MGRN1 as a Phenotypic Determinant of Human Melanoma Cells and a Potential Biomarker
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Patients and Samples
2.3. Reagents
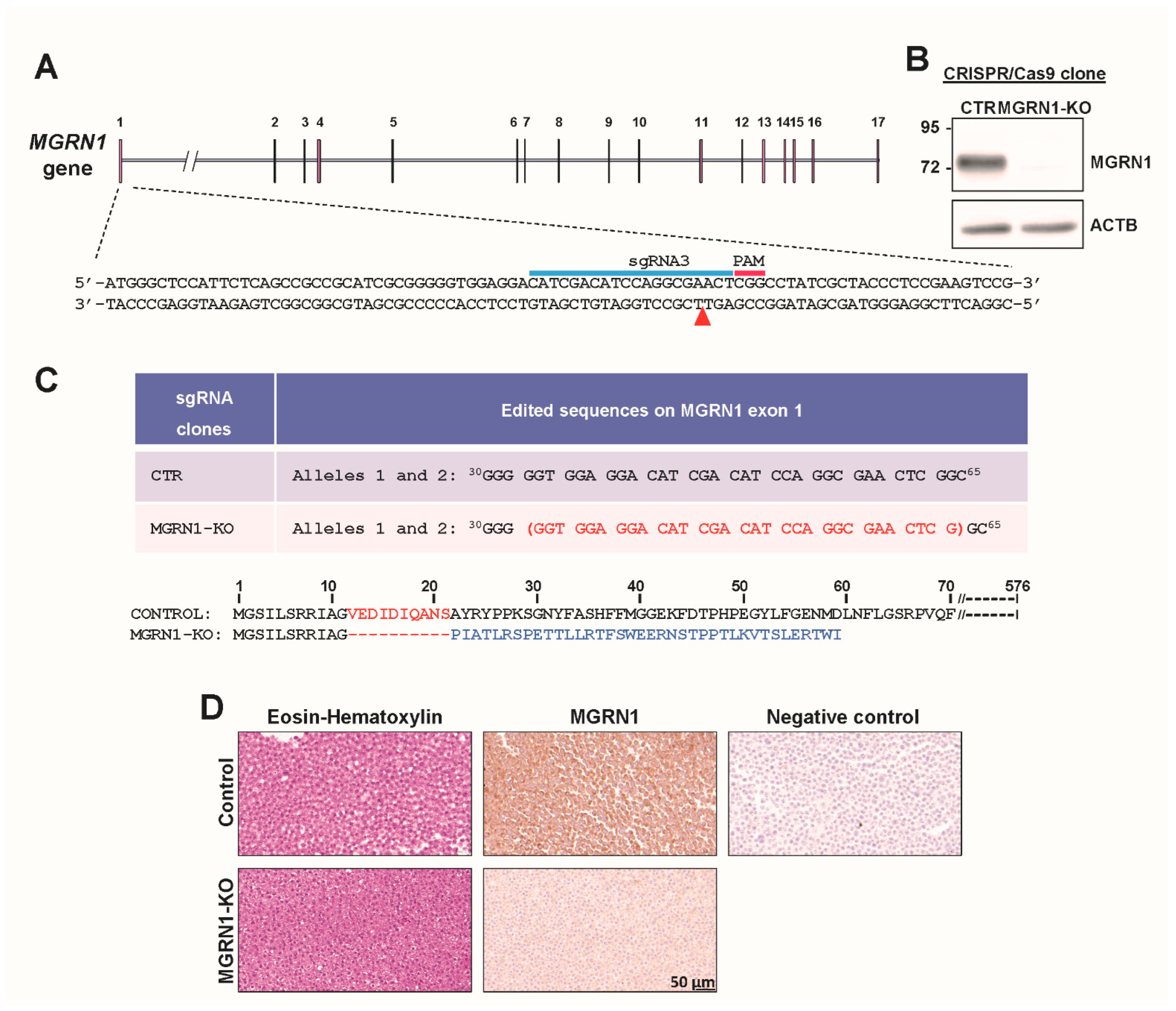
2.4. Cell Culture and Generation of MGRN1-KO Cells
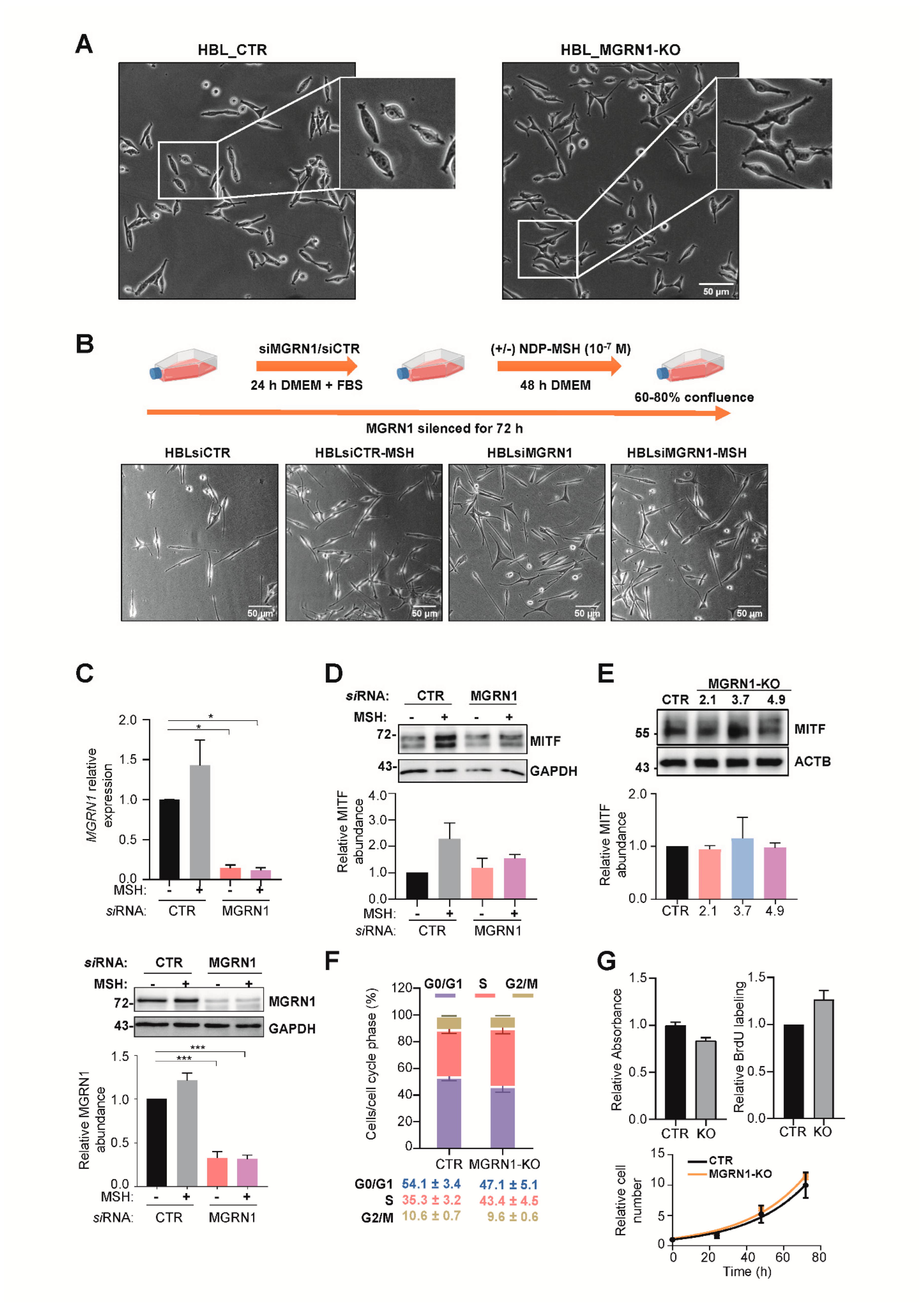
2.5. siRNA-Mediated Repression of MGRN1 in Cultured Cells
2.6. Cell Proliferation and Cell Cycle Assays
2.7. Confocal Fluorescence Microscopy and Analysis of H2AX Phosphorylation
2.8. Comet Assays
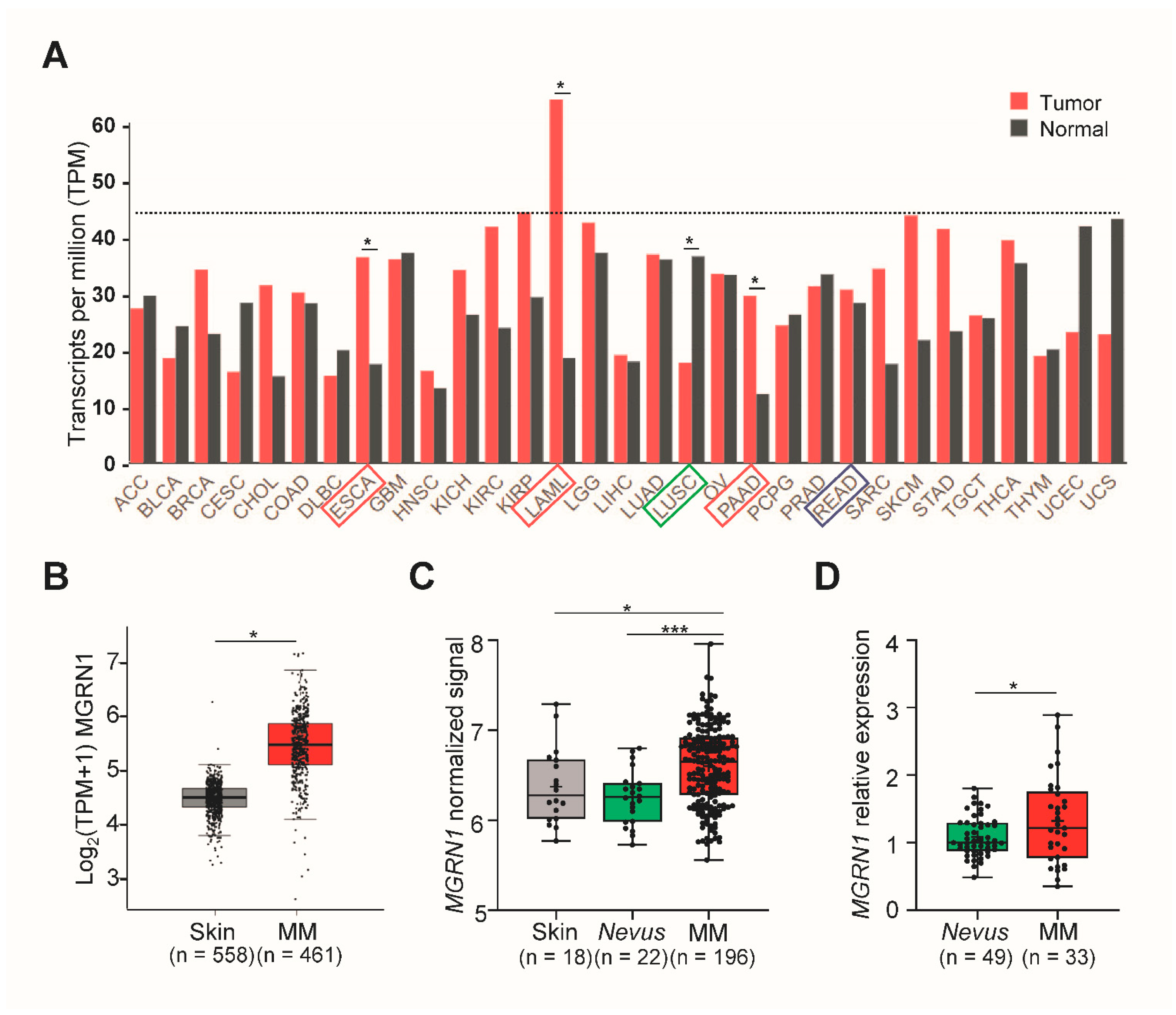
2.9. Analysis of Gene Expression from Human Databases
2.10. Real-Time RT-PCR and Digital PCR (dPCR)
2.11. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
- Transient MGRN1 depletion or permanent knockdown in human melanoma cells led to a differentiated phenotype by an apparently MITF-independent mechanism.
- Depletion of MGRN1 altered cell cycle progression and induced genomic instability by promoting the accumulation of DNA strand breaks.
- In silico analysis of melanoma datasets showed that MGRN1 expression is higher in human melanomas than in normal skin or nevi and that there is a significant correlation between lower MGRN1 expression in human melanoma with longer patient survival. This was confirmed by the estimation of MGRN1 expression in a cohort of nevi and skin melanoma specimens.
- Therefore, analysis of MGRN1 expression might provide useful clinical information. Accordingly, MGRN1 should be further analyzed as a potential melanoma biomarker.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parker, E.R. The Influence of Climate Change on Skin Cancer Incidence—A Review of the Evidence. Int. J. Women’s Dermatol. 2021, 7, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Lopes, F.C.P.S.; Sleiman, M.G.; Sebastian, K.; Bogucka, R.; Jacobs, E.A.; Adamson, A.S. UV Exposure and the Risk of Cutaneous Melanoma in Skin of Color. JAMA Dermatol. 2021, 157, 213. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, S.; Suppa, M.; Gandini, S. Melanoma Epidemiology and Sun Exposure. Acta Derm. Venereol. 2020, 100, adv00136. [Google Scholar] [CrossRef]
- O’Leary, M.A.; Wang, S.J. Epidemiology and Prevention of Cutaneous Cancer. Otolaryngol. Clin. N. Am. 2021, 54, 247–257. [Google Scholar] [CrossRef] [PubMed]
- García-Borrón, J.C.; Abdel-Malek, Z.; Jiménez-Cervantes, C. MC1R, the CAMP Pathway, and the Response to Solar UV: Extending the Horizon beyond Pigmentation. Pigment Cell Melanoma Res. 2014, 27, 699–720. [Google Scholar] [CrossRef]
- Cui, R.; Widlund, H.R.; Feige, E.; Lin, J.Y.; Wilensky, D.L.; Igras, V.E.; D’Orazio, J.; Fung, C.Y.; Schanbacher, C.F.; Granter, S.R.; et al. Central Role of P53 in the Suntan Response and Pathologic Hyperpigmentation. Cell 2007, 128, 853–864. [Google Scholar] [CrossRef] [Green Version]
- Castejón-Griñán, M.; Herraiz, C.; Olivares, C.; Jiménez-Cervantes, C.; García-Borrón, J.C. CAMP-Independent Non-Pigmentary Actions of Variant Melanocortin 1 Receptor: AKT-Mediated Activation of Protective Responses to Oxidative DNA Damage. Oncogene 2018, 37, 3631–3646. [Google Scholar] [CrossRef]
- Tagliabue, E.; Gandini, S.; Bellocco, R.; Maisonneuve, P.; Newton-Bishop, J.; Polsky, D.; Lazovich, D.; Kanetsky, P.A.; Ghiorzo, P.; Gruis, N.A.; et al. MC1R Variants as Melanoma Risk Factors Independent of At-Risk Phenotypic Characteristics: A Pooled Analysis from the M-SKIP Project. Cancer Manag. Res. 2018, 10, 1143–1154. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Laorden, B.L.; Sánchez-Más, J.; Martínez-Alonso, E.; Martínez-Menárguez, J.; García-Borrón, J.C.; Jiménez-Cervantes, C. Dimerization of the Human Melanocortin 1 Receptor: Functional Consequences and Dominant-Negative Effects. J. Investig. Dermatol. 2006, 126, 172–181. [Google Scholar] [CrossRef] [Green Version]
- Herraiz, C.; Jiménez-Cervantes, C.; Zanna, P.; García-Borrón, J.C. Melanocortin 1 Receptor Mutations Impact Differentially on Signalling to the CAMP and the ERK Mitogen-Activated Protein Kinase Pathways. FEBS Lett. 2009, 583, 3269–3274. [Google Scholar] [CrossRef] [Green Version]
- Herraiz, C.; Journé, F.; Abdel-Malek, Z.; Ghanem, G.; Jiménez-Cervantes, C.; García-Borrón, J.C. Signaling from the Human Melanocortin 1 Receptor to ERK1 and ERK2 Mitogen-Activated Protein Kinases Involves Transactivation of CKIT. Mol. Endocrinol. 2011, 25, 138–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, M.C.; Wakamatsu, K.; Ito, S.; Kadekaro, A.L.; Kobayashi, N.; Groden, J.; Kavanagh, R.; Takakuwa, T.; Virador, V.; Hearing, V.J.; et al. Human Melanocortin 1 Receptor Variants, Receptor Function and Melanocyte Response to UV Radiation. J. Cell Sci. 2002, 115, 2349–2355. [Google Scholar] [CrossRef] [PubMed]
- Leonard, J.H.; Marks, L.H.; Chen, W.; Cook, A.L.; Boyle, G.M.; Smit, D.J.; Brown, D.L.; Stow, J.L.; Parsons, P.G.; Sturm, R.A. Screening of Human Primary Melanocytes of Defined Melanocortin-1 Receptor Genotype: Pigmentation Marker, Ultrastructural and UV-Survival Studies. Pigment Cell Res. 2003, 16, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Frändberg, P.-A.; Doufexis, M.; Kapas, S.; Chhajlani, V. Human Pigmentation Phenotype: A Point Mutation Generates Nonfunctional MSH Receptor. Biochem. Biophys. Res. Commun. 1998, 245, 490–492. [Google Scholar] [CrossRef]
- Schiöth, H.B.; Phillips, S.R.; Rudzish, R.; Birch-Machin, M.; Wikberg, J.E.; Rees, J.L. Loss of Function Mutations of the Human Melanocortin 1 Receptor Are Common and Are Associated with Red Hair. Biochem. Biophys. Res. Commun. 1999, 260, 488–491. [Google Scholar] [CrossRef]
- Ringholm, A.; Klovins, J.; Rudzish, R.; Phillips, S.; Rees, J.L.; Schiöth, H.B. Pharmacological Characterization of Loss of Function Mutations of the Human Melanocortin 1 Receptor That Are Associated with Red Hair. J. Investig. Dermatol. 2004, 123, 917–923. [Google Scholar] [CrossRef] [Green Version]
- Herraiz, C.; Garcia-Borron, J.C.; Jiménez-Cervantes, C.; Olivares, C. MC1R Signaling. Intracellular Partners and Pathophysiological Implications. Biochim. Biophys. Acta 2017, 1863, 2448–2461. [Google Scholar] [CrossRef]
- Walker, W.P.; Gunn, T.M. Shades of Meaning: The Pigment-Type Switching System as a Tool for Discovery. Pigment Cell Melanoma Res. 2010, 23, 485–495. [Google Scholar] [CrossRef]
- Pérez-Oliva, A.B.; Olivares, C.; Jiménez-Cervantes, C.; García-Borrón, J.C. Mahogunin Ring Finger-1 (MGRN1) E3 Ubiquitin Ligase Inhibits Signaling from Melanocortin Receptor by Competition with Galphas. J. Biol. Chem. 2009, 284, 31714–31725. [Google Scholar] [CrossRef] [Green Version]
- Phan, L.K.; Lin, F.; LeDuc, C.; Chung, W.K.; Leibel, R.L. The Mouse Mahoganoid Coat Color Mutation Disrupts a Novel C3HC4 RING Domain Protein. J. Clin. Investig. 2002, 110, 1449–1459. [Google Scholar] [CrossRef]
- Jiao, J.; Kim, H.Y.; Liu, R.R.; Hogan, C.; Sun, K.; Tam, L.M.; Gunn, T.M. Transgenic Analysis of the Physiological Functions of Mahogunin Ring Finger-1 Isoforms. Genesis 2009, 47, 524–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chhangani, D.; Nukina, N.; Kurosawa, M.; Amanullah, A.; Joshi, V.; Upadhyay, A.; Mishra, A. Mahogunin Ring Finger 1 Suppresses Misfolded Polyglutamine Aggregation and Cytotoxicity. Biochim. Biophys. Acta 2014, 1842, 1472–1484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abrisqueta, M.; Olivares, C.; Herraiz, C.; Castejón-Griñán, M.; Sirés-Campos, J.; García-Borrón, J.C.; Jiménez-Cervantes, C. Human Melanocortin 1 Receptor-Mediated Ubiquitination of Nonvisual Arrestins. Role of Mahogunin Ring Finger 1 E3 Ligase. Biochim. Et. Biophys. Acta—Mol. Cell Res. 2018, 1865, 76–94. [Google Scholar] [CrossRef]
- He, L.; Lu, X.-Y.; Jolly, A.F.; Eldridge, A.G.; Watson, S.J.; Jackson, P.K.; Barsh, G.S.; Gunn, T.M. Spongiform Degeneration in Mahoganoid Mutant Mice. Science 2003, 299, 710–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, J.H.; Young, C.B.; Pusapati, G.; Patel, C.B.; Ho, S.; Krishnan, A.; Lin, J.-H.I.; Devine, W.; Moreau de Bellaing, A.; Athni, T.S.; et al. A Membrane-Tethered Ubiquitination Pathway Regulates Hedgehog Signaling and Heart Development. Dev. Cell 2020, 55, 432–449.e12. [Google Scholar] [CrossRef] [PubMed]
- Sirés-Campos, J.; Lambertos, A.; Delevoye, C.; Raposo, G.; Bennett, D.C.; Sviderskaya, E.; Jiménez-Cervantes, C.; Olivares, C.; García-Borrón, J.C. Mahogunin Ring Finger 1 Regulates Pigmentation by Controlling the PH of Melanosomes in Melanocytes and Melanoma Cells. Cell. Mol. Life Sci. 2021, 79, 47. [Google Scholar] [CrossRef]
- Martínez-vicente, I.; Abrisqueta, M.; Herraiz, C.; Sirés-campos, J.; Castejón-griñán, M.; Bennett, D.C.; Olivares, C.; García-borrón, J.C.; Jiménez-cervantes, C. Mahogunin Ring Finger 1 Is Required for Genomic Stability and Modulates the Malignant Phenotype of Melanoma Cells. Cancers 2020, 12, 2840. [Google Scholar] [CrossRef]
- Sevilla, A.; Morales, M.C.; Ezkurra, P.A.; Rasero, J.; Velasco, V.; Cancho-Galan, G.; Sánchez-Diez, A.; Mujika, K.; Penas, C.; Smith, I.; et al. BRAF V600E Mutational Load as a Prognosis Biomarker in Malignant Melanoma. PLoS ONE 2020, 15, e0230136. [Google Scholar] [CrossRef] [Green Version]
- Oliveros, J.C.; Franch, M.; Tabas-Madrid, D.; San-León, D.; Montoliu, L.; Cubas, P.; Pazos, F. Breaking-Cas-Interactive Design of Guide RNAs for CRISPR-Cas Experiments for ENSEMBL Genomes. Nucleic Acids Res. 2016, 44, W267–W271. [Google Scholar] [CrossRef] [Green Version]
- Goding, C.R.; Arnheiter, H. Mitf—the First 25 Years. Genes Dev. 2019, 33, 983–1007. [Google Scholar] [CrossRef] [Green Version]
- Arozarena, I.; Wellbrock, C. Phenotype Plasticity as Enabler of Melanoma Progression and Therapy Resistance. Nat. Rev. Cancer 2019, 19, 377–391. [Google Scholar] [CrossRef] [Green Version]
- Podhorecka, M.; Skladanowski, A.; Bozko, P. H2AX Phosphorylation: Its Role in DNA Damage Response and Cancer Therapy. J. Nucleic Acids 2010, 2010, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penas, C.; Apraiz, A.; Muñoa, I.; Arroyo-Berdugo, Y.; Rasero, J.; Ezkurra, P.A.; Velasco, V.; Subiran, N.; Bosserhoff, A.K.; Alonso, S.; et al. RKIP Regulates Differentiation-Related Features in Melanocytic Cells. Cancers 2020, 12, 1451. [Google Scholar] [CrossRef] [PubMed]
- Abrisqueta, M.; Herraiz, C.; Pérez Oliva, A.B.; Sanchez-Laorden, B.L.; Olivares, C.; Jiménez-Cervantes, C.; García-Borrón, J.C. Differential and Competitive Regulation of Human Melanocortin 1 Receptor Signaling by β-Arrestin Isoforms. J. Cell Sci. 2013, 126, 3724–3737. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.; Wan, L.; Hacker, E.; Dai, X.; Lenna, S.; Jimenez-Cervantes, C.; Wang, Y.; Leslie, N.R.; Xu, G.X.; Widlund, H.R.; et al. MC1R Is a Potent Regulator of PTEN after UV Exposure in Melanocytes. Mol. Cell 2013, 51, 409–422. [Google Scholar] [CrossRef] [Green Version]
- Cota, C.D.; Bagher, P.; Pelc, P.; Smith, C.O.; Bodner, C.R.; Gunn, T.M. Mice with Mutations in Mahogunin Ring Finger-1 (Mgrn1) Exhibit Abnormal Patterning of the Left-Right Axis. Dev. Dyn. 2006, 235, 3438–3447. [Google Scholar] [CrossRef] [PubMed]
- Shain, A.H.; Bastian, B.C. From Melanocytes to Melanomas. Nat. Rev. Cancer 2016, 16, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Santinon, F.; Flores González, R.E.; del Rincón, S. Melanoma Plasticity: Promoter of Metastasis and Resistance to Therapy. Front. Oncol. 2021, 11, 3703. [Google Scholar] [CrossRef]
- Rothberg, B.E.G.; Bracken, M.B.; Rimm, D.L. Tissue Biomarkers for Prognosis in Cutaneous Melanoma: A Systematic Review and Meta-Analysis. JNCI J. Natl. Cancer Inst. 2009, 101, 452–474. [Google Scholar] [CrossRef]


| siRNA | Sequence (5′-3′) |
|---|---|
| Non-Targeting siRNA (siCTR) | UAAGGCUAUGAAGAGAUAC |
| siMGRN1 1 | CGAAGGAGAUGUGGUGGAA |
| siMGRN1 2 | GAGCACCUUGUCCCUUUA |
| siMGRN1 3 | GAACUCGGCCUAUCGCUAC |
| siMGRN1 4 | AAGAUUGACUUCUCGGAAU |
| Gene | Sequence (5′-3′) |
|---|---|
| ACTB, forward primer | GACAGGATGCAGAAGGAGATCA |
| ACTB, reverse primer | GCTCAGGAGGAGCAATGATCTT |
| MGRN1, forward primer | TGGTGAACATCCGCAAAGACT |
| MGRN1, reverse primer | ATCGGCGTCGAAGGTGAAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abrisqueta, M.; Cerdido, S.; Sánchez-Beltrán, J.; Martínez-Vicente, I.; Herraiz, C.; Lambertos, A.; Olivares, C.; Sevilla, A.; Alonso, S.; Boyano, M.D.; et al. MGRN1 as a Phenotypic Determinant of Human Melanoma Cells and a Potential Biomarker. Life 2022, 12, 1118. https://doi.org/10.3390/life12081118
Abrisqueta M, Cerdido S, Sánchez-Beltrán J, Martínez-Vicente I, Herraiz C, Lambertos A, Olivares C, Sevilla A, Alonso S, Boyano MD, et al. MGRN1 as a Phenotypic Determinant of Human Melanoma Cells and a Potential Biomarker. Life. 2022; 12(8):1118. https://doi.org/10.3390/life12081118
Chicago/Turabian StyleAbrisqueta, Marta, Sonia Cerdido, José Sánchez-Beltrán, Idoya Martínez-Vicente, Cecilia Herraiz, Ana Lambertos, Conchi Olivares, Arrate Sevilla, Santos Alonso, María Dolores Boyano, and et al. 2022. "MGRN1 as a Phenotypic Determinant of Human Melanoma Cells and a Potential Biomarker" Life 12, no. 8: 1118. https://doi.org/10.3390/life12081118
APA StyleAbrisqueta, M., Cerdido, S., Sánchez-Beltrán, J., Martínez-Vicente, I., Herraiz, C., Lambertos, A., Olivares, C., Sevilla, A., Alonso, S., Boyano, M. D., García-Borrón, J. C., & Jiménez-Cervantes, C. (2022). MGRN1 as a Phenotypic Determinant of Human Melanoma Cells and a Potential Biomarker. Life, 12(8), 1118. https://doi.org/10.3390/life12081118








