Pre-Clinical Validation of A Novel Continuous Intra-Abdominal Pressure Measurement Equipment (SERENNO)
Abstract
1. Introduction
2. Materials and Methods
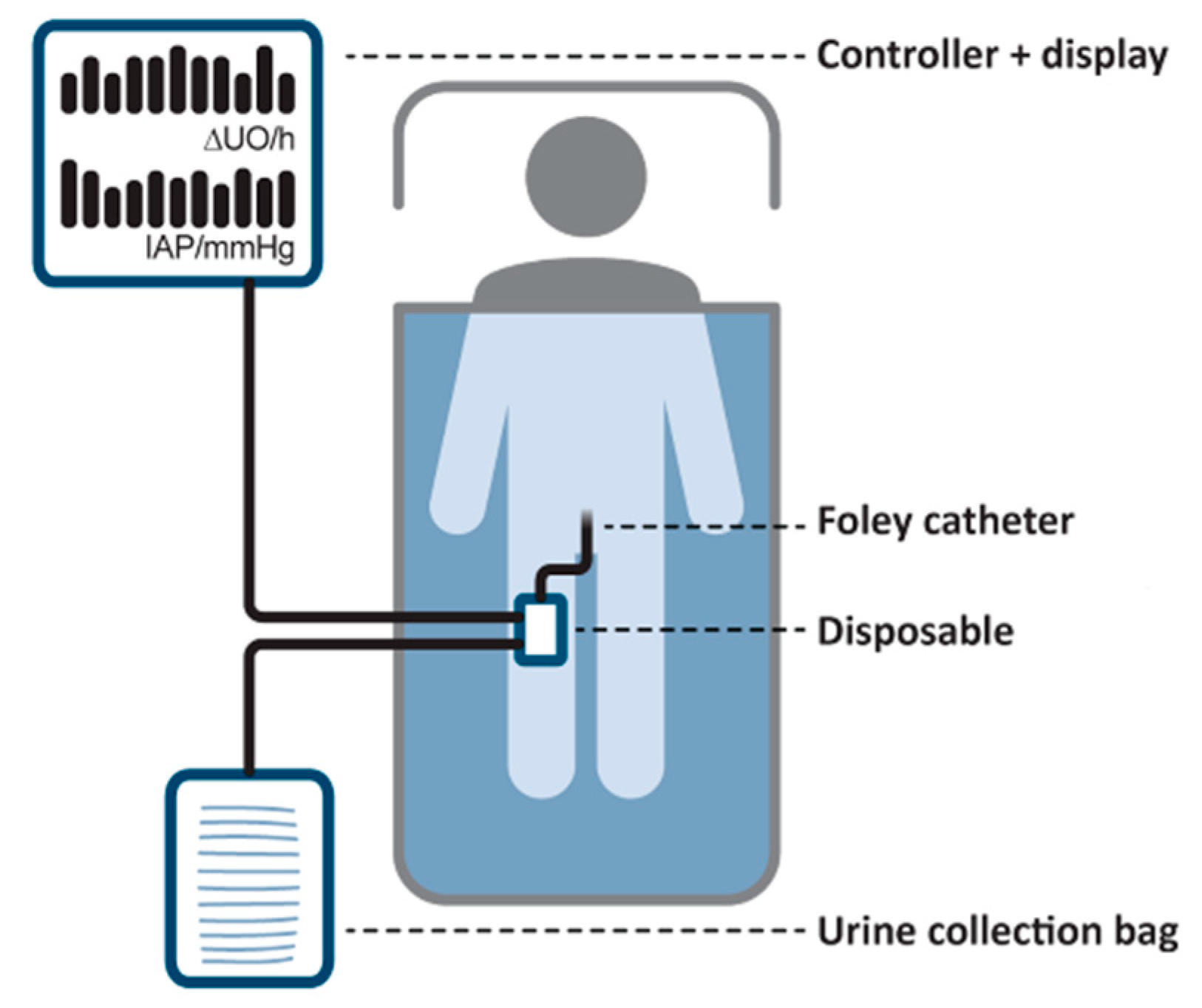
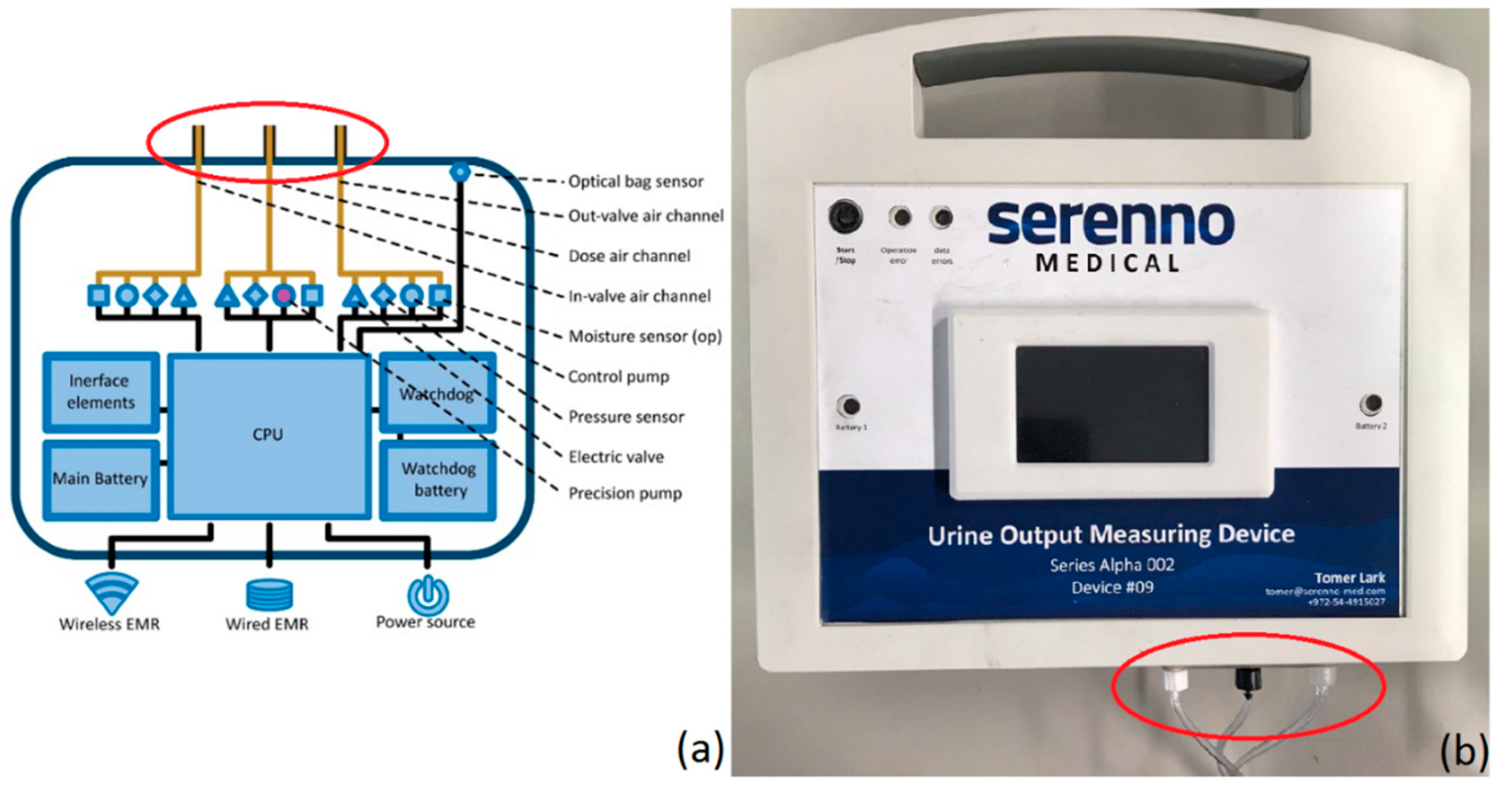
2.1. SERENNO System
2.2. Spiegelberg System
2.3. CiMON System
2.4. Human Phantom
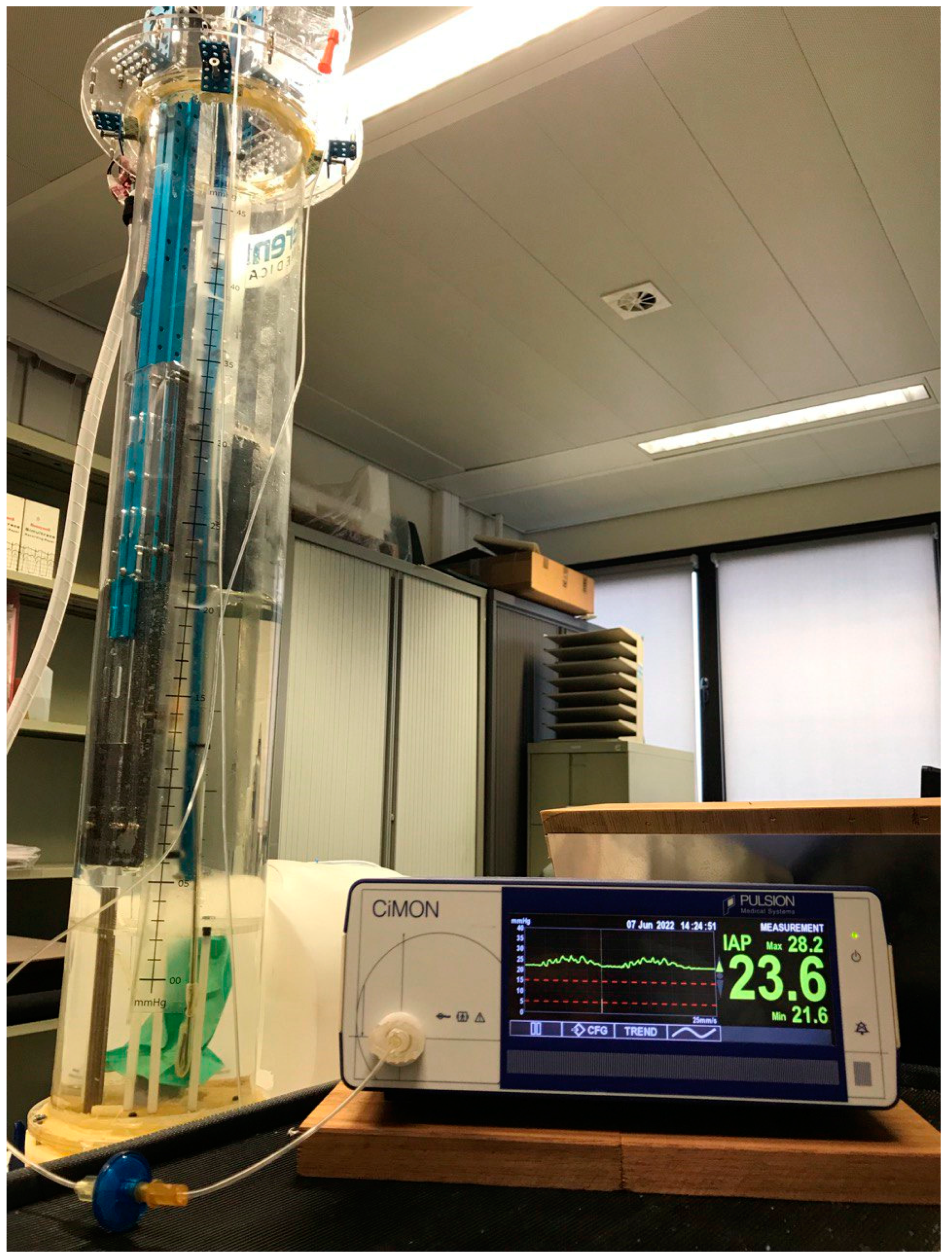
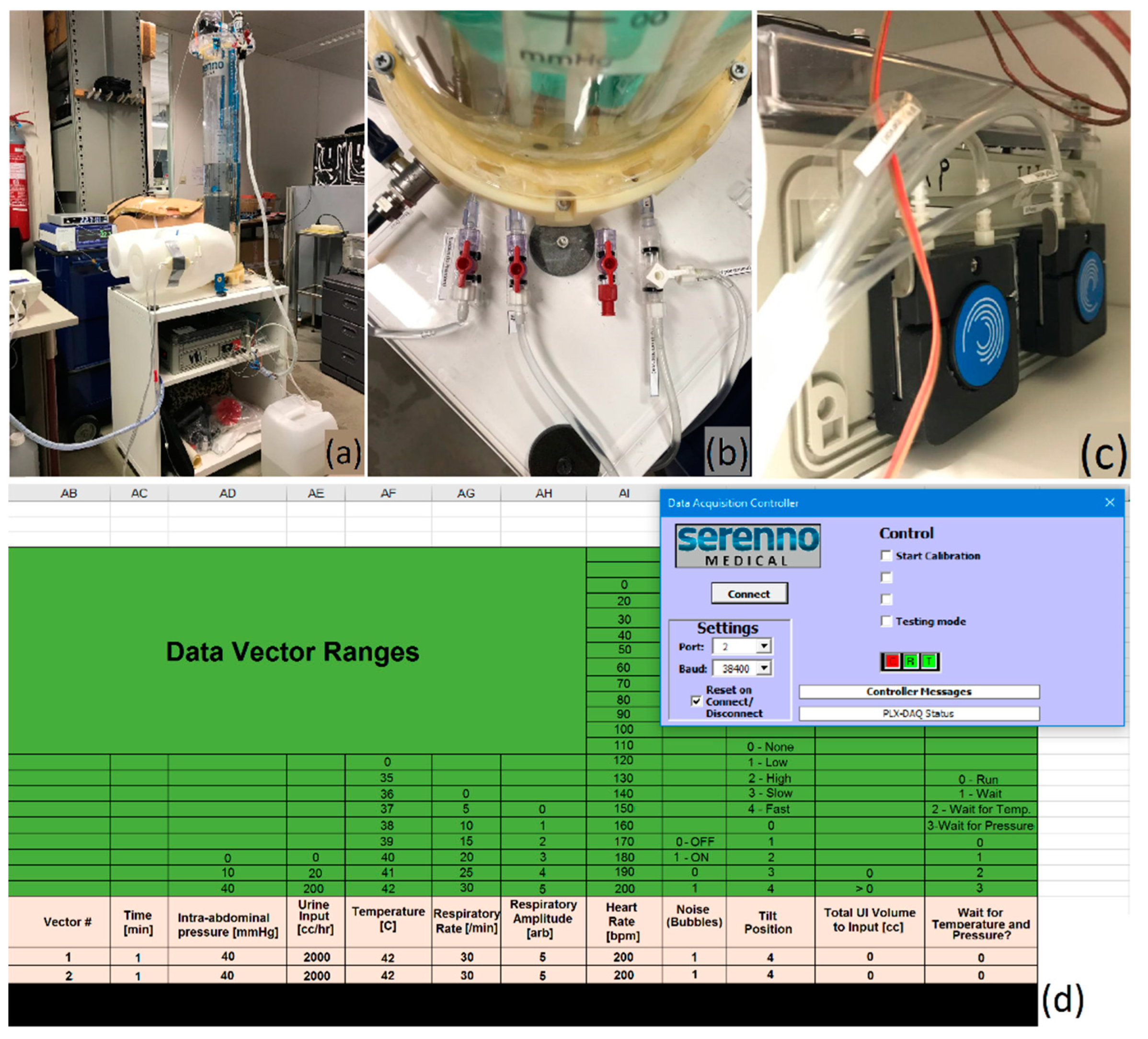
2.5. Validation Study Set-Up
2.6. Statistical Analysis
3. Results
3.1. Dynamic Respiratory IAP Variations
3.2. IAP Correlations
3.3. Bland and Altman’s Analysis and Percentage Error
3.4. Concordance Analysis
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malbrain, M.L.; Cheatham, M.L.; Kirkpatrick, A.; Sugrue, M.; Parr, M.; De Waele, J.; Balogh, Z.; Leppäniemi, A.; Olvera, C.; Ivatury, R.; et al. Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. I. Definitions. Intensive Care Med. 2006, 32, 1722–1732. [Google Scholar] [CrossRef] [PubMed]
- Malbrain, M.L. Different techniques to measure intra-abdominal pressure (IAP): Time for a critical re-appraisal. Intensive Care Med. 2004, 30, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Malbrain, M.L.; De Laet, I.; De Waele, J.J.; Sugrue, M.; Schachtrupp, A.; Duchesne, J.; Van Ramshorst, G.; De Keulenaer, B.; Kirkpatrick, A.W.; Ahmadi-Noorbakhsh, S.; et al. The role of abdominal compliance, the neglected parameter in critically ill patients—A consensus review of 16. Part 2: Measurement techniques and management recommendations. Anaesthesiol. Intensive Ther. 2014, 46, 406–432. [Google Scholar] [CrossRef] [PubMed]
- Malbrain, M.L.; Chiumello, D.; Cesana, B.M.; Reintam Blaser, A.; Starkopf, J.; Sugrue, M.; Pelosi, P.; Severgnini, P.; Hernandez, G.; Brienza, N.; et al. WAKE-Up! Investigators. A systematic review and individual patient data meta-analysis on intra-abdominal hypertension in critically ill patients: The wake-up project. World initiative on Abdominal Hypertension Epidemiology, a Unifying Project (WAKE-Up!). Minerva Anestesiol. 2014, 80, 293–306. [Google Scholar] [PubMed]
- Muturi, A.; Ndaguatha, P.; Ojuka, D.; Kibet, A. Prevalence and predictors of intra-abdominal hypertension and compartment syndrome in surgical patients in critical care units at Kenyatta National Hospital. BMC Emerg. Med. 2017, 17, 10. [Google Scholar] [CrossRef] [PubMed]
- Kuteesa, J.; Kituuka, O.; Namuguzi, D.; Ndikuno, C.; Kirunda, S.; Mukunya, D.; Galukande, M. Intra-abdominal hypertension; prevalence, incidence and outcomes in a low resource setting; a prospective observational study. World J. Emerg. Surg. 2015, 10, 57. [Google Scholar] [CrossRef]
- Kirkpatrick, A.W.; Sugrue, M.; McKee, J.L.; Pereira, B.M.; Roberts, D.J.; De Waele, J.J.; Leppaniemi, A.; Ejike, J.C.; Reintam Blaser, A.; D’Amours, S.; et al. Update from the Abdominal Compartment Society (WSACS) on intra-abdominal hypertension and abdominal compartment syndrome: Past, present, and future beyond Banff 2017. Anaesthesiol. Intensive Ther. 2017, 49, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Khanna, A.; Minear, S.; Prabhakar, A.; Kurz, A.; Stanton, K.; Essakalli, L.; Blackwell, B.A.; Sweatt, N.; Flores, K.; Harris, L.; et al. Intra-abdominal hypertension in cardiac surgery patients: A multicenter observational pilot study. J. Clin. Monit. Comput. 2022; accepted for publication. [Google Scholar]
- Tayebi, S.; Gutierrez, A.; Mohout, I.; Smets, E.; Wise, R.; Stiens, J.; Malbrain, M.L.N.G. A concise overview of non-invasive intra-abdominal pressure measurement techniques: From bench to bedside. J. Clin. Monit. Comput. 2021, 35, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Tayebi, S.; Pourkazemi, A.; Malbrain, M.L.N.G.; Stiens, J. Non-Invasive Intra-Abdominal Pressure Measurement by Means of Transient Radar Method: In Vitro Validation of a Novel Radar-Based Sensor. Sensors 2021, 21, 5999. [Google Scholar] [CrossRef] [PubMed]
- Balogh, Z.; De Waele, J.J.; Malbrain, M.L. Continuous intra-abdominal pressure monitoring. Acta Clin. Belg. 2007, 62 (Suppl. S1), 26–32. [Google Scholar] [CrossRef] [PubMed]
- David, M.; Raviv, A.; Guttel, A.; García Reyes, V.; Simini, F.; Pracca, F. Non-invasive indirect monitoring of intra-abdominal pressure using microwave reflectometry: System design and proof-of-concept clinical trial. J. Clin Monit. Comput. 2021, 35, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Buchnik, S.; Gelgor, M.; Pracca, F.; David, M. Towards the detection of intraintestinal gas by microwave reflectometry in critical patients. In Proceedings of the 2021 IEEE URUCON, Montevideo, Uruguay, 24–26 November 2021; pp. 291–293. [Google Scholar] [CrossRef]
- David, M.; Amran, O.; Peretz, A.; Raviv, A.; Pracca, F. Optimized electrical bioimpedance measurements of abdominal wall on a porcine model for the continuous non-invasive assessment of intra-abdominal pressure. J. Clin. Monit. Comput. 2020, 34, 1209–1214. [Google Scholar] [CrossRef] [PubMed]
- De Potter, T.J.R.; Dits, H.; Malbrain, M.L. Intra-and interobserver variability during in vitro validation of two novel methods for intra-abdominal pressure monitoring. Intensive Care Med. 2005, 31, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Wauters, J.; Spincemaille, L.; Dieudonne, A.S.; Van Zwam, K.; Wilmer, A.; Malbrain, M.L. A Novel Method (CiMON) for Continuous Intra-Abdominal Pressure Monitoring: Pilot Test in a Pig Model. Crit. Care Res. Pract. 2012, 181563. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Gaidukov, K.M.; Raibuzhis, E.N.; Hussain, A.; Teterin, A.Y.; Smetkin, A.A.; Kuzkov, V.V.; Malbrain, M.L.; Kirov, M.Y. Effect of intra-abdominal pressure on respiratory function in patients undergoing ventral hernia repair. World J. Crit. Care Med. 2013, 2, 9–16. [Google Scholar] [CrossRef] [PubMed]
- De Waele, J.J.; Cheatham, M.L.; Malbrain, M.L.; Kirkpatrick, A.W.; Sugrue, M.; Balogh, Z.; Ivatury, R.; De Keulenaer, B.; Kimball, E.J. Recommendations for research from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. Acta Clin. Belg. 2009, 64, 203–209. [Google Scholar] [CrossRef] [PubMed]




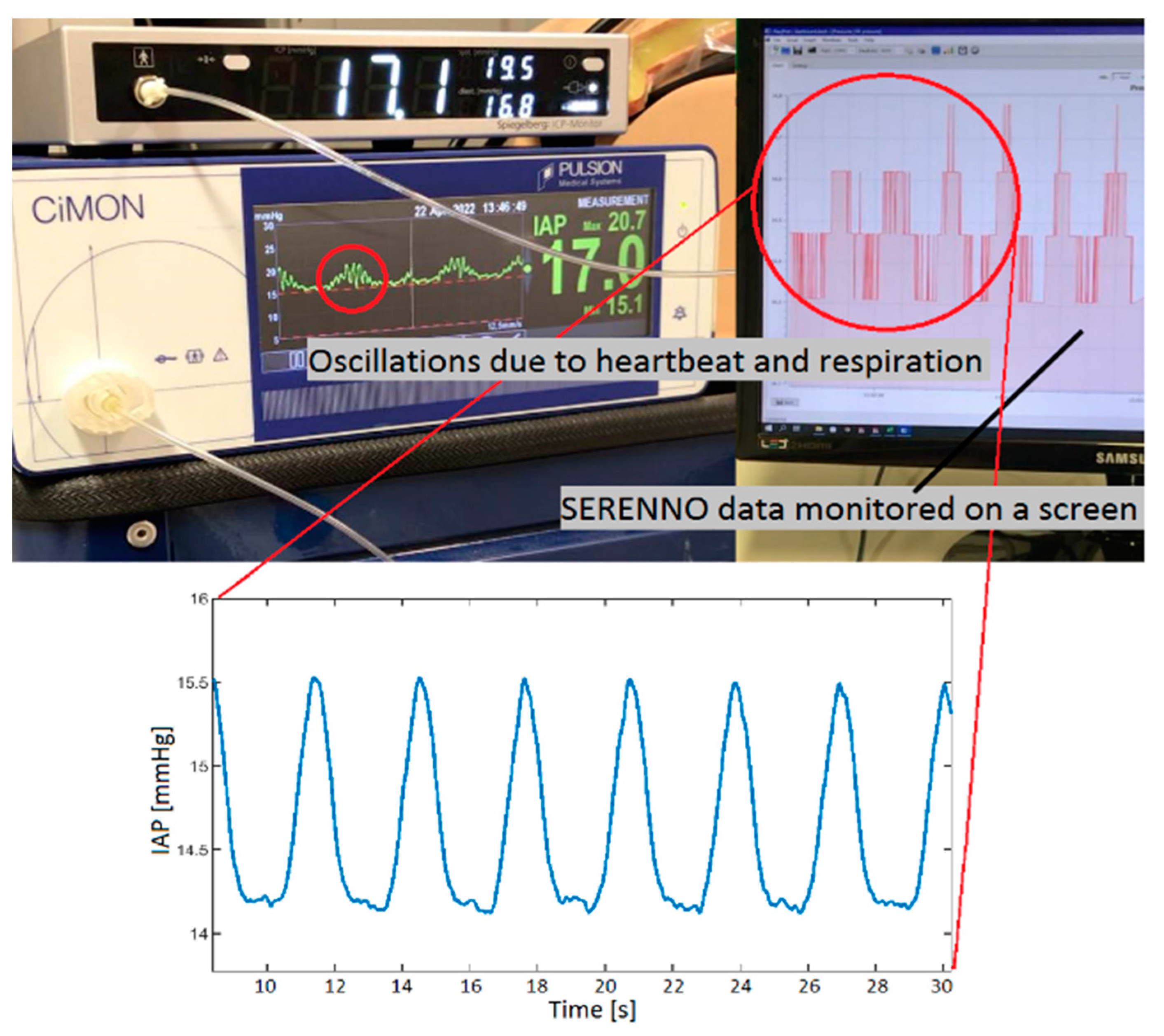
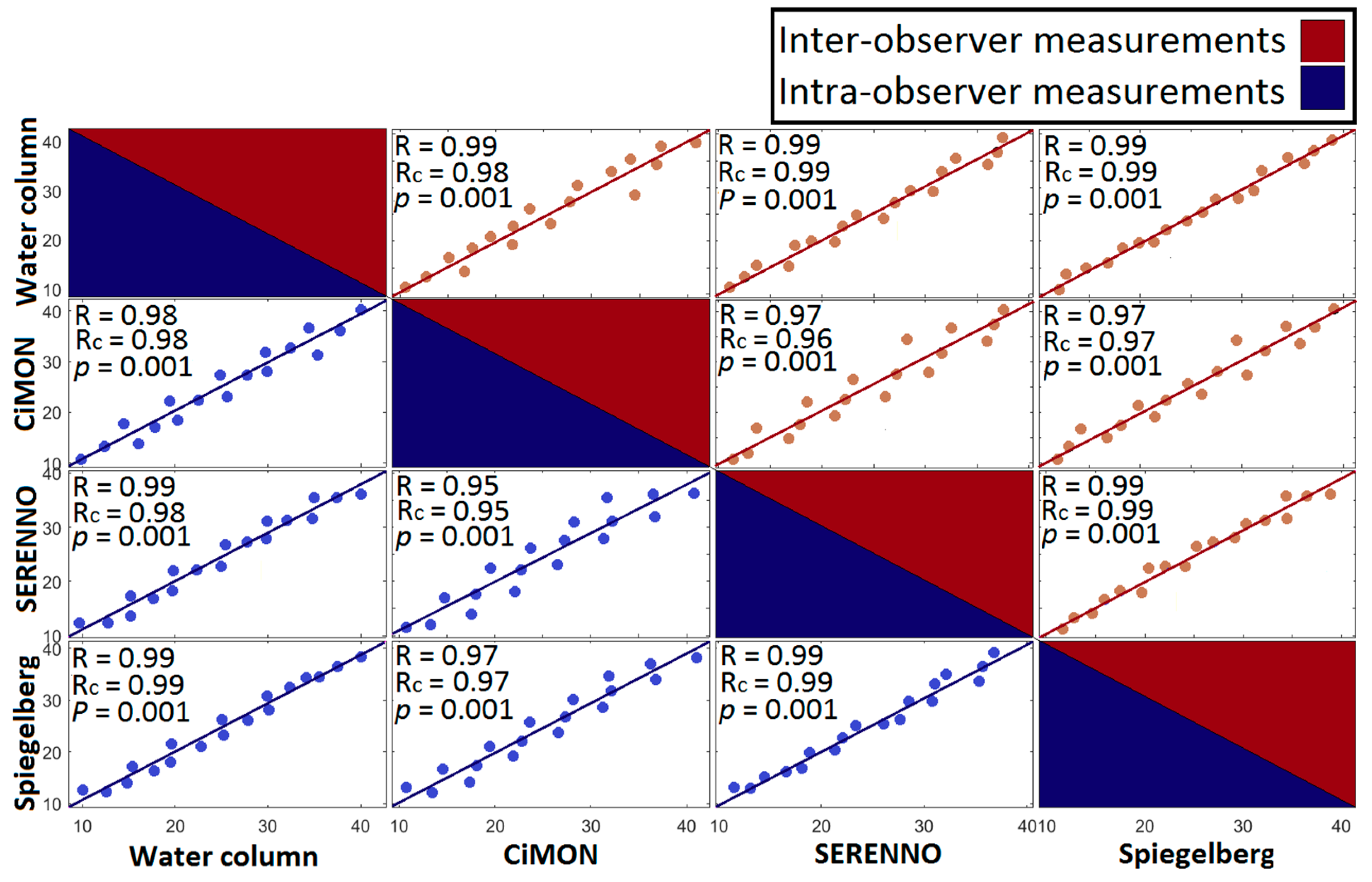
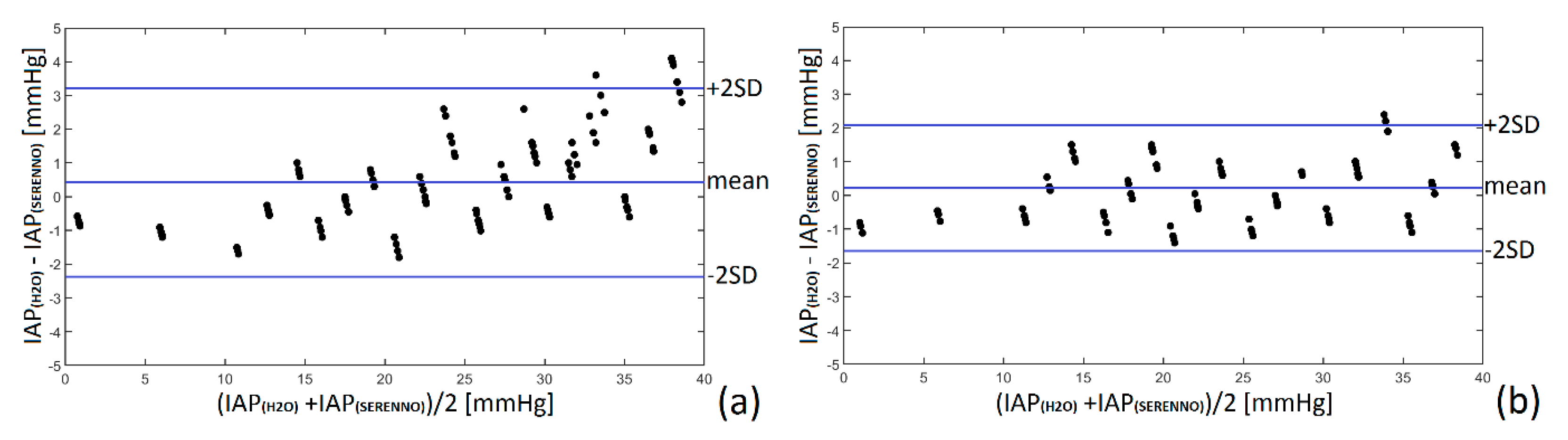
| IAPH2O | IAPCiM | IAPSER | IAPSPIE | |||
|---|---|---|---|---|---|---|
| Gold Standard [mmHg] | Intra- Observer [mmHg] | Inter- Observer [mmHg] | Intra- Observer [mmHg] | Inter- Observer [mmHg] | Intra- Observer [mmHg] | Inter- Observer [mmHg] |
| 0 | 0.3 ± 0.0 CV = 0% | 1.5 ± 0.1 CV = 6.6% | 0.9 ± 0.0 CV = 0% | 1.0 ± 0.0 CV = 0% | 0.8 ± 0.0 CV = 0% | 1.1 ± 0.0 CV = 0% |
| 5 | 5.4 ± 0.1 CV = 1.8% | 6.7 ± 0.0 CV = 0% | 6.0 ± 0.0 CV = 0% | 5.7 ± 0.1 CV = 1.7% | 5.8 ± 0.0 CV = 0% | 6.2 ± 0.1 CV = 1.6% |
| 10 | 13.2 ± 0.1 CV = 0.7% | 12.5 ± 0.4 CV = 3.2% | 12.9 ± 0.1 CV = 0.7% | 12.6 ± 0.2 CV = 1.6% | 12.7 ± 0.1 CV = 0.8% | 12.7 ± 0.4 CV = 3.4% |
| 15 | 17.8 ± 0.2 CV = 1.1% | 17.7 ± 0.3 CV = 1.7% | 17.6 ± 0.2 CV = 1.4% | 17.8 ± 0.3 CV = 1.7% | 17.2 ±0.1 CV = 0.6% | 17.9 ± 0.2 CV = 1.1% |
| 20 | 22.7 ± 0.3 CV = 1.3% | 22.0 ± 0.2 CV = 1.4% | 22.3 ± 0.3 CV = 1.3% | 22.2 ± 0.2 CV = 0.9% | 22.1 ± 0.4 CV = 1.8% | 22.3 ± 0.2 CV = 1.7% |
| 25 | 27.3 ± 0.2 CV = 0.7% | 27.6 ± 0.1 CV = 0.4% | 27.3 ± 0.3 CV = 1.1% | 27.1 ± 0.1 CV = 0.4% | 26.8 ± 0.3 CV = 1.1% | 27.4 ± 0.1 CV = 0.4% |
| 30 | 32.3 ± 0.4 CV = 1.2% | 32.2 ± 0.2 CV = 0.6% | 31.2 ± 0.2 CV = 0.6% | 31.8 ± 0.2 CV = 0.6% | 32.0 ± 0.4 CV = 1.3% | 32.1 ± 0.1 CV = 0.3% |
| 35 | 36.6 ± 0.2 CV = 0.5% | 37.3 ± 0.2 CV = 0.5% | 35.8 ± 0.3 CV = 0.8% | 36.7 ± 0.1 CV = 0.3% | 36.3 ± 0.1 CV = 0.3% | 37.2 ± 0.1 CV = 0.3% |
| Study Method | Mean IAP [mmHg] | Bias (Difference) [mmHg] | Precision (SD) [mmHg] | LLA [mmHg] | ULA [mmHg] | PE [%] |
|---|---|---|---|---|---|---|
| Intra-observer variability | ||||||
| CiMON | 18.66 | −0.25 | 1.28 | −2.76 | +2.26 | 13.71 |
| SERENNO | 19.44 | +0.34 | 1.39 | −2.39 | +3.06 | 14.30 |
| Spiegelberg | 19.39 | −0.04 | 0.87 | −1.74 | +1.67 | 8.97 |
| Inter-observer variability | ||||||
| CiMON | 19.70 | −0.75 | 1.54 | −3.76 | +2.26 | 15.63 |
| SERENNO | 20.40 | +0.12 | 0.94 | −1.72 | +1.96 | 9.21 |
| Spiegelberg | 19.64 | −0.58 | 0.67 | −1.89 | +0.73 | 6.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tayebi, S.; Wise, R.; Pourkazemi, A.; Stiens, J.; Malbrain, M.L.N.G. Pre-Clinical Validation of A Novel Continuous Intra-Abdominal Pressure Measurement Equipment (SERENNO). Life 2022, 12, 1161. https://doi.org/10.3390/life12081161
Tayebi S, Wise R, Pourkazemi A, Stiens J, Malbrain MLNG. Pre-Clinical Validation of A Novel Continuous Intra-Abdominal Pressure Measurement Equipment (SERENNO). Life. 2022; 12(8):1161. https://doi.org/10.3390/life12081161
Chicago/Turabian StyleTayebi, Salar, Robert Wise, Ali Pourkazemi, Johan Stiens, and Manu L. N. G. Malbrain. 2022. "Pre-Clinical Validation of A Novel Continuous Intra-Abdominal Pressure Measurement Equipment (SERENNO)" Life 12, no. 8: 1161. https://doi.org/10.3390/life12081161
APA StyleTayebi, S., Wise, R., Pourkazemi, A., Stiens, J., & Malbrain, M. L. N. G. (2022). Pre-Clinical Validation of A Novel Continuous Intra-Abdominal Pressure Measurement Equipment (SERENNO). Life, 12(8), 1161. https://doi.org/10.3390/life12081161








