Abstract
Background: Maternal obesity is associated with several adverse reproductive outcomes. It is a growing public health burden in sub-Saharan Africa, a region with low resources and capacity to care for the large, affected population. Objectives: To assess the evidence of maternal obesity as a risk factor for caesarean delivery in women in sub-Saharan Africa. Methods: A systematic review of relevant original articles using PubMed, MEDLINE, and CINAHL was performed. Google Scholar and the reference lists of relevant systematic reviews and meta-analyses were also searched for other eligible studies. Observational studies assessing maternal body mass index (BMI) ≥ 30 kg/m2 before or during gestation and caesarean delivery as birth outcome were included. Results: All 17 studies were published between 2009 and 2021 and included 227,675 (236–153,102) participants. The prevalence of maternal obesity ranged from 3.9 to 44%. All except two studies (88%) indicated an association of obesity and risk of caesarean delivery in pregnant women in sub-Saharan Africa. Overweight/obese women had up to 4-fold increased risk of caesarean delivery compared to normal weight women. Three studies also reported a direct relationship between morbid obesity and prevalence of caesarean delivery in the sub-region. The risk of caesarean delivery appears to increase with increasing BMI e.g., >5 times in women with BMI ≥ 40 kg/m2 than in normal weight women. Conclusions: In sub-Saharan Africa, increased BMI in pregnancy is a risk factor for subsequent caesarean delivery. The risk of caesarean delivery appears to increase with increasing BMI. A robust meta-analysis and other patho-mechanistic studies can be conducted to confirm causal association. Culturally appropriate weight management and nutritional interventions should be implemented to reduce the incidence of obesity-induced caesarean delivery in sub-Saharan Africa.
1. Introduction
Obesity is a major global public health burden that contributes to more than 2 million preventable deaths every year [1,2]. It is an inflammatory disease [3] that is commonly defined as body mass index (BMI) ≥ 30 kg/m2 [4]. There is a higher prevalence of obesity in developed countries, however, it has become increasingly more prevalent in developing countries [5]. In Africa, obesity is often erroneously associated with high socioeconomic status (wealth) and health, particularly among women [6]. African women are about four times more likely to be obese than their male counterparts [7].
Pregnancy is a widely recognised catalyst for obesity in women [8]. Although maternal obesity is assessed differently worldwide, pre-pregnancy and first trimester BMI are the most commonly employed evaluation tools [9]. However, studies have reported maternal BMI at different gestational time points because in low resource settings, the first prenatal visit, when maternal BMI is commonly determined, often occurs after first trimester [10].
The prevalence of maternal obesity in Africa can be as high as 50% [11]. Older and multiparous women have a greater risk of being obese [11]. The prevalence of excessive gestational weight gain according to the American Institute of Medicine Gestational Weight Gain guidelines can also be as high as 37% [12]. There is also postpartum retention of excess weight gained during gestation with attendant risk of obesity later in life [13], and complications in the next pregnancy [14].
Gestational weight gain, which is sometimes induced by inflammation, is vital to the success of normal pregnancy [15]. The increase in BMI, especially at the later stages of gestation (i.e., third trimester), is believed to encourage energy storage in fat cells for rapid foetal growth and development, and in readiness for subsequent energy-demanding processes such as labour and lactation [15]. Despite this supposed beneficial effect of gestational weight gain [15], maternal obesity is associated with adverse reproductive and overall health outcomes and complications [16]. Significant adverse effects of maternal obesity on maternal, labour, and child outcomes in the African continent [11,17] as well as globally [14,18,19,20,21] have been reported. Overweight and obese women have increased risk of maternal complications including gestational hypertensive disorders, pre-eclampsia and eclampsia, gestational diabetes mellitus, induction of labour, prolonged labour, instrumental vaginal delivery, caesarean delivery, spontaneous and medically indicated preterm birth, miscarriage, postpartum haemorrhage, pulmonary embolism, genitourinary tract infection, postpartum weight retention, and maternal mortality. Their offsprings are also at increased risk of complications including congenital abnormalities, stillbirth, macrosomia (birthweight ≥ 4000 g), shoulder dystocia, respiratory distress syndrome, and intrauterine (foetal) and neonatal death [14,18,19,20,21,22,23,24,25,26,27,28]. In addition, both mother and infant are at a greater risk of developing subsequent non-communicable diseases such as obesity and cardiometabolic diseases later in life [18,19,22,24]. These complications can result in disabilities or mortalities especially in poor resource settings such as Africa where the capacity to manage such complications is limited [25].
The relationship between maternal obesity and delivery by caesarean section is well established in high resource settings [29,30], but less so in low income settings such as many countries in Africa [11]. Moreover, in Africa, studies assessing medical and obstetric interventions for obese pregnant women and evaluating the awareness or attitudes of pregnant women towards maternal obesity are scarce [11]. Therefore, in this systematic review, we investigated maternal obesity as a risk factor for caesarean delivery in adult women in sub-Saharan Africa. This was to specifically assess the relationship between maternal obesity and delivery by caesarean section in women in sub-Saharan Africa in comparison to pregnant women with normal or optimal BMI.
2. Materials and Methods
2.1. Literature Search
We performed a systematic review according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Relevant original articles were identified through a systematic search of PubMed, MEDLINE via Web of Science and CINAHL via EBSCO electronic databases without a date restriction, i.e., from the inception of the databases to April 2022. Google Scholar and the reference lists of relevant reviews and meta-analyses were also searched for other potentially eligible studies. The review question was broken down into concepts relating to the population, exposure, study design, and outcomes. The searches were conducted using a comprehensive search strategy focused on maternal obesity (exposure) and delivery by caesarean section (outcome). The search strategy was formulated by combining synonyms of the following key words and free text search terms: “maternal weight”, “caesarean delivery” and “Africa or sub-Saharan Africa” together with Boolean operators (OR/AND) to refine the search results. The search criteria from MEDLINE via Web of Science is presented in Table 1. Only peer-reviewed studies reported in English language were included. The process of study selection was based on the main inclusion and exclusion criteria employed, which are shown in Table 2.

Table 1.
Search strategy.

Table 2.
Inclusion and exclusion criteria for study selection.
2.2. Study Selection
Using the inclusion criteria (Table 2), two review authors independently screened the titles and abstracts of studies retrieved to determine those that required further assessment. After duplicates were removed, the review authors further assessed the potential studies that were identified from the search strategy. When relevance could not be determined by title and abstract alone, the full-texts of the articles were retrieved and assessed for possible inclusion based on study design, type of participants, exposure and outcome measures. All studies that were excluded after assessment at the full-text stage were summarised with reasons for their exclusion in Supplementary Table S1. The stages of study selection were also presented on a PRISMA flowchart (Figure 1).
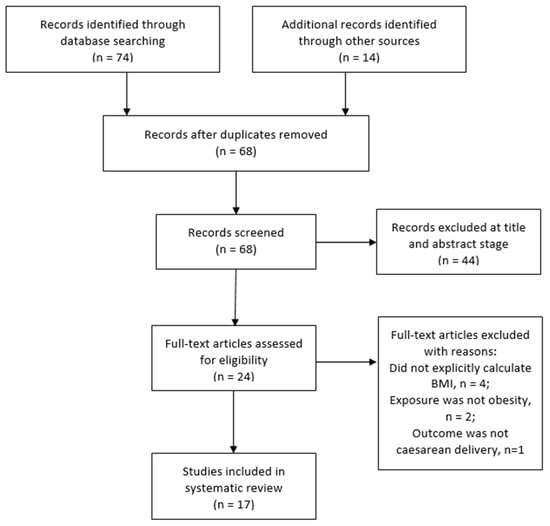
Figure 1.
PRISMA flow chart of the study selection.
2.3. Data Extraction and Quality Assessment
Data extraction was performed together by both authors using a data extraction form adapted from the Centre for Reviews and Dissemination (CRD) guidance. This form was used to summarise key details of each study that met the inclusion criteria. The included studies were further quality assessed by the two authors using the Critical Appraisals Skills Programme (CASP) Checklists for observational studies. These checklist criteria were modified to include assessment of cross-sectional studies. The following key details: study identifier, study characteristics, participant characteristics, exposure and setting, outcome data or results, and summary outcome data, were extracted from each study that met the inclusion criteria. A completed sample of the data extraction form and CASP Checklist can be found in Supplementary Tables S2 and S3, respectively.
2.4. Data Synthesis
In studies where they were reported, odds (ORs) and risk ratios (or relative risk, RR) as well as 95% Confidence Interval (CI) were calculated from the frequency (reported as percentage) or prevalence rates of caesarean delivery. If the unadjusted ORs and CIs were not reported, the respective adjusted parameters including p values were reported. The authors of the included studies were not contacted for any missing data. A narrative synthesis was used to summarise the findings of the studies included as it allowed for exploration of comparisons between the studies. Descriptive information about each study’s population, exposure, comparators, outcomes and measure of association were presented in text and tables and a forest plot. The country or countries where the included studies were conducted were noted, and the gestational age at which maternal obesity was measured was also recorded and described. Heterogeneity among included studies was assessed clinically and methodologically, and it was determined that a meta-analysis was not suitable for this data set due to the variation in study designs, methodology, and measurements of exposure between the studies.
3. Results
3.1. Literature Search
A total of 88 potentially relevant records (articles) were identified through the search process (Figure 1). The searches identified 40 records from MEDLINE, 18 from PubMed, and 16 from CINAHL. Fourteen additional records were identified through Google Scholar and the reference list of a systematic review and meta-analysis on maternal obesity in Africa [11]. Of the 88 total records, 20 were duplicates and were excluded. Sixty-eight records were screened by title and abstract, and 44 were excluded. Twenty-four full text articles were further screened for eligibility for inclusion in the review. After assessment, 7 studies [31,32,33,34,35,36,37] were excluded because they did not meet the inclusion criteria. The excluded studies and reasons for their exclusion are presented in Supplementary Table S1. The remaining 17 studies were included in this review and analysed further.
3.2. Study Characteristics
3.2.1. Study Setting
Of the 17 studies, only 1 study used a pooled data set from 31 sub–Saharan African countries [38]. Most of the studies were conducted in Nigeria (6/17) [39,40,41,42,43,44]; and South Africa (3/17) [45,46,47] (Table 3). Twelve of the included studies were conducted either in tertiary health centres or maternity clinics [10,39,40,41,42,43,45,46,48,49,50,51]; while the other 5 studies were conducted in community or neighbourhood settings [38,44,47,52,53].

Table 3.
Characteristics of the included studies.
3.2.2. Study Design
All the included studies were observational studies except for Davies et al., [47] which was a sub-study of a randomised controlled trial (Table 3). Three of the studies used a case-control design [39,40,45], six studies used a cross-sectional design [38,44,46,50,52,53], and seven studies used a cohort design [10,41,42,43,48,49,51].
3.3. Population
This review included studies comprising a total of 227,675 participants with sample sizes ranging from 236 to 153,102 (Table 3 and Table 4). BMI was recorded at various gestational time points across all the studies (from pre-pregnancy to 5 years post-delivery). Three studies recruited participants in the first trimester [40,43,51]. Seven studies recorded participants’ BMI at the first antenatal booking without stating a specific gestational age [40,41,45,46,47,48,50]. Minsart et al., [10] compared caesarean outcomes for BMI at ≤14 weeks, ≤22 weeks, and at delivery. Two other studies measured maternal BMI at ≤25 weeks [42] and ≤32 weeks [39]. Four cross-sectional studies accepted BMI measurements from participants who delivered up to 5 years before the study was conducted [38,44,52,53]. Only one retrospective study compared caesarean delivery outcomes in participants with maternal BMI recorded before pregnancy [49].

Table 4.
Summary results of included studies showing the prevalence of obesity and association between maternal obesity and caesarean delivery.
3.4. Types of Exposure
All studies reported BMI (kg/m2) as a measure of maternal obesity (Table 4). The maternal BMI exposure categories for overweight, obese, and morbidly obese varied according to the studies. Most studies used a BMI ≥ 30 kg/m2 as the cut-off for obesity except for Davies et al., [47], which used a BMI ≥ 29 kg/m2. Three studies combined overweight and obese participants and compared them to normal or optimal weight individuals (BMI = 18.5–24.9 kg/m2) [50,51,52]; while four other studies compared participants in different categories of obesity to those with normal/optimal weight [10,38,42,46].
3.5. Outcome Measure
All included studies reported delivery by caesarean section as an outcome measure (Table 2), along with several other maternal and neonatal outcomes and complications. The prevalence of obesity ranged from 3.9% to 44%. Delivery by caesarean section was associated with obesity in all of the studies except Basu et al., [46] and Fouelifack et al., [49], which did not identify an association (Table 4). Ugwuja et al., [42] (BMI > 35.0 kg/m2), Ngoga et al., [45] (BMI ≥ 40 kg/m2), and Cresswell et al., [38] (BMI > 35.0 and ≥ 40 kg/m2) reported an association between caesarean delivery and morbid obesity (Table 4). The prevalence of caesarean delivery in overweight and obese women was 1.5–4.3 times that of normal weight women. This margin rose up to 5.9 times in women with (BMI ≥ 40 kg/m2) [38]. Six studies did not report odds or risk ratios for caesarean delivery outcomes [39,41,42,45,46,47]. One study subdivided the delivery outcome into elective and emergency caesarean sections, which were both directly related to obesity [48]. These studies with missing and subdivided outcomes (n = 7) were not included in the forest plot (Figure 2).
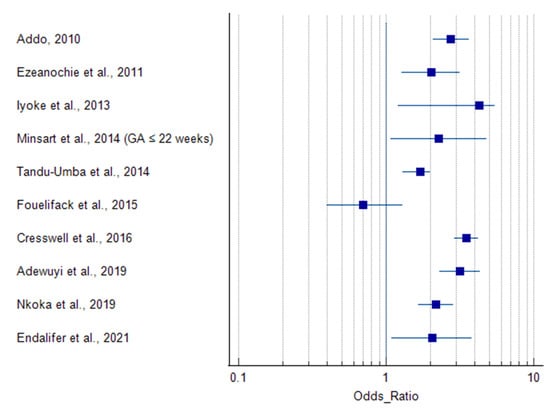
Figure 2.
Summary results of the original articles showing that maternal obesity increases the risk of caesarean delivery. Only studies that reported odd ratios [10,38,40,43,44,49,50,51,52,53] were included in the forest plot. The results from studies without odds and risk ratios (n = 6) [39,41,42,45,46,47] and studies with sub-categories of caesarean delivery (n = 1) [48] were not included in the forest plot. Minsart et al., [10] recruited participants at two gestational time points but chose BMI ≤ 22 weeks as the main variable because it had a lesser amount of missing data compared to BMI ≤ 14 weeks, and BMI at first prenatal visit (which usually occurs after first trimester) is commonly used as the main variable regardless of gestational age [10].
3.6. Association of Maternal Obesity and Caesarean Delivery
In 13 of the studies that had obese as a BMI category, 7 reported a significantly higher incidence/prevalence of caesarean delivery in obese women (BMI ≥ 30 kg/m2) compared to their non-obese counterparts. In two studies, there was no association between obesity and caesarean section [46,49]. However, in three other studies, morbidly obese women with a BMI > 35.0 kg/m2 [38,42] or BMI ≥ 40 kg/m2 [38,45], had significantly increased rates of caesarean delivery compared to non-obese women (20–25 kg/m2) (Table 4). Only one study categorized caesarean delivery into elective and emergency, and the obese women had greater risk of both elective [RR = 2.4 (1.88, 3.6)] and emergency [RR = 1.53 (1.34, 1.75)] caesarean sections [48]. In three studies, there was a significant increase in caesarean delivery in both overweight and obese women compared to non-obese women [50,51,52]. In the study that pooled results from 31 sub-Saharan African countries, women in each obesity class (i.e., Class I, II, and III) were more likely to have experienced a caesarean delivery compared to normal weight women [38]. Six studies did not report either an odds or risk ratio [39,41,42,45,46,47].
4. Discussion
This systematic review collated and assessed the available evidence of an association of maternal obesity and incidence of caesarean delivery in women in sub-Saharan Africa. All 17 included studies were published between 2009 and 2021 and included a total of 227,675 (236–153,102) participants. The prevalence of maternal obesity ranged from 3.9 to 44% across the studies. This wide range could be due to differences in study design, settings, and/or classification of obesity. Most (12/17) of the studies were conducted in healthcare facilities while 5 were community-based studies. All except two studies (88%) showed evidence of obesity as a risk factor for caesarean delivery in pregnant women in sub-Saharan Africa. That is, in sub-Saharan Africa, obesity significantly increases a woman’s risk of delivering by caesarean section. Overweight and obese women can have up to 4-fold increased risk of caesarean delivery compared to their normal weight counterparts. The risk of caesarean delivery appears to increase with increasing BMI. For instance, it is greater than 5 times in women with BMI ≥ 40 kg/m2 than in normal weight women. Most of the data (evidence, 7/17) were obtained from studies conducted in West Africa (i.e., Nigeria = 6, and Ghana = 1) within the last decade (2010–2019).
Obesity, including maternal obesity, is a growing public health burden in sub-Saharan Africa, a region with low resources/capacity to care for the large, affected population liable to poor reproductive outcomes. While it focusses on sub-Saharan Africa, our study also validates and updates the findings of a previous meta-analysis on the effect of maternal obesity on six labour outcomes including caesarean delivery in Africa [11]. The meta-analysis reported that obese women were more likely to deliver by caesarean section than women who were not obese (RR 1.87, 1.64–2.12, n = 8) [11].
A clear dose-response relationship between maternal obesity and risk of caesarean delivery has been observed in sub-Saharan Africa [38]. That is, the likelihood of a woman delivering by caesarean section increases with an increasing BMI. For instance, morbidly obese (BMI ≥ 40 kg/m2) women can have more than a three-fold risk of delivering by caesarean section than normal weight women [38]. The prevalence of obesity in sub-Saharan Africa is projected to increase to 17.5% by 2030 [54]. With the increasing demand for caesarean section, which has already surpassed available capacity, pre- and post-pregnancy weight loss should be recommended for overweight and obese women of reproductive age in the sub-region [38,55].
We identified two studies [46,49] that did not indicate an association between maternal obesity and delivery by caesarean section. Although Basu et al., [46] did not identify an association between maternal obesity and delivery by caesarean section in their cohort, they recorded a high prevalence of obesity (44%), which was associated with other complications including gestational diabetes, urinary tract infection, and failed induction of labour. Similarly, Fouelifack et al., [49] did not observe a direct association between maternal obesity and delivery by caesarean section but reported that gestational weight gain above the American Institute of Medicine Gestational Weight Gain Recommendations [56] increased the risk of poor maternal outcomes including caesarean delivery, obstetrical haemorrhage or preeclampsia by 1.7-fold. The prevalence of maternal obesity was also relatively high (14%) compared to other studies.
Taken together, the findings of both studies corroborate the association of maternal obesity, gestational weight gain, adverse reproductive outcomes, and overall health complications in women, especially those living in the sub-region. The multiple comorbidities and complications associated with maternal obesity can be reduced if overweight, obesity, and excess gestational weight gain can be reduced in women of reproductive age [24]. Medical and obstetric management of obesity-associated complications during gestation, especially of blood glucose [14] and hypertension, should be accompanied with promotion of public health recommendations for a healthy diet and engaging in moderate physical exercise during pregnancy [12,21,28,57].
Limitations
There are several limitations that may impact the interpretation and implications of the findings of this study. This study included mainly observational studies, some of which did not adjust for confounders. Those that did adjust for confounders differed by type and number of the confounding variables. Although we strictly followed a robust list of inclusion/exclusion criteria, we recognize that obesity may not have been the sole indication for caesarean delivery in the included studies. The BMI of the obese group in one of the studies [47], a sub-study from a RCT, may have also been misclassified. The included studies also measured BMI at different gestational time points, most of which were unspecified. Furthermore, there could be a difference in outcome between pre-pregnancy BMI and BMI measured during gestation. Generalisability of the findings is also impacted by the dominance of data from the West African sub-region compared to other sub-regions. There are 46 countries in sub-Saharan Africa (United Nations Development Programme) and only original articles from 8 countries in the sub-region were included in this study. The report from Demographic and Health Surveys pooled from 31 countries in the sub-region [38] could not ascertain the prevalence of elective vs. emergency caesarean sections, which could be impacted by BMI. Data extraction and quality assessment for this review was conducted by only two reviewers, therefore, an objective appraisal may not have been fully obtained. In addition, a meta-analysis on more homogenous studies could provide more precise evidence on the effect of maternal obesity on caesarean delivery.
5. Conclusions
This review provides further evidence and validation that maternal obesity is a risk factor for caesarean section in sub-Saharan Africa. Obesity exerts significant influence on the mode of delivery, independent of other risk factors such as macrosomia, pre-eclampsia, and gestational diabetes, etc. The risk of caesarean delivery increases as BMI increases, plausibly more so for emergency caesarean section. Although this study has contributed more evidence to the limited literature on maternal obesity and its associated pregnancy outcomes in the African population, a robust meta-analysis and other patho-mechanistic studies can be conducted to confirm causal association. Culturally appropriate weight management and nutritional interventions should be implemented to reduce the incidence of obesity-induced caesarean delivery in sub-Saharan Africa.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/life12060906/s1, Table S1: Full-text articles excluded with reasons. Table S2: Sample of data extraction form. Table S3: Sample of quality assessment checklist.
Author Contributions
J.B.B. conceptualised the review idea. J.B.B. and E.A. performed the literature search, data extraction, and analysis. J.B.B. prepared the initial draft that was reviewed and edited by E.A. Both authors edited subsequent drafts of the manuscript and approved the final draft for submission. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by the University of Sheffield as part of J.B.B.’s Master of Public Health course.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank Andrew Booth and Sue Harnan for their feedback during the conceptualisation and literature search. We would also like to thank The Playbook Factory (theplaybookfactory@gmail.com) for contributing to the design of the graphic image.
Conflicts of Interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Chan, R.S.M.; Woo, J. Prevention of overweight and obesity: How effective is the current public health approach. Int. J. Environ. Res. Public Health 2010, 7, 765–783. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Amabebe, E.; Robert, F.O.; Agbalalah, T.; Orubu, E.S.F. Microbial dysbiosis-induced obesity: Role of gut microbiota in homoeostasis of energy metabolism. Br. J. Nutr. 2020, 123, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation; WHO Technical Report Series; WHO: Geneva, Switzerland, 2000; Volume 894, pp. i–xii, 1–253. [Google Scholar]
- Popkin, B.M. Part II. What is unique about the experience in lower-and middle-income less-industrialised countries compared with the very-highincome industrialised countries?: The shift in stages of the nutrition transition in the developing world differes from past experiences! Public Health Nutr. 2002, 5, 205–214. [Google Scholar] [CrossRef]
- Fezeu, L.; Minkoulou, E.; Balkau, B.; Kengne, A.P.; Awah, P.; Unwin, N.; Alberti, G.K.; Mbanya, J.C. Association between socioeconomic status and adiposity in urban Cameroon. Int. J. Epidemiol. 2006, 35, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Sodjinou, R.; Agueh, V.; Fayomi, B.; Delisle, H. Obesity and cardio-metabolic risk factors in urban adults of Benin: Relationship with socio-economic status, urbanisation, and lifestyle patterns. BMC Public Health 2008, 8, 84. [Google Scholar] [CrossRef]
- Davis, E.M.; Zyzanski, S.J.; Olson, C.M.; Stange, K.C.; Horwitz, R.I. Racial, ethnic, and socioeconomic differences in the incidence of obesity related to childbirth. Am. J. Public Health 2009, 99, 294–299. [Google Scholar] [CrossRef]
- Kleinman, K.P.; Oken, E.; Radesky, J.S.; Rich-Edwards, J.W.; Peterson, K.E.; Gillman, M.W. How should gestational weight gain be assessed? A comparison of existing methods and a novel method, area under the weight gain curve. Int. J. Epidemiol. 2007, 36, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Minsart, A.F.; N’Guyen, T.-S.; Dimtsu, H.; Ratsimanresy, R.; Dada, F.; Ali Hadji, R. Maternal obesity and rate of cesarean delivery in Djibouti. Int. J. Gynaecol. Obstet. 2014, 127, 167–170. [Google Scholar] [CrossRef]
- Onubi, O.J.; Marais, D.; Aucott, L.; Okonofua, F.; Poobalan, A.S. Maternal obesity in Africa: A systematic review and meta-analysis. J. Public Health 2016, 38, e218–e231. [Google Scholar] [CrossRef] [PubMed]
- Madlala, H.P.; Steyn, N.P.; Kalk, E.; Davies, M.-A.; Nyemba, D.; Malaba, T.R.; Mehta, U.; Petro, G.; Boulle, A.; Myer, L. Association between food intake and obesity in pregnant women living with and without HIV in Cape Town, South Africa: A prospective cohort study. BMC Public Health 2021, 21, 1504. [Google Scholar] [CrossRef]
- Rooney, B.L.; Schauberger, C.W. Excess pregnancy weight gain and long-term obesity: One decade later. Obstet. Gynecol. 2002, 100, 245–252. [Google Scholar] [CrossRef]
- Poston, L.; Caleyachetty, R.; Cnattingius, S.; Corvalán, C.; Uauy, R.; Herring, S.; Gillman, M.W. Preconceptional and maternal obesity: Epidemiology and health consequences. Lancet Diabetes Endocrinol. 2016, 4, 1025–1036. [Google Scholar] [CrossRef]
- Amabebe, E.; Anumba, D.O. Diabetogenically beneficial gut microbiota alterations in third trimester of pregnancy. Reprod. Fertil. 2021, 2, R1–R12. [Google Scholar] [CrossRef]
- Thangaratinam, S.; Rogozińska, E.; Jolly, K.; Glinkowski, S.; Duda, W.; Borowiack, E.; Roseboom, T.; Tomlinson, J.; Walczak, J.; Kunz, R.; et al. Interventions to reduce or prevent obesity in pregnant women: A systematic review. Health Technol. Assess. 2012, 16, iii–iv, 1–191. [Google Scholar] [CrossRef]
- Ozodiegwu, I.D.; Mamudu, H.M.; Wang, L.; Wallace, R.; Quinn, M.; Liu, Y.; Doctor, H.V. Country-Level Analysis of the Association between Maternal Obesity and Neonatal Mortality in 34 Sub-Saharan African Countries. Ann. Glob. Health 2019, 85, 139. [Google Scholar] [CrossRef]
- Nurul-Farehah, S.; Rohana, A.J. Maternal obesity and its determinants: A neglected issue? Malays. Fam. Physician 2020, 15, 34–42. [Google Scholar]
- Leddy, M.A.; Power, M.L.; Schulkin, J. The impact of maternal obesity on maternal and fetal health. Rev. Obstet. Gynecol. 2008, 1, 170–178. [Google Scholar]
- Neal, K.; Ullah, S.; Glastras, S.J. Obesity Class Impacts Adverse Maternal and Neonatal Outcomes Independent of Diabetes. Front. Endocrinol. 2022, 13, 832678. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.C.W.; Schmidt, M.I.; Tam, W.H.; McIntyre, H.D.; Catalano, P.M. Clinical management of pregnancy in the obese mother: Before conception, during pregnancy, and post partum. Lancet Diabetes Endocrinol. 2016, 4, 1037–1049. [Google Scholar] [CrossRef]
- Poston, L.; Harthoorn, L.F.; van der Beek, E.M.; On Behalf of Contributors to The ILSI Europe Workshop. Obesity in Pregnancy: Implications for the Mother and Lifelong Health of the Child. A Consensus Statement. Pediatr. Res. 2011, 69, 175–180. [Google Scholar] [CrossRef]
- Alfadhli, E.M. Maternal obesity influences birth weight more than gestational diabetes. BMC Pregnancy Childbirth 2021, 21, 111. [Google Scholar] [CrossRef]
- Grieger, J.A.; Hutchesson, M.J.; Cooray, S.D.; Bahri Khomami, M.; Zaman, S.; Segan, L.; Teede, H.; Moran, L.J. A review of maternal overweight and obesity and its impact on cardiometabolic outcomes during pregnancy and postpartum. Ther. Adv. Reprod. Health 2021, 15, 2633494120986544. [Google Scholar] [CrossRef]
- Betran, A.; Torloni, M.; Zhang, J.; Gülmezoglu, A.; WHO Working Group on Caesarean Section. WHO Statement on Caesarean Section Rates. BJOG Int. J. Obstet. Gynaecol. 2016, 123, 667–670. [Google Scholar] [CrossRef]
- Sebire, N.J.; Jolly, M.; Harris, J.P.; Wadsworth, J.; Joffe, M.; Beard, R.W.; Regan, L.; Robinson, S. Maternal obesity and pregnancy outcome: A study of 287,213 pregnancies in London. Int. J. Obes. 2001, 25, 1175–1182. [Google Scholar] [CrossRef]
- Damodaran, S.; Swaminathan, K. 6—Obesity and Contraception. In Obesity; Mahmood, T., Arulkumaran, S., Eds.; Elsevier: Oxford, UK, 2013; pp. 69–89. [Google Scholar]
- Shrestha, A.; Prowak, M.; Berlandi-Short, V.-M.; Garay, J.; Ramalingam, L. Maternal Obesity: A Focus on Maternal Interventions to Improve Health of Offspring. Front. Cardiovasc. Med. 2021, 8, 674. [Google Scholar] [CrossRef]
- Poobalan, A.S.; Aucott, L.S.; Gurung, T.; Smith, W.C.; Bhattacharya, S. Obesity as an independent risk factor for elective and emergency caesarean delivery in nulliparous women--systematic review and meta-analysis of cohort studies. Obes. Rev. 2009, 10, 28–35. [Google Scholar] [CrossRef]
- Chu, S.Y.; Kim, S.Y.; Schmid, C.H.; Dietz, P.M.; Callaghan, W.M.; Lau, J.; Curtis, K.M. Maternal obesity and risk of cesarean delivery: A meta-analysis. Obes. Rev. 2007, 8, 385–394. [Google Scholar] [CrossRef]
- Olayemi, O.O.; Umuerri, C.O.; Aimakhu, C.O. Obstetric Performance of Nigerian Obese Parturients. Trop. J. Obstet. Gynaecol. 2002, 19, 17–20. [Google Scholar] [CrossRef]
- Puotege, S.k.; Seidu, M.; Kangkpi, L.; Yussif, A.R.M.; Yakong, V.N.; Mumuni, A.N. Maternal body mass index, gestational weight gain and birth outcome among puerperal women in the Tamale metropolis of Ghana. Afr. J. Midwifery Women’s Health 2020, 14, 1–13. [Google Scholar] [CrossRef]
- Akinola, O.I.; Fabamwo, A.O.; Tayo, A.O.; Rabiu, K.A.; Oshodi, Y.A.; Alokha, M.E. Caesarean section—An appraisal of some predictive factors in Lagos Nigeria. BMC Pregnancy Childbirth 2014, 14, 217. [Google Scholar] [CrossRef]
- Edomwonyi, N.P.; Osaigbovo, P.E. Incidence of obesity in parturients scheduled for caesarean section, intra-operative complications, management and outcome. East Afr. Med. J. 2006, 83, 112–119. [Google Scholar] [CrossRef]
- Efiong, E.I. Pregnancy in the overweight Nigerian. Br. J. Obstet. Gynaecol. 1975, 82, 903–906. [Google Scholar] [CrossRef]
- Moraes, A.N.; Likwa, R.N.; Nzala, S.H. A retrospective analysis of adverse obstetric and perinatal outcomes in adolescent pregnancy: The case of Luapula Province, Zambia. Matern. Health Neonatol. Perinatol. 2018, 4, 20. [Google Scholar] [CrossRef]
- Mpotora, J.C.; Yahaya, J.J.; Ngw’eshemi, S.K.; Mwampagatwa, I.H. Rationale of indications for caesarean delivery and associated factors among primigravidae in Tanzania. J. Taibah Univ. Med. Sci. 2021, 16, 350–358. [Google Scholar] [CrossRef]
- Cresswell, J.A.; Campbell, O.M.; De Silva, M.J.; Slaymaker, E.; Filippi, V. Maternal obesity and Caesarean delivery in sub-Saharan Africa. Trop. Med. Int. Health 2016, 21, 879–885. [Google Scholar] [CrossRef]
- Adesina, K.; Aderibigbe, S.; Fawole, A.; Ijaiya, M.; Olarinoye, A. Pregnancy outcome of the obese in Ilorin. Obstet. Med. 2011, 4, 160–163. [Google Scholar] [CrossRef]
- Ezeanochie, M.C.; Ande, A.B.; Olagbuji, B.N. Maternal obesity in early pregnancy and subsequent pregnancy outcome in a Nigerian population. Afr. J. Reprod. Health 2011, 15, 55–59. [Google Scholar]
- Israel, J.; Solomon, N.; Chris, A.; Nwadiuto, A. Pregnancy outcome among obese paturients at the University of Port Harcourt Teaching Hospital, Nigeria. J. Med. Med. Sci. 2011, 2, 1152–1156. [Google Scholar]
- Ugwuja, E.I.; Akubugwo, E.I.; Obidoa, O.; Ibiam, A.U. Maternal BMI during Pregnancy: Effect on trace elements Status and Pregnancy Outcomes. Int. J. Health Res. 2011, 3, 71–78. [Google Scholar] [CrossRef]
- Iyoke, C.A.; Ugwu, G.O.; Ezugwu, F.O.; Lawani, O.L.; Onyebuchi, A.K. Retrospective cohort study of the effects of obesity in early pregnancy on maternal weight gain and obstetric outcomes in an obstetric population in Africa. Int. J. Womens Health 2013, 5, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Adewuyi, E.O.; Auta, A.; Khanal, V.; Tapshak, S.J.; Zhao, Y. Cesarean delivery in Nigeria: Prevalence and associated factors―A population-based cross-sectional study. BMJ Open 2019, 9, e027273. [Google Scholar] [CrossRef]
- Ngoga, E.; Hall, D.; Mattheyse, F.; Grové, D. Outcome of pregnancy in the morbidly obese woman. SA Fam. Pract. 2009, 51, 39–41. [Google Scholar] [CrossRef]
- Basu, J.K.; Jeketera, C.M.; Basu, D. Obesity and its outcomes among pregnant South African women. Int. J. Gynaecol. Obstet. 2010, 110, 101–104. [Google Scholar] [CrossRef]
- Davies, H.; Visser, J.; Tomlinson, M.; Rotheram-Borus, M.; Gissane, C.; Harwood, J.; LeRoux, I. An investigation into utilising gestational body mass index as a screening tool for adverse birth outcomes and maternal morbidities in a group of pregnant women in Khayelitsha. S. Afr. J. Clin. Nutr. 2013, 26, 116–122. [Google Scholar] [CrossRef][Green Version]
- Mwanamsangu, A.H.; Mahande, M.J.; Mazuguni, F.S.; Bishanga, D.R.; Mazuguni, N.; Msuya, S.E.; Mosha, D. Maternal obesity and intrapartum obstetric complications among pregnant women: Retrospective cohort analysis from medical birth registry in Northern Tanzania. Obes. Sci. Pract. 2020, 6, 171–180. [Google Scholar] [CrossRef]
- Fouelifack, F.Y.; Fouedjio, J.H.; Fouogue, J.T.; Sando, Z.; Fouelifa, L.D.; Mbu, R.E. Associations of body mass index and gestational weight gain with term pregnancy outcomes in urban Cameroon: A retrospective cohort study in a tertiary hospital. BMC Res. Notes 2015, 8, 806. [Google Scholar] [CrossRef]
- Tandu-Umba, B.; Mbangama, M.A.; Kamongola, K.M.B.; Kamgang Tchawou, A.G.; Kivuidi, M.P.; Kasonga Munene, S.; Kambashi Meke, I.; Kapuku Kabasele, O.; Kondoli, B.J.; Kikuni, K.R.; et al. Pre-pregnancy high-risk factors at first antenatal visit: How predictive are these of pregnancy outcomes? Int. J. Womens Health 2014, 6, 1011–1018. [Google Scholar] [CrossRef]
- Addo, V.N. Body Mass Index, Weight Gain during Pregnancy and Obstetric Outcomes. Ghana Med. J. 2010, 44, 64–69. [Google Scholar] [CrossRef]
- Endalifer, M.L.; Diress, G.; Almaw, H.; Endalifer, B.L. Effect of overweight/obesity on caesarean section occurrence among reproductive-aged women in Ethiopia: A secondary data analysis. BMJ Nutr. Prev. Health 2021, 4, 111–114. [Google Scholar] [CrossRef]
- Nkoka, O.; Ntenda, P.A.M.; Senghore, T.; Bass, P. Maternal overweight and obesity and the risk of caesarean birth in Malawi. Reprod. Health 2019, 16, 40. [Google Scholar] [CrossRef] [PubMed]
- Kelly, T.; Yang, W.; Chen, C.S.; Reynolds, K.; He, J. Global burden of obesity in 2005 and projections to 2030. Int. J. Obes. 2008, 32, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Modder, J.; Fitzsimons, K. CMACE/RCOG Joint Guideline: Management of Women with Obesity in Pregnancy; Centre for Maternal and Child Enquiries; Royal College of Obstetricians and Gynaecologists: London, UK, 2010. [Google Scholar]
- Davies, G.A.; Maxwell, C.; McLeod, L.; Gagnon, R.; Basso, M.; Bos, H.; Delisle, M.F.; Farine, D.; Hudon, L.; Menticoglou, S.; et al. SOGC Clinical Practice Guidelines: Obesity in pregnancy. No. 239, February 2010. Int. J. Gynaecol. Obstet. 2010, 110, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Slack, E.; Brandon, H.; Heslehurst, N. Chapter 13—Obesity and Pregnancy. In Practical Guide to Obesity Medicine; Weaver, J.U., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 143–151. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).