Fractal Dimension Analysis of Melanocytic Nevi and Melanomas in Normal and Polarized Light—A Preliminary Report
Abstract
1. Introduction
- There are no differences in area, perimeter, and fractal dimensions of lesions in normal and polarized illumination in each examined group (melanoma, dysplastic nevus, benign nevus);
- There are no differences in fractal dimensions of lesion shapes in the groups (melanoma, dysplastic nevus, benign nevus);
- There are no differences in fractal dimensions of lesion surface structures in the groups (melanoma, dysplastic nevus, benign nevus);
- There is no correlation between fractal dimensions and the area or perimeter of lesions in normal and polarized light in the groups (melanoma, dysplastic nevus, benign nevus).
2. Materials and Methods
2.1. Patients and Lesions
2.2. Photographs
2.3. Basic Image Analysis
2.4. Image Processing
2.4.1. Preparing Images for Shape Analysis
2.4.2. Preparing Images for Surface Feature Analysis
2.5. Fractal Dimension Analysis
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
- Fractal dimension analysis of images in polarized light enables distinguishing melanomas, dysplastic nevi, and benign nevi in shape. It also makes it possible to distinguish melanomas from benign and dysplastic nevi under non-polarized light.
- Fractal dimension of texture allows distinguishing melanomas from benign and dysplastic nevi under polarized light
- Polarized light is superior to non-polarized light for imaging details of pigmented lesions, especially their borders. It offers better material as the base for fractal dimension analysis than non-polarized light images.
- It is possible to distinguish between in situ and invasive melanoma in shape and surface structure (in both polarized and non-polarized light).
- Fractal dimensions of shape and texture are parameters useful for developing automatic computer-based diagnosis systems.
- There is no correlation between the fractal dimensions and area or perimeter of lesions in normal and polarized light in the melanoma and dysplastic nevus groups.
- There is a negative correlation between the fractal dimensions and area or perimeter of lesions in normal (r = −0.45) and polarized light (r = −0.72) in the benign nevus group.
- There are no differences in area, perimeter, and fractal dimensions of lesions in normal and polarized illumination in the examined group
- There are no differences in fractal dimensions of lesion shapes in the groups (melanoma, dysplastic nevus, benign nevus);
- There are no differences in fractal dimensions of lesion surface structures in the groups
- There is no correlation between the fractal dimensions and area or perimeter of lesions in normal and polarized light in the benign nevus group.
6. Study Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lentsch, G.; Valdebran, M.; Saknite, I.; Smith, J.; Linden, K.G.; König, K.; Barr, R.J.; Harris, R.M.; Tromberg, B.J.; Ganesan, A.K.; et al. Non-invasive Optical Biopsy by Multiphoton Microscopy Identifies the Live Morphology of Common Melanocytic Nevi. Pigment. Cell Melanoma Res. 2020, 33, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Damsky, W.E.; Bosenberg, M. Melanocytic Nevi and Melanoma: Unraveling a Complex Relationship. Oncogene 2017, 36, 5771–5792. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Garbe, C. Acquired Melanocytic Nevi as Risk Factor for Melanoma Development. A Comprehensive Review of Epidemiological Data. Pigment. Cell Res. 2003, 16, 297–306. [Google Scholar] [CrossRef]
- Tsao, H.; Bevona, C.; Goggins, W.; Quinn, T. The Transformation Rate of Moles (Melanocytic Nevi) into Cutaneous Melanoma: A Population-Based Estimate. Arch. Dermatol. 2003, 139, 282–288. [Google Scholar] [CrossRef]
- Bevona, C.; Goggins, W.; Quinn, T.; Fullerton, J.; Tsao, H. Cutaneous Melanomas Associated with Nevi. Arch. Dermatol. 2003, 139, 1620–1624, discussion 1624. [Google Scholar] [CrossRef] [PubMed]
- Clark, W.H.; Reimer, R.R.; Greene, M.; Ainsworth, A.M.; Mastrangelo, M.J. Origin of Familial Malignant Melanomas from Heritable Melanocytic Lesions. “The B-K Mole Syndrome”. Arch. Dermatol. 1978, 114, 732–738. [Google Scholar] [CrossRef]
- Lynch, H.T.; Frichot, B.C.; Lynch, J.F. Familial Atypical Multiple Mole-Melanoma Syndrome. J. Med. Genet. 1978, 15, 352–356. [Google Scholar] [CrossRef]
- Goldstein, A.M.; Tucker, M.A. Dysplastic Nevi and Melanoma. Cancer Epidemiol. Biomark. Prev. 2013, 22, 528–532. [Google Scholar] [CrossRef]
- Baigrie, D.; Tanner, L.S. Dysplastic Nevi. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Roh, M.R.; Eliades, P.; Gupta, S.; Tsao, H. Genetics of Melanocytic Nevi. Pigment. Cell Melanoma Res. 2015, 28, 661–672. [Google Scholar] [CrossRef]
- Garbe, C.; Amaral, T.; Peris, K.; Hauschild, A.; Arenberger, P.; Bastholt, L.; Bataille, V.; del Marmol, V.; Dréno, B.; Fargnoli, M.C.; et al. European Consensus-Based Interdisciplinary Guideline for Melanoma. Part 1: Diagnostics—Update 2019. Eur. J. Cancer 2020, 126, 141–158. [Google Scholar] [CrossRef]
- Davey, M.G.; Miller, N.; McInerney, N.M. A Review of Epidemiology and Cancer Biology of Malignant Melanoma. Cureus 2021, 13, e15087. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Marghoob, N.G.; Liopyris, K.; Jaimes, N. Dermoscopy: A Review of the Structures That Facilitate Melanoma Detection. J. Osteopath. Med. 2019, 119, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Gareau, D.S.; Scope, A.; Rajadhyaksha, M.; Mullani, N.A.; Marghoob, A.A. Polarized and Nonpolarized Dermoscopy. Arch. Dermatol. 2008, 144, 828–829. [Google Scholar] [CrossRef] [PubMed]
- Braun, R.P.; Rabinovitz, H.S.; Oliviero, M.; Kopf, A.W.; Saurat, J.-H. Dermoscopy of Pigmented Skin Lesions. J. Am. Acad. Derm. 2005, 52, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Annessi, G.; Bono, R.; Sampogna, F.; Faraggiana, T.; Abeni, D. Sensitivity, Specificity, and Diagnostic Accuracy of Three Dermoscopic Algorithmic Methods in the Diagnosis of Doubtful Melanocytic Lesions. J. Am. Acad. Dermatol. 2007, 56, 759–767. [Google Scholar] [CrossRef]
- Dinnes, J.; Deeks, J.J.; Chuchu, N.; Ferrante di Ruffano, L.; Matin, R.N.; Thomson, D.R.; Wong, K.Y.; Aldridge, R.B.; Abbott, R.; Fawzy, M.; et al. Dermoscopy, with and without Visual Inspection, for Diagnosing Melanoma in Adults. Cochrane Database Syst. Rev. 2018, 2018. [Google Scholar] [CrossRef]
- Kittler, H.; Pehamberger, H.; Wolff, K.; Binder, M. Diagnostic Accuracy of Dermoscopy. Lancet Oncol. 2002, 3, 159–165. [Google Scholar] [CrossRef]
- Shahriari, N.; Rabinovitz, H.; Oliviero, M.; Grant-Kels, J.M. Reflectance Confocal Microscopy: Melanocytic and Nonmelanocytic. Clin. Dermatol. 2021, 39, 643–656. [Google Scholar] [CrossRef]
- Shahriari, N.; Grant-Kels, J.M.; Rabinovitz, H.; Oliviero, M.; Scope, A. Reflectance Confocal Microscopy. J. Am. Acad. Dermatol. 2021, 84, 1–14. [Google Scholar] [CrossRef]
- Dinnes, J.; Deeks, J.J.; Saleh, D.; Chuchu, N.; Bayliss, S.E.; Patel, L.; Davenport, C.; Takwoingi, Y.; Godfrey, K.; Matin, R.N.; et al. Reflectance Confocal Microscopy for Diagnosing Cutaneous Melanoma in Adults. Cochrane Database Syst. Rev. 2018, 12. [Google Scholar] [CrossRef]
- Olsen, J.; Holmes, J.; Jemec, G.B.E. Advances in Optical Coherence Tomography in Dermatology—A Review. J. Biomed. Opt. 2018, 23, 040901. [Google Scholar] [CrossRef] [PubMed]
- Rajabi-Estarabadi, A.; Bittar, J.M.; Zheng, C.; Nascimento, V.; Camacho, I.; Feun, L.G.; Nasiriavanaki, M.; Kunz, M.; Nouri, K. Optical Coherence Tomography Imaging of Melanoma Skin Cancer. Lasers Med. Sci. 2019, 34, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Polańska, A.; Jenerowicz, D.; Paszyńska, E.; Żaba, R.; Adamski, Z.; Dańczak-Pazdrowska, A. High-Frequency Ultrasonography—Possibilities and Perspectives of the Use of 20 MHz in Teledermatology. Front. Med. 2021, 8, 619965. [Google Scholar] [CrossRef]
- Scotto di Santolo, M.; Sagnelli, M.; Mancini, M.; Scalvenzi, M.; Delfino, M.; Schonauer, F.; Molea, G.; Ayala, F.; Salvatore, M. High-Resolution Color-Doppler Ultrasound for the Study of Skin Growths. Arch. Dermatol. Res. 2015, 307, 559–566. [Google Scholar] [CrossRef]
- Barber, C.; Boiko, S. Tape Stripping: Investigational, Diagnostic and Therapeutic Uses in Dermatology. Clin. Dermatol. 2022. [Google Scholar] [CrossRef]
- Bollard, S.M.; Casalou, C.; Potter, S.M. Gene Expression Profiling in Melanoma: A View from the Clinic. Cancer Treat. Res. Commun. 2021, 29, 100447. [Google Scholar] [CrossRef]
- Ferrante di Ruffano, L.; Takwoingi, Y.; Dinnes, J.; Chuchu, N.; Bayliss, S.E.; Davenport, C.; Matin, R.N.; Godfrey, K.; O’Sullivan, C.; Gulati, A.; et al. Computer-Assisted Diagnosis Techniques (Dermoscopy and Spectroscopy-Based) for Diagnosing Skin Cancer in Adults. Cochrane Database Syst. Rev. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Gomory, R. Benoît Mandelbrot (1924–2010). Nature 2010, 468, 378. [Google Scholar] [CrossRef] [PubMed]
- Breki, C.-M.; Dimitrakopoulou-Strauss, A.; Hassel, J.; Theoharis, T.; Sachpekidis, C.; Pan, L.; Provata, A. Fractal and Multifractal Analysis of PET/CT Images of Metastatic Melanoma before and after Treatment with Ipilimumab. EJNMMI Res. 2016, 6, 61. [Google Scholar] [CrossRef]
- Bedin, V.; Adam, R.L.; de Sá, B.C.; Landman, G.; Metze, K. Fractal Dimension of Chromatin Is an Independent Prognostic Factor for Survival in Melanoma. BMC Cancer 2010, 10, 260. [Google Scholar] [CrossRef] [PubMed]
- Fiz, J.A.; Monte-Moreno, E.; Andreo, F.; Auteri, S.J.; Sanz-Santos, J.; Serra, P.; Bonet, G.; Castellà, E.; Manzano, J.R. Fractal Dimension Analysis of Malignant and Benign Endobronchial Ultrasound Nodes. BMC Med. Imaging 2014, 14, 22. [Google Scholar] [CrossRef] [PubMed]
- Captur, G.; Karperien, A.L.; Li, C.; Zemrak, F.; Tobon-Gomez, C.; Gao, X.; Bluemke, D.A.; Elliott, P.M.; Petersen, S.E.; Moon, J.C. Fractal Frontiers in Cardiovascular Magnetic Resonance: Towards Clinical Implementation. J. Cardiovasc. Magn. Reson. 2015, 17, 80. [Google Scholar] [CrossRef] [PubMed]
- Trafalski, M.; Kozakiewicz, M.; Jurczyszyn, K. Application of Fractal Dimension and Texture Analysis to Evaluate the Effectiveness of Treatment of a Venous Lake in the Oral Mucosa Using a 980 Nm Diode Laser—A Preliminary Study. Materials 2021, 14, 4140. [Google Scholar] [CrossRef] [PubMed]
- Plasmeijer, E.I.; Nguyen, T.-M.-U.; Olsen, C.M.; Janda, M.; Soyer, H.P.; Green, A.C. The Natural History of Common Melanocytic Nevi: A Systematic Review of Longitudinal Studies in the General Population. J. Investig. Dermatol. 2017, 137, 2017–2018. [Google Scholar] [CrossRef]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Barsouk, A. Epidemiology of Melanoma. Med. Sci. 2021, 9, 63. [Google Scholar] [CrossRef]
- Conforti, C.; Zalaudek, I. Epidemiology and Risk Factors of Melanoma: A Review. Dermatol. Pract. Concept. 2021, 11 (Suppl. S1), e2021161S. [Google Scholar] [CrossRef]
- Liu-Smith, F.; Farhat, A.M.; Arce, A.; Ziogas, A.; Taylor, T.; Wang, Z.; Yourk, V.; Liu, J.; Wu, J.; McEligot, A.J.; et al. Sex Differences in the Association of Cutaneous Melanoma Incidence Rates and Geographic Ultraviolet Light Exposure. J. Am. Acad. Dermatol. 2017, 76, 499–505.e3. [Google Scholar] [CrossRef]
- Abbasi, N.R.; Yancovitz, M.; Gutkowicz-Krusin, D.; Panageas, K.S.; Mihm, M.C.; Googe, P.; King, R.; Prieto, V.; Osman, I.; Friedman, R.J.; et al. Utility of Lesion Diameter in the Clinical Diagnosis of Cutaneous Melanoma. Arch. Dermatol. 2008, 144, 469–474. [Google Scholar] [CrossRef][Green Version]
- Walter, F.M.; Prevost, A.T.; Vasconcelos, J.; Hall, P.N.; Burrows, N.P.; Morris, H.C.; Kinmonth, A.L.; Emery, J.D. Using the 7-Point Checklist as a Diagnostic Aid for Pigmented Skin Lesions in General Practice: A Diagnostic Validation Study. Br. J. Gen. Pract. 2013, 63, e345–e353. [Google Scholar] [CrossRef]
- Dickman, J.S.; Haddad, R.M.; Racette, A. Predictive Value of Positive Margins in Diagnostic Biopsies of Dysplastic Nevi. Dermatol. Res. Pract. 2020, 2020, 6716145. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.J.; Shin, T.M.; Sobanko, J.F.; Sharkey, J.M.; Grunyk, J.W.; Elenitsas, R.; Chu, E.Y.; Capell, B.C.; Ming, M.E.; Etzkorn, J.R. Risk Factors for Positive or Equivocal Margins after Wide Local Excision of 1345 Cutaneous Melanomas. J. Am. Acad. Dermatol. 2017, 77, 333–340.e1. [Google Scholar] [CrossRef]
- Kim, C.C.; Berry, E.G.; Marchetti, M.A.; Swetter, S.M.; Lim, G.; Grossman, D.; Curiel-Lewandrowski, C.; Chu, E.Y.; Ming, M.E.; Zhu, K.; et al. Risk of Subsequent Cutaneous Melanoma in Moderately Dysplastic Nevi Excisionally Biopsied but With Positive Histologic Margins. JAMA Dermatol. 2018, 154, 1401. [Google Scholar] [CrossRef] [PubMed]
- Ross, M.I.; Balch, C.M. Excision Margins of Melanoma Make a Difference: New Data Support an Old Paradigm. Ann. Surg. Oncol. 2016, 23, 1053–1056. [Google Scholar] [CrossRef] [PubMed]
- Haydu, L.E.; Stollman, J.T.; Scolyer, R.A.; Spillane, A.J.; Quinn, M.J.; Saw, R.P.M.; Shannon, K.F.; Stretch, J.R.; Bonenkamp, J.J.; Thompson, J.F. Minimum Safe Pathologic Excision Margins for Primary Cutaneous Melanomas (1–2 Mm in Thickness): Analysis of 2131 Patients Treated at a Single Center. Ann. Surg. Oncol. 2016, 23, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Losa, G.A.; Nonnenmacher, T.F. Self-Similarity and Fractal Irregularity in Pathologic Tissues. Mod. Pathol. Off. J. U. S. Can. Acad. Pathol. Inc. 1996, 9, 174–182. [Google Scholar]
- Moroni, F.; Magnoni, M.; Vergani, V.; Ammirati, E.; Camici, P.G. Fractal Analysis of Plaque Border, a Novel Method for the Quantification of Atherosclerotic Plaque Contour Irregularity, Is Associated with pro-Atherogenic Plasma Lipid Profile in Subjects with Non-Obstructive Carotid Stenoses. PLoS ONE 2018, 13, e0192600. [Google Scholar] [CrossRef]
- Minati, L.; Frasca, M.; Giustolisi, G.; Oświȩcimka, P.; Drożdż, S.; Ricci, L. High-Dimensional Dynamics in a Single-Transistor Oscillator Containing Feynman-Sierpiński Resonators: Effect of Fractal Depth and Irregularity. Chaos Interdiscip. J. Nonlinear Sci. 2018, 28, 093112. [Google Scholar] [CrossRef]
- Benvenuto-Andrade, C.; Dusza, S.W.; Agero, A.L.C.; Scope, A.; Rajadhyaksha, M.; Halpern, A.C.; Marghoob, A.A. Differences Between Polarized Light Dermoscopy and Immersion Contact Dermoscopy for the Evaluation of Skin Lesions. Arch. Dermatol. 2007, 143, 329–338. [Google Scholar] [CrossRef]
- Wang, S.Q.; Dusza, S.W.; Scope, A.; Braun, R.P.; Kopf, A.W.; Marghoob, A.A. Differences in Dermoscopic Images from Nonpolarized Dermoscope and Polarized Dermoscope Influence the Diagnostic Accuracy and Confidence Level: A Pilot Study. Dermatol. Surg. 2008, 34, 1389–1395. [Google Scholar] [CrossRef][Green Version]
- Piantanelli, A.; Maponi, P.; Scalise, L.; Serresi, S.; Cialabrini, A.; Basso, A. Fractal Characterisation of Boundary Irregularity in Skin Pigmented Lesions. Med. Biol. Eng. Comput. 2005, 43, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Carbonetto, S.H.; Lew, S.E. Characterization of Border Structure Using Fractal Dimension in Melanomas. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 4088–4091. [Google Scholar]
- Cross, S.S.; McDonagh, A.J.G.; Stephenson, T.J.; Cotton, D.W.K.; Underwood, J.C.E. Fractal and Integer-Dimensional Geometric Analysis of Pigmented Skin Lesions. Am. J. Dermatopathol. 1995, 17, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Jurczyszyn, K.; Trzeciakowski, W.; Kozakiewicz, M.; Kida, D.; Malec, K.; Karolewicz, B.; Konopka, T.; Zborowski, J. Fractal Dimension and Texture Analysis of Lesion Autofluorescence in the Evaluation of Oral Lichen Planus Treatment Effectiveness. Materials 2021, 14, 5448. [Google Scholar] [CrossRef] [PubMed]
- Moldovanu, S.; Damian Michis, F.A.; Biswas, K.C.; Culea-Florescu, A.; Moraru, L. Skin Lesion Classification Based on Surface Fractal Dimensions and Statistical Color Cluster Features Using an Ensemble of Machine Learning Techniques. Cancers 2021, 13, 5256. [Google Scholar] [CrossRef]
- Jenkins, R.W.; Fisher, D.E. Treatment of Advanced Melanoma in 2020 and Beyond. J. Investig. Dermatol. 2021, 141, 23–31. [Google Scholar] [CrossRef]
- Davis, L.E.; Shalin, S.C.; Tackett, A.J. Current State of Melanoma Diagnosis and Treatment. Cancer Biol. Ther. 2019, 20, 1366–1379. [Google Scholar] [CrossRef]
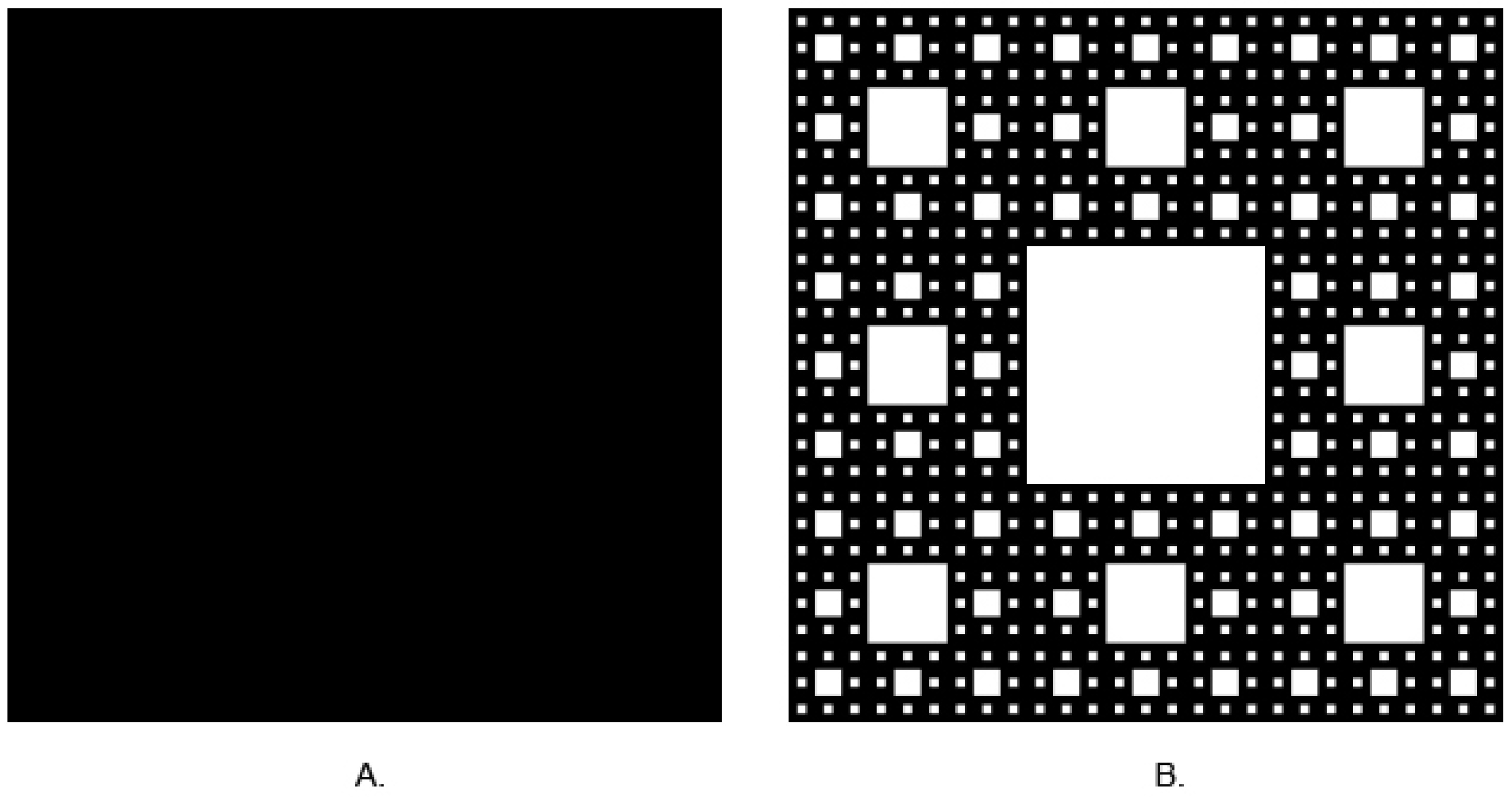
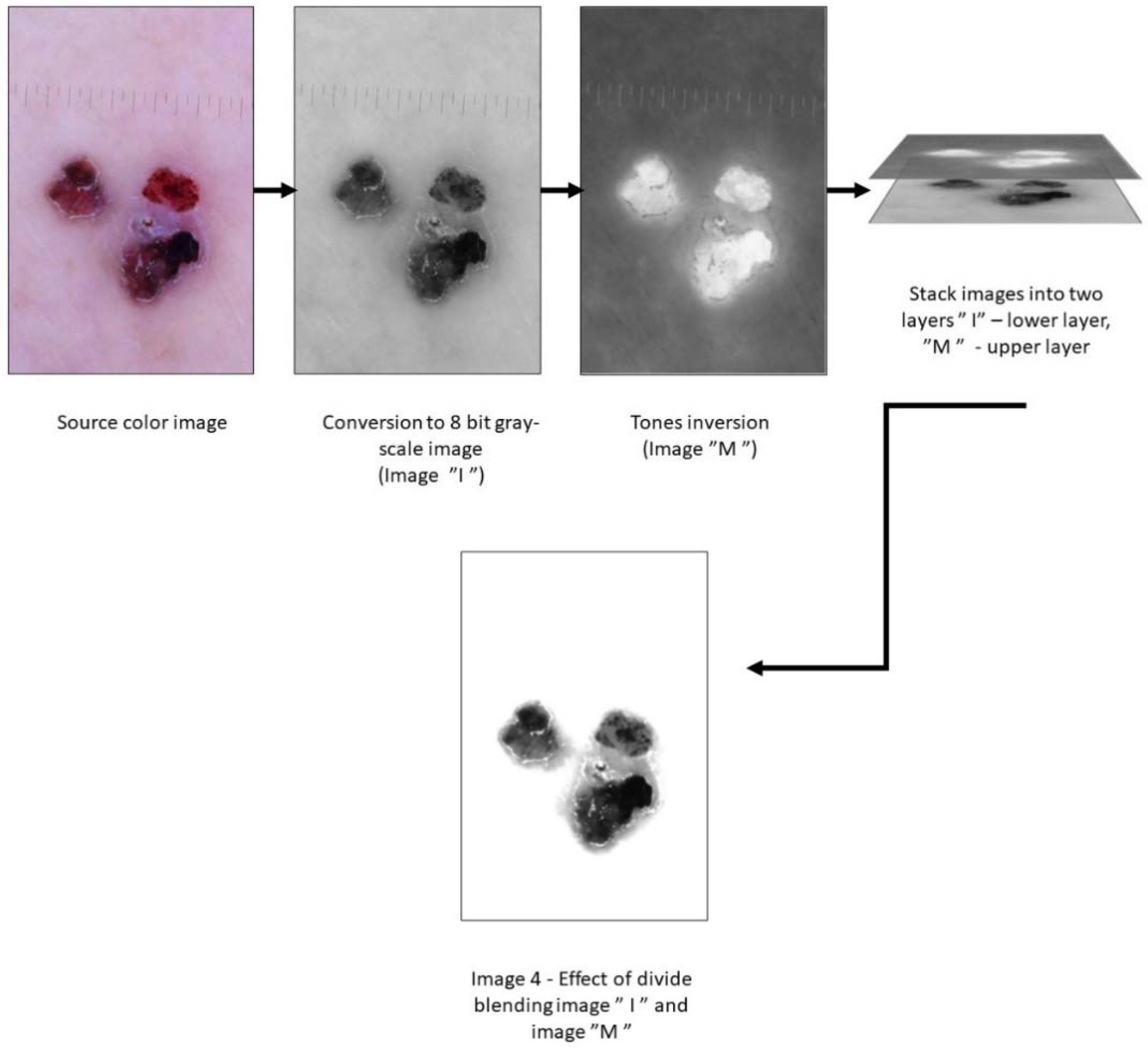
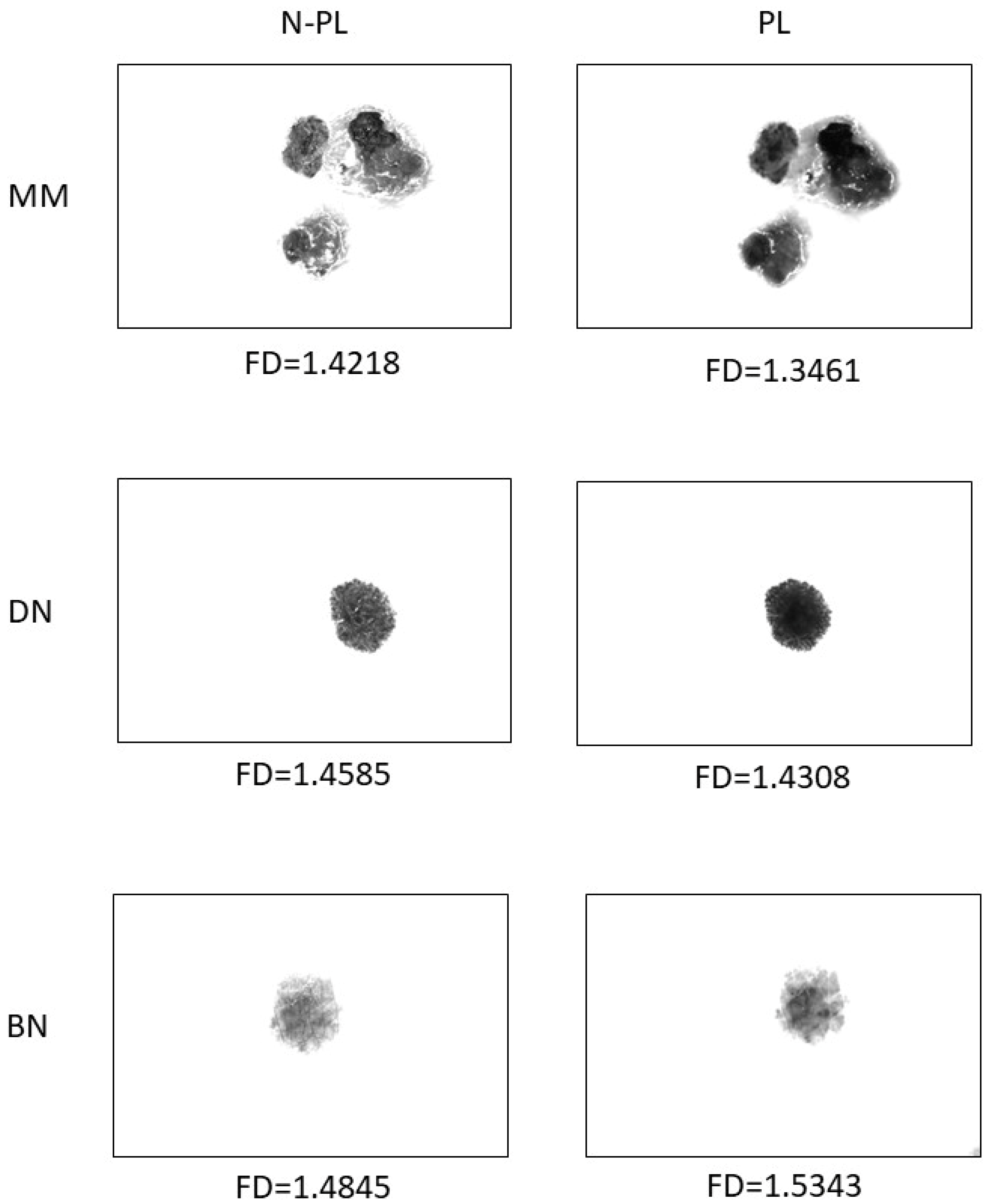
| Lesion | Sex | Age | Localization | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| F | M | Mean | SD | Min. | Max. | Head/Neck | Trunk | Upper Limb | Lower Limb | Total | |
| MM | 8 | 12 | 62.0 | 18.3 | 33 | 90 | 1 | 9 | 6 | 4 | 20 |
| DN | 10 | 8 | 55.6 | 18.1 | 23 | 81 | 2 | 17 | 2 | 2 | 23 |
| BN | 28 | 18 | 46.0 | 18.4 | 14 | 85 | 7 | 34 | 2 | 11 | 54 |
| Total | 46 | 38 | 54.6 | 18.3 | 14 | 90 | 10 | 60 | 10 | 17 | 97 |
| Lesion | Examined Feature | Mean | SD | N | Difference | p |
|---|---|---|---|---|---|---|
| MM | Area of PL [mm2] | 166.95 | 210.16 | 20 | 5.39 | 0.4781 |
| Area of N-PL [mm2] | 161.57 | 206.95 | ||||
| DN | Area of PL [mm2] | 18.43 | 12.02 | 23 | 0.92 | 0.0081 |
| Area of N-PL [mm2] | 17.51 | 11.39 | ||||
| BN | Area of PL [mm2] | 15.51 | 13.95 | 54 | 0.61 | 0.0000 |
| Area of N-PL [mm2] | 14.91 | 13.66 | ||||
| MM | Perimeter of PL [mm] | 54.73 | 33.34 | 20 | 4.16 | 0.0012 |
| Perimeter of N-PL [mm] | 50.57 | 30.61 | ||||
| DN | Perimeter of PL [mm] | 18.55 | 7.81 | 23 | 1.11 | 0.0012 |
| Perimeter of N-PL [mm] | 17.44 | 6.91 | ||||
| BN | Perimeter of PL [mm] | 14.56 | 7.29 | 54 | 0.67 | 0.0000 |
| Perimeter of N-PL [mm] | 13.89 | 6.74 |
| Polarized Light | Non-Polarized Light | ||||||
|---|---|---|---|---|---|---|---|
| Area | Area | ||||||
| MM R = 80.700 | DN R = 47.739 | BN R = 37.796 | MM R = 81.450 | DN R = 47.652 | BN R = 37.565 | ||
| MM | p = 0.000384 | p = 0.000000 | MM | p = 0.000257 | p = 0.000000 | ||
| DN | p = 0.000384 | p = 0.467883 | DN | p = 0.000257 | p = 0.448982 | ||
| BN | p = 0.000000 | p = 0.467883 | BN | p = 0.000000 | p = 0.448982 | ||
| Perimeter | Perimeter | ||||||
| MM R = 84.000 | DN R = 48.870 | BN R = 36.093 | MM R = 84.350 | DN R = 48.565 | BN R = 36.093 | ||
| MM | p = 0.000134 | p = 0.000000 | MM | p = 0.000096 | p = 0.000000 | ||
| DN | p = 0.000134 | p = 0.204818 | DN | p = 0.000096 | p = 0.225339 | ||
| BN | p = 0.000000 | p = 0.204818 | BN | p = 0.000000 | p = 0.225339 | ||
| Lesion | Examined Feature | Mean | SD | N | Difference | P |
|---|---|---|---|---|---|---|
| MM | FD PL | 1.3885 | 0.0404 | 20 | −0.0586 | 0.0000 |
| FD N-PL | 1.4471 | 0.0431 | ||||
| DN | FD PL | 1.4225 | 0.0393 | 23 | −0.0459 | 0.0000 |
| FD N-PL | 1.4685 | 0.0318 | ||||
| BN | FD PL | 1.4713 | 0.0579 | 54 | −0.0255 | 0.0001 |
| FD N-PL | 1.4968 | 0.0418 |
| Polarized Light | Non-Polarized Light | ||||||
|---|---|---|---|---|---|---|---|
| Value of Fractal Dimension for the Shape of Lesions | |||||||
| MM | DN | BN | MM | DN | BN | ||
| MM | 0.031161 | 0.000000 | MM | 0.144874 | 0.000165 | ||
| DN | 0.031161 | 0.000275 | DN | 0.144874 | 0.021539 | ||
| BN | 0.000000 | 0.000275 | BN | 0.000165 | 0.021539 | ||
| MM | DN | BN | ||||
|---|---|---|---|---|---|---|
| PL | N-PL | PL | N-PL | PL | N-PL | |
| FD vs. Area of lesion | −0.131579 | 0.303759 | −0.367589 | −0.219368 | −0.720775 | −0.453937 |
| FD vs. Perimeter of lesion | 0.001754 | 0.321805 | −0.213439 | −0.265810 | −0.712869 | −0.459186 |
| Lesion | Polarized Light | Non-Polarized Light | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| MM N = 200 | 1.4117 | 0.1639 | 1.5909 | 0.1160 |
| DN N = 107 | 1.4498 | 0.1314 | 1.6139 | 0.0880 |
| BN N = 198 | 1.5007 | 0.1314 | 1.5965 | 0.0930 |
| Polarized Light | Non-Polarized Light | ||||||
|---|---|---|---|---|---|---|---|
| Fractal Dimension Values for Lesion Surface (ROIs) | |||||||
| MM R = 300.07 | DN R = 241.16 | BN R = 211.42 | MM R = 246.04 | DN R = 275.08 | BN R = 256.10 | ||
| MM | p = 0.002331 | p = 0.000000 | MM | p = 0.300678 | p = 1.000000 | ||
| DN | p = 0.002331 | p = 0.267457 | DN | p = 300678 | p = 0.854320 | ||
| BN | p = 0.000000 | p = 0.267457 | BN | p = 1.000000 | p = 0.854320 | ||
| Examined Feature | Lesion | Mean | SD | N | p |
|---|---|---|---|---|---|
| FD PL | MM in situ | 1.4166 | 0.0165 | 8 | 0.0165 |
| MM invasive | 1.3708 | 0.0165 | 12 | ||
| FD N-PL | MM in situ | 1.4800 | 0.0173 | 8 | 0.0173 |
| MM invasive | 1.4300 | 0.0173 | 12 |
| Examined Feature | Lesion | Mean | SD | N | p |
|---|---|---|---|---|---|
| FD PL | MM in situ | 1.4676 | 0.1367 | 86 | 0.0059 |
| MM invasive | 1.3678 | 0.1500 | 114 | ||
| FD N-PL | MM in situ | 1.6241 | 0.0877 | 86 | 0.0065 |
| MM invasive | 1.5639 | 0.1557 | 114 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popecki, P.; Kozakiewicz, M.; Ziętek, M.; Jurczyszyn, K. Fractal Dimension Analysis of Melanocytic Nevi and Melanomas in Normal and Polarized Light—A Preliminary Report. Life 2022, 12, 1008. https://doi.org/10.3390/life12071008
Popecki P, Kozakiewicz M, Ziętek M, Jurczyszyn K. Fractal Dimension Analysis of Melanocytic Nevi and Melanomas in Normal and Polarized Light—A Preliminary Report. Life. 2022; 12(7):1008. https://doi.org/10.3390/life12071008
Chicago/Turabian StylePopecki, Paweł, Marcin Kozakiewicz, Marcin Ziętek, and Kamil Jurczyszyn. 2022. "Fractal Dimension Analysis of Melanocytic Nevi and Melanomas in Normal and Polarized Light—A Preliminary Report" Life 12, no. 7: 1008. https://doi.org/10.3390/life12071008
APA StylePopecki, P., Kozakiewicz, M., Ziętek, M., & Jurczyszyn, K. (2022). Fractal Dimension Analysis of Melanocytic Nevi and Melanomas in Normal and Polarized Light—A Preliminary Report. Life, 12(7), 1008. https://doi.org/10.3390/life12071008








