Onset Timing of Hyoid Muscles Activation during Cervical Flexion Is Position-Dependent: An EMG Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
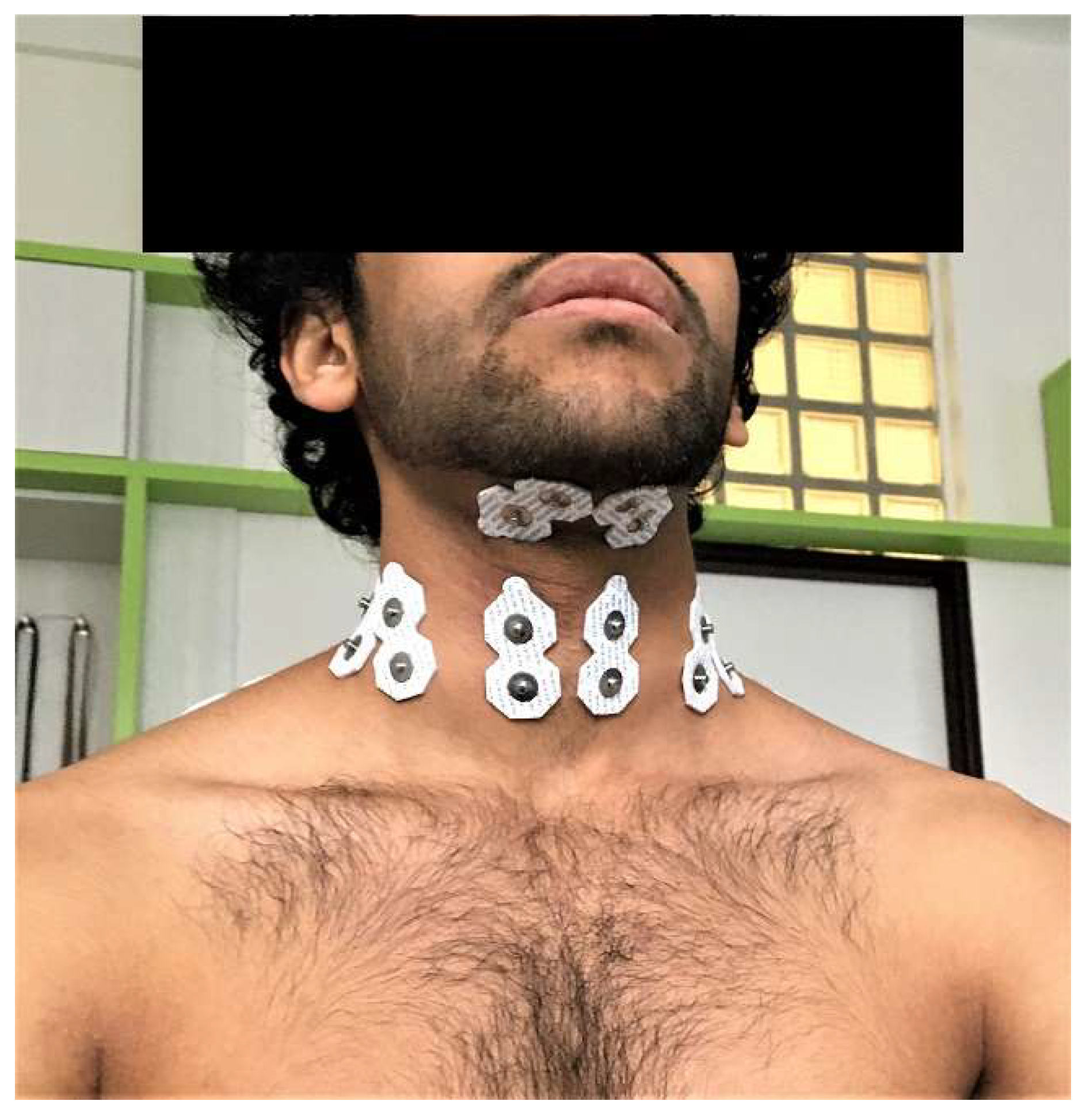
2.2. Methods
2.3. Data Proceeding
2.4. Data Analysis
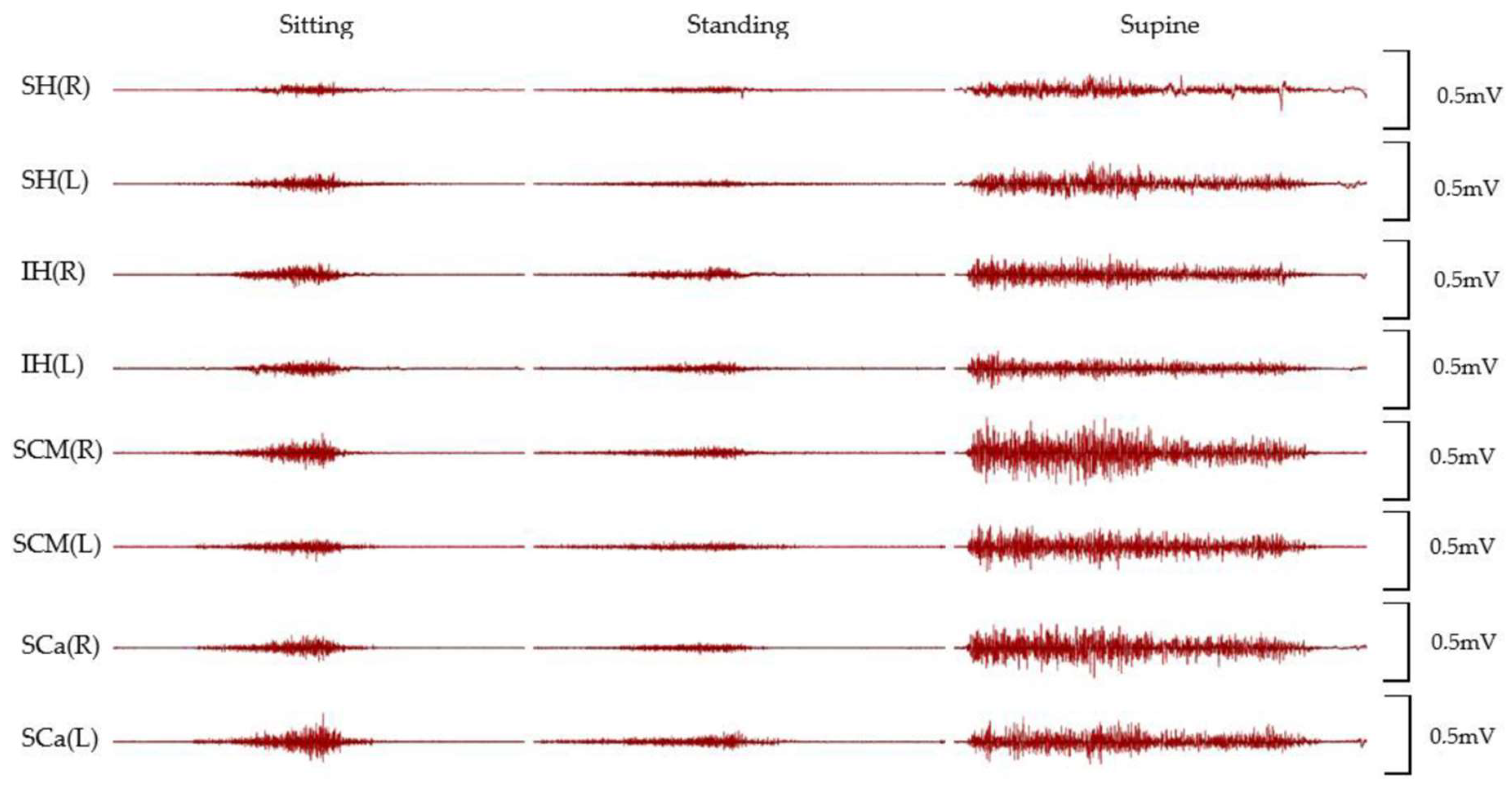
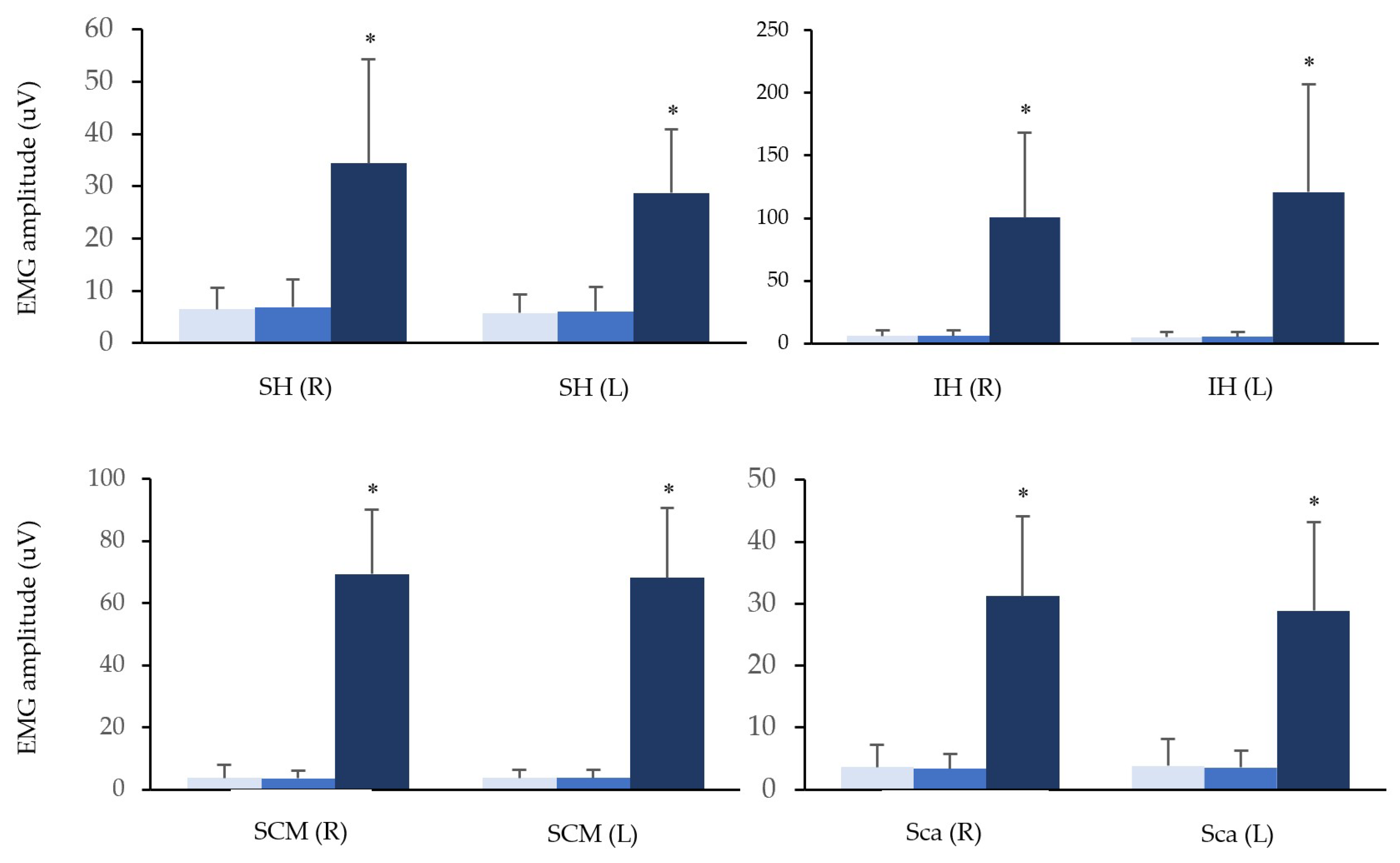
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, E.; Hoy, D.; Cross, M.; Merriman, T.R.; Vos, T.; Buchbinder, R.; Woolf, A.; March, L. The Global Burden of Gout: Estimates from the Global Burden of Disease 2010 Study. Ann. Rheum. Dis. 2014, 73, 1470–1476. [Google Scholar] [CrossRef]
- Fejer, R.; Kyvik, K.O.; Hartvigsen, J. The Prevalence of Neck Pain in the World Population: A Systematic Critical Review of the Literature. Eur. Spine J. 2006, 15, 834–848. [Google Scholar] [CrossRef] [Green Version]
- Yip, C.H.T.; Chiu, T.T.W.; Poon, A.T.K. The Relationship between Head Posture and Severity and Disability of Patients with Neck Pain. Man. Ther. 2008, 13, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Ming, Z.; Närhi, M.; Siivola, J. Neck and Shoulder Pain Related to Computer Use. Pathophysiology 2004, 11, 51–56. [Google Scholar] [CrossRef]
- McLean, S.M.; May, S.; Klaber-Moffett, J.; Sharp, D.M.; Gardiner, E. Risk Factors for the Onset of Non-Specific Neck Pain: A Systematic Review. J. Epidemiol. Community Health 2010, 64, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Kanchanomai, S.; Janwantanakul, P.; Pensri, P.; Jiamjarasrangsi, W. Risk Factors for the Onset and Persistence of Neck Pain in Undergraduate Students: 1-Year Prospective Cohort Study. BMC Public Health 2011, 11, 566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, G.R.R.; Pitangui, A.C.R.; Xavier, M.K.A.; Correia-Júnior, M.A.V.; De Araújo, R.C. Prevalence of Musculoskeletal Pain in Adolescents and Association with Computer and Videogame Use. J. Pediatr. 2016, 92, 188–196. [Google Scholar] [CrossRef] [Green Version]
- Cohen, S.P. Epidemiology, Diagnosis, and Treatment of Neck Pain. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2015; Volume 90, pp. 284–299. [Google Scholar] [CrossRef] [Green Version]
- Jull, G.A.; O’Leary, S.P.; Falla, D.L. Clinical Assessment of the Deep Cervical Flexor Muscles: The Craniocervical Flexion Test. J. Manip. Physiol. Ther. 2008, 31, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Falla, D.L.; Jull, G.A.; Hodges, P.W. Patients with Neck Pain Demonstrate Reduced Electromyographic Activity of the Deep Cervical Flexor Muscles during Performance of the Craniocervical Flexion Test. Spine 2004, 29, 2108–2114. [Google Scholar] [CrossRef]
- Jull, G.; Falla, D. Does Increased Superficial Neck Flexor Activity in the Craniocervical Flexion Test Reflect Reduced Deep Flexor Activity in People with Neck Pain? Man. Ther. 2016, 25, 43–47. [Google Scholar] [CrossRef] [Green Version]
- Page, P.; Frank, C.C.; Lardner, R. Assessment and Treatment of Muscle Imbalance: The Janda Approach; Human Kinetics: Champaign, IL, USA, 2009; ISBN 0736074007. [Google Scholar]
- Hodges, P.W.; Tucker, K. Moving Differently in Pain: A New Theory to Explain the Adaptation to Pain. Pain 2011, 152 (Suppl. S3), S90–S98. [Google Scholar] [CrossRef]
- Page, P. Cervicogenic Headaches: An Evidence-Led Approach to Clinical Management. Int. J. Sports Phys. Ther. 2011, 6, 254–266. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3201065/ (accessed on 1 November 2021).
- Sterling, M.; Jull, G.; Vicenzino, B.; Kenardy, J. Characterization of Acute Whiplash-Associated Disorders. Spine 2004, 29, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Jull, G.; Kristjansson, E.; Dall’Alba, P. Impairment in the Cervical Flexors: A Comparison of Whiplash and Insidious Onset Neck Pain Patients. Man. Ther. 2004, 9, 89–94. [Google Scholar] [CrossRef]
- O’Leary, S.; Falla, D.; Jull, G.; Vicenzino, B. Muscle Specificity in Tests of Cervical Flexor Muscle Performance. J. Electromyogr. Kinesiol. 2007, 17, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Lascurain-Aguirrebeña, I.; Newham, D.J.; Galarraga-Gallastegui, B.; Critchley, D.J. Differences in Neck Surface Electromyography, Kinematics and Pain Occurrence during Physiological Neck Movements between Neck Pain and Asymptomatic Participants. A Cross-Sectional Study. Clin. Biomech. 2018, 57, 1–9. [Google Scholar] [CrossRef]
- Forsberg, C.M.; Hellsing, E.; Linder-Aronson, S.; Sheikholeslam, A. EMG Activity in Neck and Masticatory Muscles in Relation to Extension and Flexion of the Head. Eur. J. Orthod. 1985, 7, 177–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moroney, S.P.; Schultz, A.B.; Miller, J.A.A. Analysis and Measurement of Neck Loads. J. Orthop. Res. 1988, 6, 713–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegmund, G.P.; Blouin, J.S.; Brault, J.R.; Hedenstierna, S.; Inglis, J.T. Electromyography of Superficial and Deep Neck Muscles during Isometric, Voluntary, and Reflex Contractions. J. Biomech. Eng. 2007, 129, 66–77. [Google Scholar] [CrossRef] [Green Version]
- Chancey, V.C.; Nightingale, R.W.; Van Ee, C.A.; Knaub, K.E.; Myers, B.S. Improved Estimation of Human Neck Tensile Tolerance: Reducing the Range of Reported Tolerance Using Anthropometrically Correct Muscles and Optimized Physiologic Initial Conditions. SAE Tech. Pap. 2003, 47, 135–153. [Google Scholar] [CrossRef]
- Matsuo, K.; Palmer, J.B. Anatomy and Physiology of Feeding and Swallowing: Normal and Abnormal. Phys. Med. Rehabil. Clin. N. Am. 2008, 19, 691–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitti, M.; Fujiwara, M.; Basmajian, J.V.; Iida, M. The Integrated Roles of Longus Colli and Sternocleidomastoid Muscles: An Electromyographic Study. Anat. Rec. 1973, 177, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, J.D.; Vasavada, A.N.; Merryweather, A.S. The Inclusion of Hyoid Muscles Improve Moment Generating Capacity and Dynamic Simulations in Musculoskeletal Models of the Head and Neck. PLoS ONE 2018, 13, e0199912. [Google Scholar] [CrossRef] [PubMed]
- Falla, D.; Jull, G.; O’Leary, S.; Dall’Alba, P. Further Evaluation of an EMG Technique for Assessment of the Deep Cervical Flexor Muscles. J. Electromyogr. Kinesiol. 2006, 16, 621–628. [Google Scholar] [CrossRef]
- Pialasse, J.P.; Dubois, J.D.; Pilon Choquette, M.H.; Lafond, D.; Descarreaux, M. Kinematic and Electromyographic Parameters of the Cervical Flexion-Relaxation Phenomenon: The Effect of Trunk Positioning. Ann. Phys. Rehabil. Med. 2009, 52, 49–58. [Google Scholar] [CrossRef] [Green Version]
- Pialasse, J.P.; Lafond, D.; Cantin, V.; Descarreaux, M. Load and Speed Effects on the Cervical Flexion Relaxation Phenomenon. BMC Musculoskelet. Disord. 2010, 11, 46. [Google Scholar] [CrossRef] [Green Version]
- Douglas, E.C.; Gallagher, K.M. Are the Neck Positions and Muscle Activity Observed When Reading a Tablet Similar to That of the Cervical Flexion-Relaxation Onset? IISE Trans. Occup. Ergon. Hum. Factors 2018, 6, 43–50. [Google Scholar] [CrossRef]
- Olson, M.; Solomonow, M.; Li, L. Flexion-Relaxation Response to Gravity. J. Biomech. 2006, 39, 2545–2554. [Google Scholar] [CrossRef]
- Falla, D.; Dall’Alba, P.; Rainoldi, A.; Merletti, R.; Jull, G. Location of Innervation Zones of Sternocleidomastoid and Scalene Muscles—A Basis for Clinical and Research Electromyography Applications. Clin. Neurophysiol. 2002, 113, 57–63. [Google Scholar] [CrossRef]
- Konrad, P. Noraxon: The ABC of EMG. 2006. ISBN 0-9771622-1-4. Available online: https://www.noraxon.com/wp-content/uploads/2014/12/ABC-EMG-ISBN.pdf (accessed on 12 September 2021).
- Kendall, F.P.; McCreary, E.K.; Provance, P.G.; Rodgers, M.M.I.; Romani, W.A. Muscles: Testing and Function, with Posture and Pain, 5th ed.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2005; ISBN 0781747805. [Google Scholar]
- Vikne, H.; Bakke, E.S.; Liestøl, K.; Sandbæk, G.; Vøllestad, N. The Smoothness of Unconstrained Head Movements Is Velocity-Dependent. Hum. Mov. Sci. 2013, 32, 540–554. [Google Scholar] [CrossRef]
- Hug, F. Can Muscle Coordination Be Precisely Studied by Surface Electromyography? J. Electromyogr. Kinesiol. 2011, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.H.; Lin, K.H.; Wang, J.L. Co-Contraction of Cervical Muscles during Sagittal and Coronal Neck Motions at Different Movement Speeds. Eur. J. Appl. Physiol. 2008, 103, 647–654. [Google Scholar] [CrossRef]
- Queisser, F.; Blüthner, R.; Bräuer, D.; Seidel, H. The Relationship between the Electromyogram-Amplitude and Isometric Extension Torques of Neck Muscles at Different Positions of the Cervical Spine. Eur. J. Appl. Physiol. Occup. Physiol. 1994, 68, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Sommerich, C.M.; Joines, S.M.B.; Hermans, V.; Moon, S.D. Use of Surface Electromyography to Estimate Neck Muscle Activity. J. Electromyogr. Kinesiol. 2000, 10, 377–398. [Google Scholar] [CrossRef]
- Drake, R.L. Gray’s Anatomy for Students, 4th ed.; Elsevier/Churchill Livingstone: Philadelphia, PA, USA, 2019; ISBN 0323393047. [Google Scholar]
- Gilroy, A.M.; MacPherson, B.R.; Ross, L.M.; Broman, J.; Josephson, A. Atlas of Anatomy, 4th ed.; Thieme: Stuttgart, Germany, 2020; ISBN 1684202035. [Google Scholar]
- Kapandji, A. The Physiology of the Joints—Volume 3: The Vertebral Column, Pelvic Girdle and Head, 7th ed.; Handspring Publishing: London, UK, 2019; ISBN 1912085615. [Google Scholar]
- Zheng, L.; Jahn, J.; Vasavada, A.N. Sagittal Plane Kinematics of the Adult Hyoid Bone. J. Biomech. 2012, 45, 531–536. [Google Scholar] [CrossRef] [Green Version]
- Vasavada, A.N.; Li, S.; Delp, S.L. Influence of Muscle Morphometry and Moment Arms on the Moment-Generating Capacity of Human Neck Muscles. Spine 1998, 23, 412–422. [Google Scholar] [CrossRef]
- Ackland, D.C.; Merritt, J.S.; Pandy, M.G. Moment Arms of the Human Neck Muscles in Flexion, Bending and Rotation. J. Biomech. 2011, 44, 475–486. [Google Scholar] [CrossRef]
- Kippers, V.; Parker, A.W. Posture Related to Myoelectric Silence of Erectores Spinae during Trunk Flexion. Spine 1984, 9, 740–745. [Google Scholar] [CrossRef] [Green Version]
- Dorel, S.; Guilhem, G.; Couturier, A.; Hug, F. Adjustment of Muscle Coordination during an All-out Sprint Cycling Task. Med. Sci. Sports Exerc. 2012, 44, 2154–2164. [Google Scholar] [CrossRef] [Green Version]
- Youdas, J.W.; Garrett, T.R.; Suman, V.J.; Bogard, C.L.; Hallman, H.O.; Carey, J.R. Normal Range of Motion of the Cervical Spine: An Initial Goniometric Study. Phys. Ther. 1992, 72, 770–780. [Google Scholar] [CrossRef]
- Falla, D.; Jull, G.; Dall’Alba, P.; Rainoldi, A.; Merletti, R. An Electromyographic Analysis of the Deep Cervical Flexor Muscles in Performance of Craniocervical Flexion. Phys. Ther. 2003, 83, 899–906. [Google Scholar] [CrossRef] [Green Version]
- Cresswell, A.G.; Oddsson, L.; Thorstensson, A. The Influence of Sudden Perturbations on Trunk Muscle Activity and Intra-Abdominal Pressure While Standing. Exp. Brain Res. 1994, 98, 336–341. [Google Scholar] [CrossRef]
- Hodges, P.W.; Richardson, C.A. Contraction of the Abdominal Muscles Associated with Movement of the Lower Limb. Phys. Ther. 1997, 77, 132–144. [Google Scholar] [CrossRef]

| Age | Height (m) | Weight (kg) | BMI | |
|---|---|---|---|---|
| Participants (n = 20) | 23.3 (2.70) | 1.70 (0.08) | 66.8 (12.4) | 23.0 (3.22) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sageshima, H.; Pavlů, D.; Dvořáčková, D.; Pánek, D. Onset Timing of Hyoid Muscles Activation during Cervical Flexion Is Position-Dependent: An EMG Study. Life 2022, 12, 949. https://doi.org/10.3390/life12070949
Sageshima H, Pavlů D, Dvořáčková D, Pánek D. Onset Timing of Hyoid Muscles Activation during Cervical Flexion Is Position-Dependent: An EMG Study. Life. 2022; 12(7):949. https://doi.org/10.3390/life12070949
Chicago/Turabian StyleSageshima, Hirofumi, Dagmar Pavlů, Dominika Dvořáčková, and David Pánek. 2022. "Onset Timing of Hyoid Muscles Activation during Cervical Flexion Is Position-Dependent: An EMG Study" Life 12, no. 7: 949. https://doi.org/10.3390/life12070949
APA StyleSageshima, H., Pavlů, D., Dvořáčková, D., & Pánek, D. (2022). Onset Timing of Hyoid Muscles Activation during Cervical Flexion Is Position-Dependent: An EMG Study. Life, 12(7), 949. https://doi.org/10.3390/life12070949






