Panfolliculoma: Report of the Youngest Case and Literature Review of Its Histopathologic Variants
Abstract
:1. Introduction
2. Case Report
3. Methods
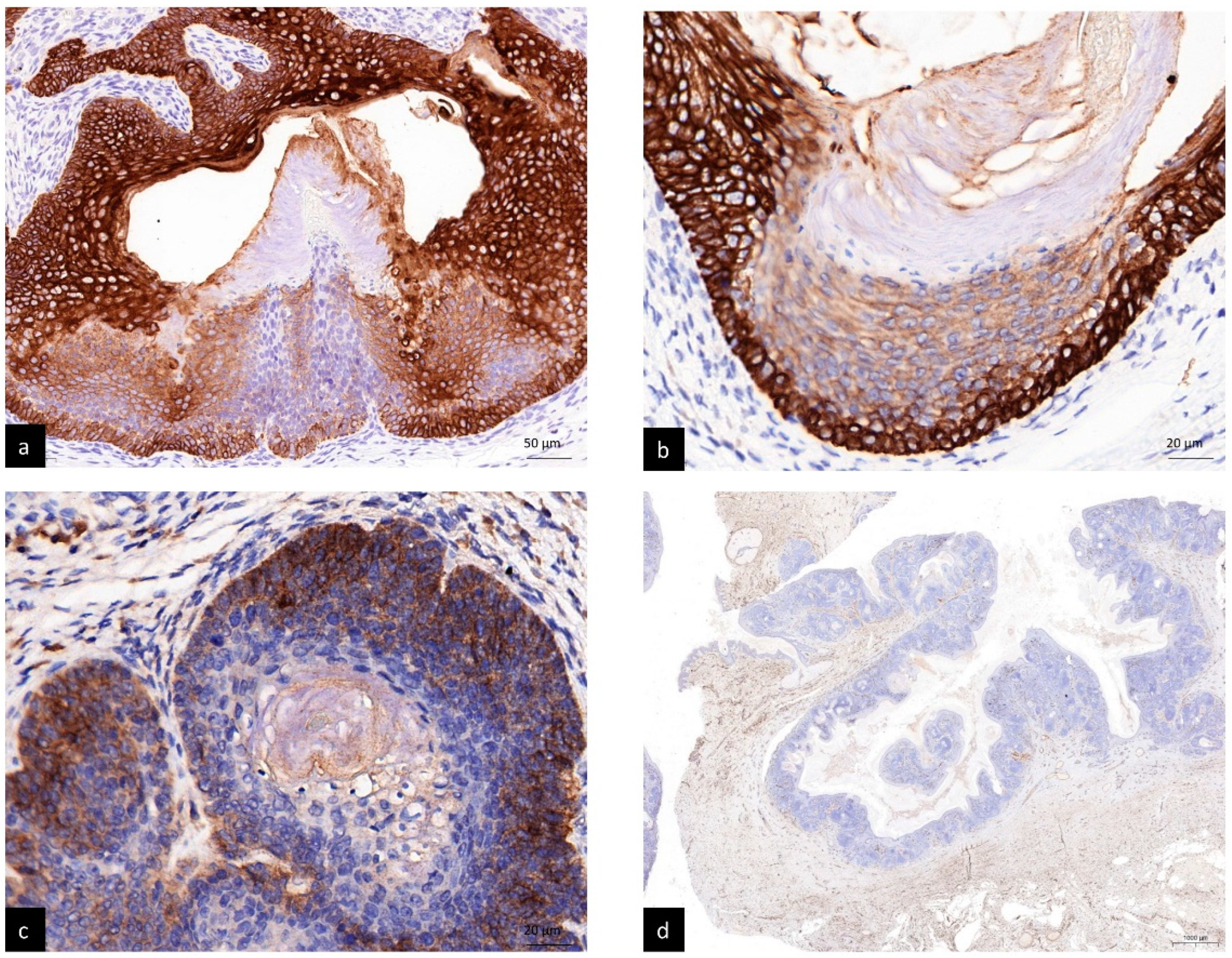
4. Results
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ackerman, A.B.; de Viragh, P.A.; Chongchitnant, N. Neoplasms with Follicular Differentiation; Lea & Febiger: London, UK, 1993. [Google Scholar]
- Schirren, C.G.; Rutten, A.; Plewig, G. Panfolliculoma. Clinical and immunohistochemical findings in 4 cases. Hautarzt 1996, 47, 610–615. (In German) [Google Scholar] [CrossRef] [PubMed]
- Marini, M.A.; Starck, F.; Duhm, G.; Baldrich, M.A.; Magariños, G. Panfolliculoma: Case presentation. Arch. Argent. Dermatol. 2006, 56, 71–73. (In Spanish) [Google Scholar]
- Hoang, M.P.; Levenson, B.M. Cystic panfolliculoma. Arch. Pathol. Lab. Med. 2006, 130, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Wu, Y.H. Panfolliculoma: Report of two cases. Dermatol. Sín. 2010, 28, 73–76. [Google Scholar] [CrossRef]
- Harris, A.; Faulkner-Jones, B.; Zimarowski, M.J. Epidermal panfolliculoma: A report of 2 cases. Am. J. Dermatopathol. 2011, 33, e7–e10. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Villaverde, R.; Elosegui-Martinez, F.; Luque-Barona, R. Panfolliculoma, an unusual cutaneous adnexal neoplasm. Eur. J. Dermatol. 2011, 21, 450–451. [Google Scholar] [CrossRef] [PubMed]
- Kacerovska, D.; Michal, M.; Kazakov, D.V. Panfolliculoma with sebaceous differentiation: A case report. Am. J. Dermatopathol. 2012, 34, e90–e93. [Google Scholar] [CrossRef] [PubMed]
- Idriss, M.H.; Khalil, A.; Long, W.; Elston, D.M. Epidermal panfolliculoma: An adnexal proliferation with advanced follicular differentiation confined to the epidermis. J. Cutan. Pathol. 2013, 40, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Alkhalidi, H.S.; Alhumaidy, A.A. Cystic panfolliculoma of the scalp: Report of a very rare case and brief review. Indian J. Pathol. Microbiol. 2013, 56, 437–439. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.J.; Guo, Y. Panfolliculoma and histopathologic variants: A study of 19 cases. Am. J. Dermatopathol. 2014, 36, 965. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.B.S.; Jacobson-Dunlop, E.; Elston, D.M. An unusual cystic lesion on the helix. Indian Dermatol. Online J. 2014, 5, 326–327. [Google Scholar] [PubMed]
- Neill, B.; Bingham, C.; Braudis, K.; Zurowski, S. A rare cutaneous adnexal neoplasm: Cystic panfolliculoma. J. Cutan. Pathol. 2016, 43, 1183–1185. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, S.; Hamasaki, Y.; Otani, T.; Izumi, M. A Case of Panfolliculoma. Nishi. Nihon. Hifuka. 2016, 78, 621–624. (In Japanese) [Google Scholar] [CrossRef]
- Terushkin, V.; Meehan, S.; Shahabi, L.; Brinster, N. Panfolliculoma with an endophytic architecture resembling a hair follicle: A report of three cases. Cutan. Pathol. 2016, 43, 1074–1076. [Google Scholar] [CrossRef] [PubMed]
- Parvinnejad, N.; Orr, C.; Wang, A.; Hurlbut, D. Panfolliculoma: Report of a rare cutaneous neoplasm and literature review. Cana. Dian. J. Pathol. 2017, 9 (Suppl. 9), 26. [Google Scholar]
- Estrada-Castañón, R.; Vega-Memije, M.E.; Cuevas-González, J.C.; Chávez-López, M.G. Panfolliculoma: A Clinical Case Report. Int. J. Trichol. 2017, 9, 187–189. [Google Scholar]
- Fukuyama, M.; Sato, Y.; Yamazaki, Y.; Ohyama, M. Immunohistochemical dissection of cystic panfolliculoma focusing on the ex pression of multiple hair follicle lineage markers with an insight into the pathogenesis. J. Cutan. Pathol. 2017, 44, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Rivera, V.; Campos, M. Cystic panfolliculoma: Case report. Med. Leg. Costa Rica 2018, 35, n.2. (In Spanish) [Google Scholar]
- Poblet, E.; Jiménez, F.; Godínez, J.M.; Pascual-Martín, A.; Izeta, A. The immunohistochemical expression of CD34 in human hair follicles: A comparative study with the bulge marker CK15. Clin. Exp. Dermatol. 2006, 31, 807–812. [Google Scholar] [CrossRef] [PubMed]


| Author, Year | Case Number | Age | Sex | Tumor Location | Clinical Impression | Lesion Size (cm) | Histopathology Variants |
|---|---|---|---|---|---|---|---|
| Schirren et al. 1996 [2] | 1 | 53 | M | infraclavicular | follicular cyst | 2~3 | NPF |
| 2 | N/A | N/A | N/A | N/A | N/A | N/A | |
| 3 | 22 | F | scalp | cylindroma | 2~3 | NPF | |
| 4 | 49 | F | right eyebrow | BCC | 2~3 | NPF | |
| Marini et al. 2006 [3] | 5 | 46 | F | nostril | N/A | N/A | N/A |
| Hoang et al. 2006 [4] | 6 | 33 | F | scalp | cyst | 3 × 2 × 2 | CPF |
| Huang et al. 2010 [5] | 7 | 41 | F | scalp | EC, pilar cyst | N/A | NPF |
| 8 | 51 | M | left eyebrow | ALHE, leiomyoma | N/A | NPF | |
| Harris et al. 2011 [6] | 9 | 81 | M | right medial thigh | inflammed SK | 0.6 | SPF |
| 10 | 61 | F | rightl lateral thigh | SCC | 0.3 | SPF | |
| Ruiz-Villaverde et al. 2011 [7] | 11 | 56 | M | Right tibia | EC | <3 | NPF |
| Kacerovska et al. 2012 [8] | 12 | 53 | M | occipital scalp | atheroma | N/A | NPF with sebaceous differentiation |
| Idriss et al. 2013 [9] | 13 | 55 | M | right leg | N/A | 0.8 | SPF |
| Alkhalidi et al. 2013 [10] | 14 | 19 | F | scalp | pilar cyst, lipoma | 0.9 × 0.8 | CPF |
| Shan et al. 2014 [11] | 15 | 76 | F | Left forearm | BCC | 0.5 × 0.4 | SPF |
| 16 | 65 | M | Left cheek | SK, AK, BCC | 0.4 × 0.3 | SPF | |
| 17 | 79 | M | Left nasal ala | BCC, dermal cyst | 0.6 × 0.5 | CPF | |
| 18 | 32 | M | Left scalp | Sebaceous cyst | 0.6 × 0.5 | CPF | |
| 19 | 55 | M | Right leg | VV | 0.5 × 0.4 | SPF | |
| 20 | 82 | M | Upper back | BCC | 1.2 × 0.8 | SPF | |
| 21 | 53 | M | Right low lip | Cyst | 0.3 × 0.3 | CPF | |
| 22 | 70 | F | Right lateral forehead | SCC, BCC | 0.8 × 0.6 | CPF | |
| 23 | 22 | F | Scalp | Cyst | 0.7 × 0.6 | CPF | |
| 24 | 33 | F | Left parietal scalp | BCC, nevus | 0.8 × 0.8 | NPF | |
| 25 | 49 | M | Right nostril | Cyst, BCC | 0.5 × 0.4 | CPF | |
| 26 | 70 | M | Central forehead | SCC, BCC | 0.7 × 0.6 | SPF | |
| 27 | 72 | M | Left post auricular | Wart, cyst | 0.4 × 0.4 | SPF | |
| 28 | 59 | F | Right lateral leg | Wart | 0.4 × 0.3 | SPF | |
| 29 | 71 | M | Forehead | BCC | 0.4 × 0.3 | SPF | |
| 30 | 83 | F | Left scalp | SCC | 4 × 3 | CPF | |
| 31 | 87 | M | Right temple | SCC, BCC | 0.8 × 0.7 | NPF | |
| 32 | 70 | M | Nose | Cyst, BCC | 0.4 × 0.3 | NPF | |
| 33 | 67 | M | Right lower leg | SCC | 0.4 × 0.3 | SPF | |
| Patel et al. 2014 [12] | 34 | 73 | M | Left helix | EC | N/A | CPF |
| Neill et al. 2016 [13] | 35 | 64 | F | right forearm | SCC, amelanotic melanoma, poroma | 0.8 | CPF |
| Nishikawa et al. 2016 [14] | 36 | 36 | F | back | N/A | 2.3 × 3 | NPF |
| Terushkin et al. 2016 [15] | 37 | 56 | F | Left cheek | BCC | N/A | endophytic |
| 38 | 88 | M | right anti-helix | cyst | N/A | endophytic | |
| 39 | 53 | F | right pretibial leg | foreign body | N/A | endophytic | |
| Parvinnejad et al.2017 [16] | 40 | 74 | M | abdomen | BCC | N/A | CPF |
| Estrada-Castañón et al. 2017 [17] | 41 | 55 | F | eyelid | comedone | N/A | CPF |
| Fukuyama et al. 2017 [18] | 42 | 70 | M | occipital scalp | trichilemmal cyst | 2.4 | CPF |
| Rivera et al.2018 [19] | 43 | 35 | M | right occipital scalp | pilar cyst | 1.3 | CPF |
| Present case | 44 | 14 | M | back | follicular cyst | 6 × 4 | CPF |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juang, S.-J.; Win, K.-T.; Lai, F.-J. Panfolliculoma: Report of the Youngest Case and Literature Review of Its Histopathologic Variants. Life 2022, 12, 881. https://doi.org/10.3390/life12060881
Juang S-J, Win K-T, Lai F-J. Panfolliculoma: Report of the Youngest Case and Literature Review of Its Histopathologic Variants. Life. 2022; 12(6):881. https://doi.org/10.3390/life12060881
Chicago/Turabian StyleJuang, Shiow-Jen, Khin-Than Win, and Feng-Jie Lai. 2022. "Panfolliculoma: Report of the Youngest Case and Literature Review of Its Histopathologic Variants" Life 12, no. 6: 881. https://doi.org/10.3390/life12060881
APA StyleJuang, S.-J., Win, K.-T., & Lai, F.-J. (2022). Panfolliculoma: Report of the Youngest Case and Literature Review of Its Histopathologic Variants. Life, 12(6), 881. https://doi.org/10.3390/life12060881





