR) Imaging of Vascular Malformations. PJR 2017, 82, 731–741. [Google Scholar] [CrossRef] [Green Version]
- Bertani, R.; Garret, B.; Perret, C.M.; Batista, S.; Koester, S.W.; Azeredo, R.; Guimarães Cavalcante Carlos de Carvalho, T.; Almeida, J.A. Undiagnosed Sturge-Weber Syndrome as a Differential Diagnosis of Seizures and Deep Cerebral Venous System Dilation: A Case Report. Cureus 2021, 13, e19111. [Google Scholar] [CrossRef]
- Gross, B.A.; Du, R. Diagnosis and Treatment of Vascular Malformations of the Brain. Curr. Treat. Options Neurol. 2014, 16, 279. [Google Scholar] [CrossRef]
- Settecase, F.; Hetts, S.W.; Nicholson, A.D.; Amans, M.R.; Cooke, D.L.; Dowd, C.F.; Higashida, R.T.; Halbach, V.V. Superselective Intra-Arterial Ethanol Sclerotherapy of Feeding Artery and Nidal Aneurysms in Ruptured Cerebral Arteriovenous Malformations. AJNR Am. J. Neuroradiol. 2016, 37, 692–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.-J.; Norat, P.; Ding, D.; Mendes, G.A.C.; Tvrdik, P.; Park, M.S.; Kalani, M.Y. Transvenous Embolization of Brain Arteriovenous Malformations: A Review of Techniques, Indications, and Outcomes. Neurosurgical. Focus 2018, 45, E13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rangel, C.C.; Cordova JS, Z.; Noriega, A.R.; Sanchez, J.M.M.; Mejia Frías, A.A. Foramen magnum meningioma: Surgical planning Analysis with 3d printing. J. Neuro Brain Res. 2021, 1, 1–4. [Google Scholar] [CrossRef]
- Tao, Y.; Sun, X.; You, Y.; Chen, J.; Wang, J.; Wang, S.; Lin, N.; Liang, B.; Zhao, J. Symptomatic large or giant capillary telangiectasias: Management and outcome in 5 cases. J. Neurosurg. 2016, 125, 160–166. [Google Scholar]
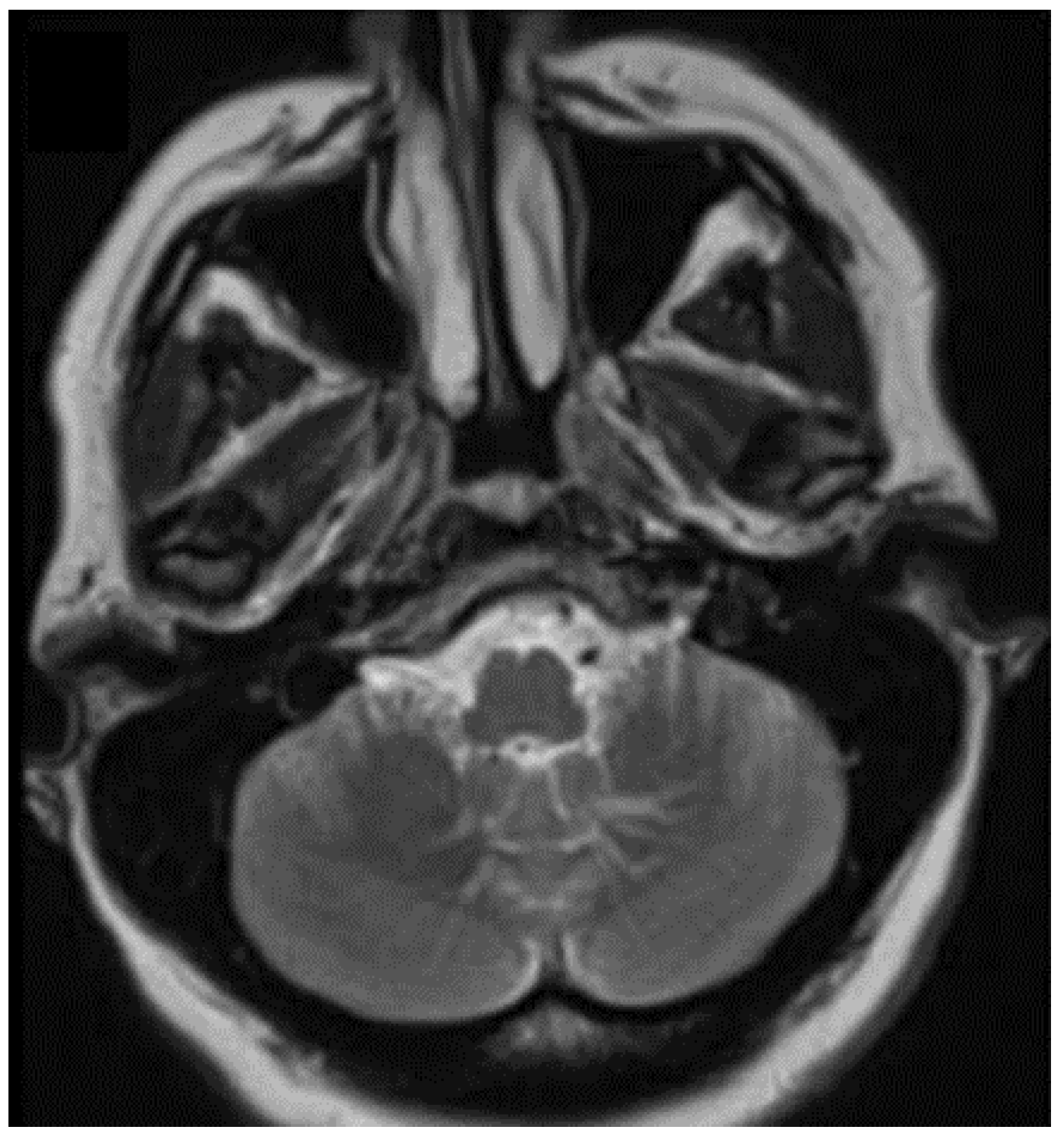
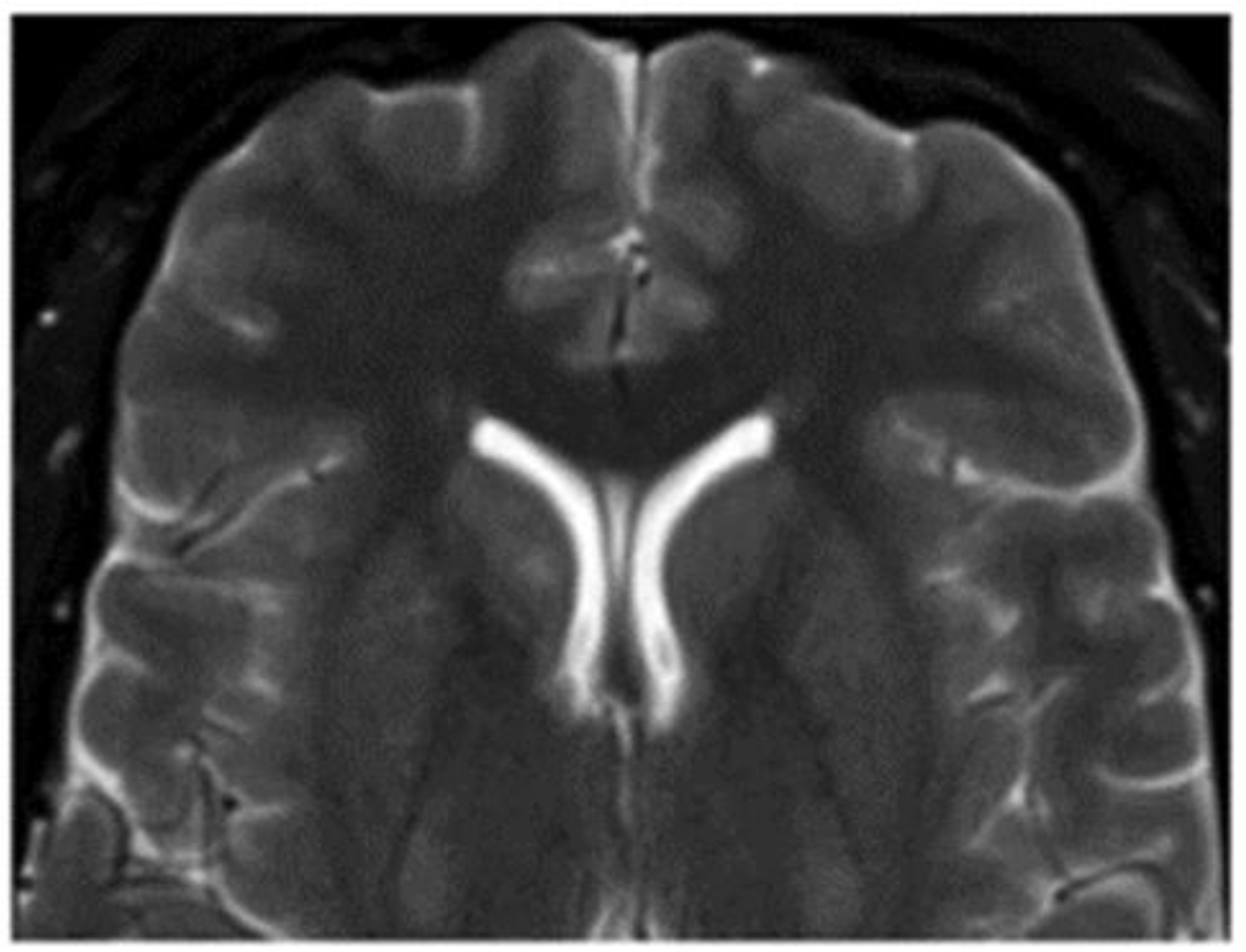
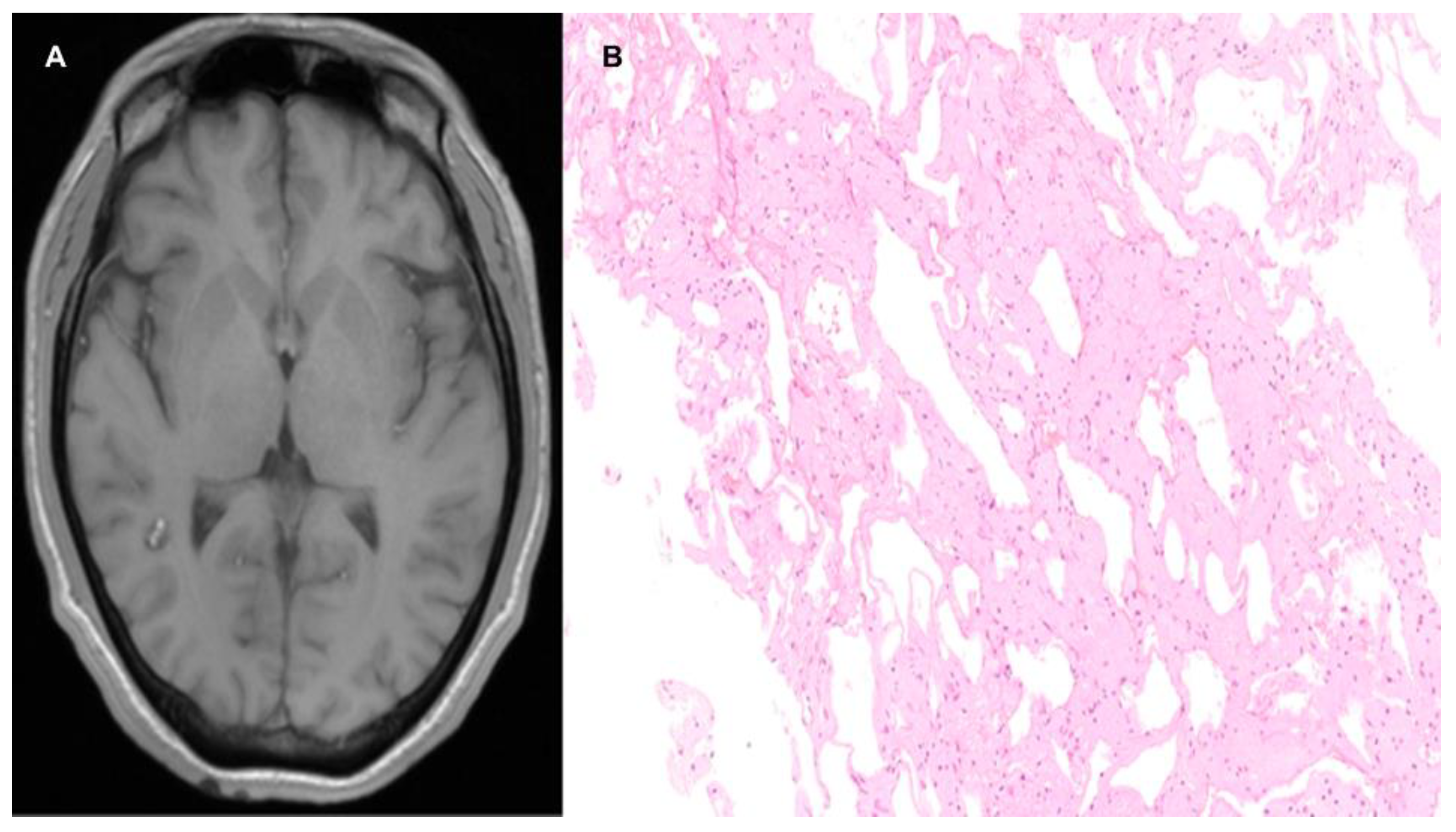
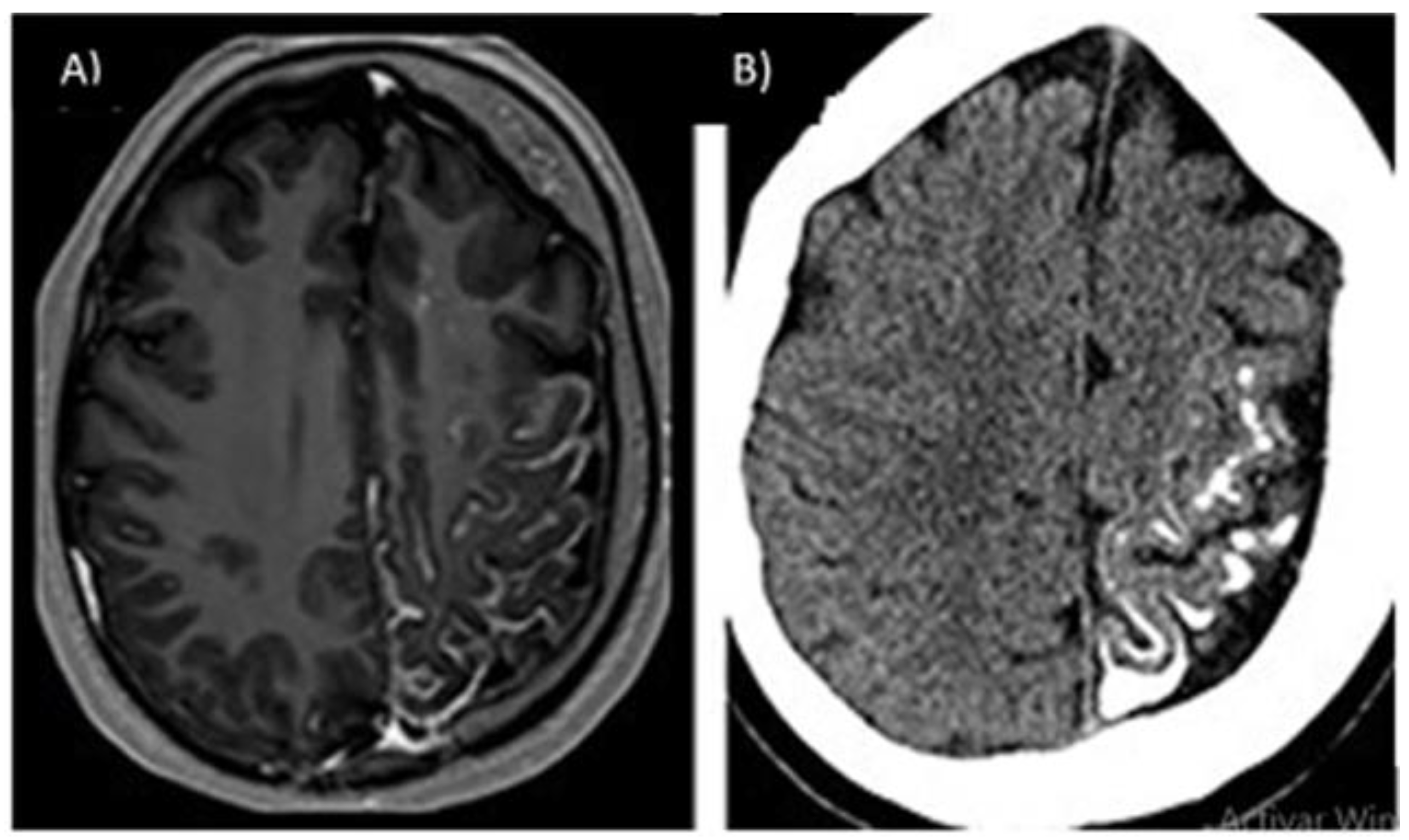
| Location | Effects |
|---|---|
| Mesencephalon-pontine | Ataxia, imbalance, multiple falls, headache, blurred vision, and dysarthria [35]. |
| Insular cortex | Sensory, taste and speech symptoms [36]. |
| Insular putamen | Hemichorea–hemiballism [37]. |
| Frontal lobe | Cognitive impairment [38]. |
| Pontocerebellar | Hemifacial spasm, headache [39]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo-Rangel, C.; Marín, G.; Hernandez-Contreras, K.A.; Zarate-Calderon, C.; Vichi-Ramirez, M.M.; Cortez-Saldias, W.; Rodriguez-Florido, M.A.; Riley-Moguel, Á.E.; Pichardo, O.; Torres-Pineda, O.; et al. Atlas of Nervous System Vascular Malformations: A Systematic Review. Life 2022, 12, 1199. https://doi.org/10.3390/life12081199
Castillo-Rangel C, Marín G, Hernandez-Contreras KA, Zarate-Calderon C, Vichi-Ramirez MM, Cortez-Saldias W, Rodriguez-Florido MA, Riley-Moguel ÁE, Pichardo O, Torres-Pineda O, et al. Atlas of Nervous System Vascular Malformations: A Systematic Review. Life. 2022; 12(8):1199. https://doi.org/10.3390/life12081199
Chicago/Turabian StyleCastillo-Rangel, Carlos, Gerardo Marín, Karla Aketzalli Hernandez-Contreras, Cristofer Zarate-Calderon, Micheel Merari Vichi-Ramirez, Wilmar Cortez-Saldias, Marco Antonio Rodriguez-Florido, Ámbar Elizabeth Riley-Moguel, Omar Pichardo, Osvaldo Torres-Pineda, and et al. 2022. "Atlas of Nervous System Vascular Malformations: A Systematic Review" Life 12, no. 8: 1199. https://doi.org/10.3390/life12081199
APA StyleCastillo-Rangel, C., Marín, G., Hernandez-Contreras, K. A., Zarate-Calderon, C., Vichi-Ramirez, M. M., Cortez-Saldias, W., Rodriguez-Florido, M. A., Riley-Moguel, Á. E., Pichardo, O., Torres-Pineda, O., Vega-Quesada, H. G., Lopez-Elizalde, R., Ordoñez-Granja, J., Alvarado-Martinez, H. H., Vega-Quesada, L. A., & Aranda-Abreu, G. E. (2022). Atlas of Nervous System Vascular Malformations: A Systematic Review. Life, 12(8), 1199. https://doi.org/10.3390/life12081199








