Association of the Genetic Variation in the Long Non-Coding RNA FENDRR with the Risk of Developing Hypertrophic Cardiomyopathy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Controls
2.2. Next Generation Sequencing
2.3. Genotyping Studies
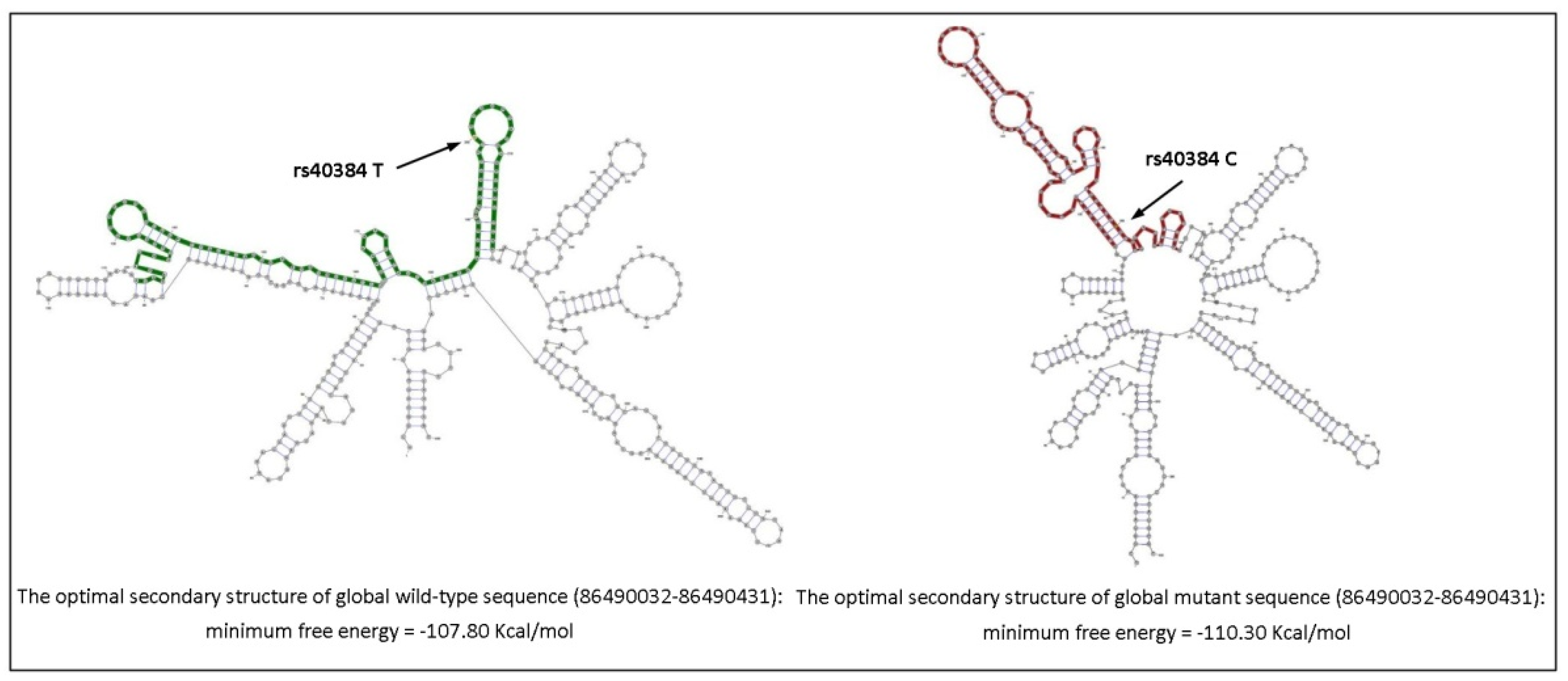
2.4. FENDRR Promoter Sanger Sequencing
2.5. Statistic Analysis
3. Results
3.1. Next Generation Sequencing in Discovery Cohort
3.2. Genotyping Studies in Validation Cohort
3.3. FENDRR Promoter Sanger Sequencing
4. Discussion
5. Limitations of the Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maron, B.J.; Maron, M.S. Hypertrophic cardiomyopathy. Lancet 2013, 381, 242–255. [Google Scholar] [CrossRef]
- Gómez, J.; Lorca, R.; Reguero, J.R.; Morís, C.; Martín, M.; Tranche, S.; Alonso, B.; Iglesias, S.; Alvarez, V.; Díaz-Molina, B.; et al. Screening of the Filamin C Gene in a Large Cohort of Hypertrophic Cardiomyopathy Patients. Circ. Cardiovasc. Genet. 2017, 10, e001584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marian, A.J.; Braunwald, E. Hypertrophic Cardiomyopathy: Genetics, Pathogenesis, Clinical Manifestations, Diagnosis, and Therapy. Circ. Res. 2017, 121, 749–770. [Google Scholar] [CrossRef] [PubMed]
- Lorca, R.; Gómez, J.; Martín, M.; Cabanillas, R.; Calvo, J.; León, V.; Pascual, I.; Morís, C.; Coto, E.; Reguero, J.J.R. Insights Into Hypertrophic Cardiomyopathy Evaluation Through Follow-up of a Founder Pathogenic Variant. Rev. EspCardiol 2019, 72, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Bajic, V.B.; Zhang, Z. On the classification of long non-coding RNAs. RNA Biol. 2013, 10, 925–933. [Google Scholar] [CrossRef]
- Kopp, F.; Mendell, J.T. Functional Classification and Experimental Dissection of Long Noncoding RNAs. Cell 2018, 172, 393–407. [Google Scholar] [CrossRef] [Green Version]
- Grote, P.; Wittler, L.; Hendrix, D.; Koch, F.; Währisch, S.; Beisaw, A.; Macura, K.; Bläss, G.; Kellis, M.; Werber, M.; et al. The tissue-specific lncRNA Fendrr is an essential regulator of heart and body wall development in the mouse. Dev. Cell 2013, 24, 206–214. [Google Scholar] [CrossRef] [Green Version]
- Grote, P.; Herrmann, B.G. The long non-coding RNA Fendrr links epigenetic control mechanisms to gene regulatory networks in mammalian embryogenesis. RNA Biol. 2013, 10, 1579–1585. [Google Scholar] [CrossRef] [Green Version]
- Han, P.; Li, W.; Lin, C.-H.; Yang, J.; Shang, C.; Nurnberg, S.T.; Jin, K.K.; Xu, W.; Lin, C.-Y.; Lin, C.-J.; et al. A long noncoding RNA protects the heart from pathological hypertrophy. Nature 2014, 514, 102–106. [Google Scholar] [CrossRef] [Green Version]
- Ounzain, S.; Micheletti, R.; Arnan, C.; Plaisance, I.; Cecchi, D.; Schroen, B.; Reverter, F.; Alexanian, M.; Gonzales, C.; Ng, S.-Y.; et al. CARMEN, a human super enhancer-associated long noncoding RNA controlling cardiac specification, differentiation and homeostasis. J. Mol. Cell. Cardiol. 2015, 89, 98–112. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Li, L.; Peng, L. MAPK1 up-regulates the expression of MALAT1 to promote the proliferation of cardiomyocytes through PI3K/AKT signaling pathway. Int. J. Clin. Exp. Pathol. 2015, 8, 15947–15953. [Google Scholar] [PubMed]
- Liu, L.; An, X.; Li, Z.; Song, Y.; Li, L.; Zuo, S.; Liu, N.; Yang, G.; Wang, H.; Cheng, X.; et al. The H19 long noncoding RNA is a novel negative regulator of cardiomyocyte hypertrophy. Cardiovasc. Res. 2016, 111, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.H.; Yuan, Y.X.; Rao, S.L.; Wang, P. LncRNA MIAT enhances cardiac hypertrophy partly through sponging miR-150. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 3653–3660. [Google Scholar] [PubMed]
- Haemmig, S.; Simion, V.; Yang, D.; Deng, Y.; Feinberg, M.W. Long noncoding RNAs in cardiovascular disease, diagnosis, and therapy. Curr. Opin. Cardiol. 2017, 32, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Coto, E.; Calvo, D.; Reguero, J.R.; Morís, C.; Rubín, J.M.; Díaz-Corte, C.; Gil-Peña, H.; Alosno, B.; Iglesias, S.; Gómez, J. Differential methylation of lncRNA KCNQ1OT1 promoter polymorphism was associated with symptomatic cardiac long QT. Epigenomics 2017, 9, 1049–1057. [Google Scholar] [CrossRef]
- Shao, M.; Chen, G.; Lv, F.; Liu, Y.; Tian, H.; Tao, R.; Jiang, R.; Zhang, W.; Zhuo, C. LncRNA TINCR attenuates cardiac hypertrophy by epigenetically silencing CaMKII. Oncotarget 2017, 8, 47565–47573. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Fang, Z.; He, S.; Wang, J.; Yang, X. ANRIL polymorphism rs4977574 is associated with increased risk of coronary artery disease in Asian populations: A meta-analysis of 12,005 subjects. Medicine 2018, 97, e12641. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, Q.; Wang, J.; Fan, D.; Zhou, H.; Yuan, Y.; Shen, D. Long non-coding RNA Pvt1 modulates the pathological cardiac hypertrophy via miR-196b-mediated OSMR regulation. Cell. Signal. 2021, 86, 110077. [Google Scholar] [CrossRef]
- Gómez, J.; Lorca, R.; Reguero, J.R.; Martín, M.; Morís, C.; Alonso, B.; Iglesias, S.; Díaz-Molina, B.; Avanzas, P.; Coto, E. Genetic variation at the long noncoding RNA H19 gene is associated with the risk of hypertrophic cardiomyopathy. Epigenomics 2018, 10, 865–873. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [Green Version]
- Gómez, J.; Reguero, J.R.; Morís, C.; Martín, M.; Alvarez, V.; Alonso, B.; Iglesias, S.; Coto, E. Mutation analysis of the main hypertrophic cardiomyopathy genes using multiplex amplification and semiconductor next-generation sequencing. Circ. J. 2014, 78, 2963–2971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabarinathan, R.; Tafer, H.; Seemann, S.E.; Hofacker, I.L.; Stadler, P.F.; Gorodkin, J. The RNAsnp web server: Predicting SNP effects on local RNA secondary structure. Nucleic Acids Res. 2013, 41, W475–W479. [Google Scholar] [CrossRef] [Green Version]
- Szafranski, P.; Stankiewicz, P. Long Non-Coding RNA FENDRR: Gene Structure, Expression, and Biological Relevance. Genes 2021, 12, 177. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Li, H.; Zheng, J.; Xu, J.; Gao, P.; Wang, J. FENDRR Sponges miR-424-5p to Inhibit Cell Proliferation, Migration and Invasion in Colorectal Cancer. Technol. Cancer Res. Treat. 2020, 19, 1–9. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhang, Q.; Yu, X.; He, Y.; Guo, W. FENDRR: A pivotal, cancer-related, long non-coding RNA. Biomed. Pharmacother. 2021, 137, 111390. [Google Scholar] [CrossRef] [PubMed]
- Yuxuan, C.; Wang, B.; Cai, Y.; Chengfu, Y.; Meng, E.; Guo, C.; Zhou, G.; Yuan, C. The Therapeutic Value and Molecular Mechanisms of lncRNA FENDRR in Human Cancer. Curr. Pharm. Des. 2021, 27, 4100–4106. [Google Scholar] [CrossRef]
- Li, X.; Ni, L.; Wang, W.; Zong, L.; Yao, B. LncRNA Fendrr inhibits hypoxia/reoxygenation-induced cardiomyocyte apoptosis by downregulating p53 expression. J. Pharm. Pharmacol. 2020, 72, 1211–1220. [Google Scholar] [CrossRef]
- GTEx Consortium. The Genotype-Tissue Expression (GTEx) project. Nat. Genet. 2013, 45, 580–585. [Google Scholar] [CrossRef]
- Fagerberg, L.; Hallström, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteom. 2014, 13, 397–406. [Google Scholar] [CrossRef] [Green Version]
- Kontaraki, J.E.; Marketou, M.E.; Kochiadakis, G.E.; Maragkoudakis, S.; Konstantinou, J.; Vardas, P.E.; Parthenakis, F.I. The long non-coding RNAs MHRT, FENDRR and CARMEN, their expression levels in peripheral blood mononuclear cells in patients with essential hypertension and their relation to heart hypertrophy. Clin. Exp. Pharmacol. Physiol. 2018, 45, 1213–1217. [Google Scholar] [CrossRef]
- Gong, L.; Zhu, L.; Yang, T. Fendrr involves in the pathogenesis of cardiac fibrosis via regulating miR-106b/SMAD3 axis. Biochem. Biophys. Res. Commun. 2020, 524, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Gardin, J.M.; Flack, J.M.; Gidding, S.S.; Kurosaki, T.T.; Bild, D.E. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation 1995, 92, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Olivotto, I.; Maron, M.S.; Adabag, S.; Casey, S.A.; Vargiu, D.; Link, M.S.; Udelson, J.E.; Cecchi, F.; Maron, B.J. Gender-related differences in the clinical presentation and outcome of hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2005, 46, 480–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Towbin, J.A. Hypertrophic cardiomyopathy. Pacing Clin. Electrophysiol. 2009, 32 (Suppl. S2), S23–S31. [Google Scholar] [CrossRef]
- Geske, J.B.; Ong, K.C.; Siontis, K.C.; Hebl, V.B.; Ackerman, M.J.; Hodge, D.O.; Miller, V.M.; Nishimura, R.A.; Oh, J.K.; Schaff, H.; et al. Women with hypertrophic cardiomyopathy have worse survival. Eur. Heart J. 2017, 38, 3434–3440. [Google Scholar] [CrossRef] [Green Version]
- Huang, F.Y.; Shah, J.P.; Pu, X.B.; Hagar, A.; Chen, S.J. Influence of Gender on Clinical Characteristics and Outcomes in Chinese Patients with Hypertrophic Cardiomyopathy. Am. J. Med. Sci. 2020, 360, 517–524. [Google Scholar] [CrossRef]
- Seo, J.; Hong, Y.J.; Kim, Y.J.; Lkhagvasuren, P.; Cho, I.; Shim, C.Y.; Ha, J.-W.; Hong, G.-R. Prevalence, functional characteristics, and clinical significance of right ventricular involvement in patients with hypertrophic cardiomyopathy. Sci. Rep. 2020, 10, 21908. [Google Scholar] [CrossRef]
- Butters, A.; Lakdawala, N.K.; Ingles, J. Sex Differences in Hypertrophic Cardiomyopathy: Interaction with Genetics and Environment. Curr. Heart Fail. Rep. 2021, 18, 264–273. [Google Scholar] [CrossRef]
- Miller, S.A.; Dykes, D.D.; Polesky, H.F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988, 16, 1215. [Google Scholar] [CrossRef]
| lncRNAs | Mechanism of Action | References |
|---|---|---|
| FENDRR | Cardiovascular embryogenesis, interaction with PRC2 | [7,8] |
| MHRT | Antagonizes the role of Brg1 in cardiac hypertrophy | [9] |
| CARMEN | Regulation of cardiac differentiation | [10] |
| MALAT1 | Regulates the proliferation of endothelial cells and cardiomyocytes, epigenetic and alternative splicing regulation | [11] |
| H19 | Regulates cardiac hypertrophy through miR-675 | [12,19] |
| MIAT | Regulates cardiac hypertrophy, miR-150 molecular sponge | [13] |
| KCNQ1OT1 | Long QT syndrome, regulator of gene expression | [15] |
| TINCR | Regulates cardiac hypertrophy, epigenetic silencing of CaMKII | [16] |
| ANRIL | Structure and function of vascular smooth muscle, regulator of gene expression | [17] |
| PVT1 | Modulates the pathological cardiac hypertrophy via miR-196b | [18] |
| Discovery | Validation | |||||
|---|---|---|---|---|---|---|
| Controls | Patients | p-Value | Controls | Patients | p-Value | |
| (N = 212) | (N = 238) | (N = 923) | (N = 962) | |||
| Age | 72.0 [64.0, 76.0] | 61.0 [51.0, 69.0] | <0.001 | 66.0 [55.8, 78.0] | 60.0 [48.0, 69.0] | <0.001 |
| Sex | ||||||
| Men | 91 (45.0%) | 148 (63.0%) | <0.001 | 518 (56.1%) | 620 (64.4%) | <0.001 |
| Women | 111 (55.0%) | 87 (37.0%) | 405 (43.9%) | 342 (35.6%) | ||
| Sarcomere | ||||||
| No | - | 186 (78.2%) | - | - | 763 (79.3%) | - |
| Yes | - | 52 (21.8%) | - | 199 (20.7%) | ||
| Patients | Controls | Total Patients | Total Controls | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | ≥50 | < 50 | Men | Women | ≥50 | <50 | |||
| (N = 620) | (N = 342) | (N = 691) | (N = 271) | (N = 518) | (N = 405) | (N = 763) | (N = 160) | (N = 962) | (N = 923) | |
| rs74035787 | ||||||||||
| GG (n (%)) | 607 (97.9%) | 338 (98.8%) | 680 (98.4%) | 265 (97.8%) | 499 (96.3%) | 382 (94.3%) | 727 (95.3%) | 154 (96.3%) | 945 (98.2%) | 881 (95.4%) |
| GA (n (%)) | 13 (2.10%) | 4 (1.17%) | 11 (1.59%) | 6 (2.21%) | 19 (3.67%) | 23 (5.68%) | 36 (4.72%) | 6 (3.75%) | 17 (1.77%) | 42 (4.55%) |
| G (Freq.) | 0.990 | 0.994 | 0.992 | 0.989 | 0.982 | 0.972 | 0.976 | 0.981 | 0.991 | 0.977 |
| A (Freq.) | 0.011 | 0.006 | 0.008 | 0.011 | 0.018 | 0.028 | 0.024 | 0.019 | 0.009 | 0.023 |
| rs1424019 | ||||||||||
| AA (n (%)) | 53 (8.55%) | 26 (7.60%) | 55 (7.96%) | 24 (8.86%) | 42 (8.11%) | 30 (7.41%) | 58 (7.60%) | 14 (8.75%) | 79 (8.21%) | 72 (7.80%) |
| AG (n (%)) | 212 (34.2%) | 109 (31.9%) | 222 (32.1%) | 99 (36.5%) | 208 (40.2%) | 133 (32.8%) | 286 (37.5%) | 55 (34.4%) | 321 (33.4%) | 341 (36.9%) |
| GG (n (%)) | 355 (57.3%) | 207 (60.5%) | 414 (59.9%) | 148 (54.6%) | 268 (51.7%) | 242 (59.8%) | 419 (54.9%) | 91 (56.9%) | 562 (58.4%) | 510 (55.3%) |
| A (Freq.) | 0.256 | 0.235 | 0.240 | 0.271 | 0.282 | 0.238 | 0.263 | 0.259 | 0.249 | 0.263 |
| G (Freq.) | 0.744 | 0.765 | 0.760 | 0.729 | 0.718 | 0.762 | 0.737 | 0.741 | 0.751 | 0.737 |
| Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|
| Predictors | Odds Ratio | CI | p-Value | Odds Ratio | CI | p-Value | |
| rs74035787 | GG | Reference | Reference | ||||
| GA | 0.38 | 0.21–0.66 | 0.001 | 0.38 | 0.21–0.67 | 0.001 | |
| Women | 0.71 | 0.59–0.85 | <0.001 | ||||
| G | Reference | Reference | |||||
| A | 0.38 | 0.21–0.66 | 0.001 | 0.39 | 0.21–0.67 | 0.001 | |
| Women | 0.71 | 0.62–0.81 | <0.001 | ||||
| rs1424019 | GG | Reference | Reference | ||||
| AA o AG | 0.88 | 0.73–1.05 | 0.165 | 0.86 | 0.72–1.04 | 0.112 | |
| Women | 0.70 | 0.58–0.84 | <0.001 | ||||
| G | Reference | Reference | |||||
| A | 0.93 | 0.80–1.08 | 0.333 | 0.92 | 0.79–1.06 | 0.248 | |
| Women | 0.70 | 0.62–0.80 | <0.001 | ||||
| Predictors | Women | Men | |||||
| Odds Ratio | CI | p-Value | Odds Ratio | CI | p-Value | ||
| rs74035787 | GA | 0.20 | 0.06–0.52 | 0.003 | 0.56 | 0.27–1.14 | 0.115 |
| A | 0.20 | 0.06–0.53 | 0.003 | 0.57 | 0.27–1.14 | 0.118 | |
| rs1424019 | GG | 1.03 | 0.77–1.39 | 0.830 | 1.25 | 0.99–1.58 | 0.063 |
| G | 1.02 | 0.80–1.29 | 0.896 | 1.14 | 0.94–1.37 | 0.173 | |
| Predictors | ≥50 yo | <50 yo | |||||
| Odds Ratio | CI | p-Value | Odds Ratio | CI | p-Value | ||
| rs74035787 | GA | 0.33 | 0.16–0.63 | 0.001 | 0.70 | 0.21–2.36 | 0.559 |
| Women | 0.87 | 0.71–1.08 | 0.0208 | 0.39 | 0.25–0.60 | <0.001 | |
| A | 0.33 | 0.16–0.63 | 0.001 | 0.71 | 0.21–2.35 | 0.563 | |
| Women | 0.87 | 0.75–1.01 | 0.073 | 0.39 | 0.29–0.53 | <0.001 | |
| rs1424019 | GG | 1.24 | 1.01–1.53 | 0.045 | 0.92 | 0.61–1.38 | 0.686 |
| Women | 0.86 | 0.70–1.06 | 0.162 | 0.39 | 0.25–0.60 | <0.001 | |
| G | 1.14 | 0.96–1.35 | 0.129 | 0.93 | 0.68–1.28 | 0.674 | |
| Women | 0.87 | 0.75–1.01 | 0.062 | 0.39 | 0.29–0.53 | <0.001 | |
| Polymorphism | Haplotype Frequency | |||||
|---|---|---|---|---|---|---|
| rs74035787 G>A | rs1424019 A>G | Patients N = 962 | Controls N = 923 | p-Value | OR | 95%CI |
| G | G | 1441 (0.749) | 1341 (0.726) | 0.1160 | 1.13 | 0.97–1.30 |
| G | A | 466 (0.242) | 464 (0.251) | 0.5147 | 0.95 | 0.82–1.10 |
| A | G | 4 (0.002) | 19 (0.010) | 0.0035 | 0.20 | 0.07–0.59 |
| A | A | 13 (0.007) | 22 (0.012) | 0.1031 | 0.56 | 0.28–1.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuesta-Llavona, E.; Lorca, R.; Rolle, V.; Alonso, B.; Iglesias, S.; Rodríguez-Reguero, J.; Duarte-Herrera, I.D.; Pérez-Oliveira, S.; Junco-Vicente, A.; Lago, C.G.; et al. Association of the Genetic Variation in the Long Non-Coding RNA FENDRR with the Risk of Developing Hypertrophic Cardiomyopathy. Life 2022, 12, 818. https://doi.org/10.3390/life12060818
Cuesta-Llavona E, Lorca R, Rolle V, Alonso B, Iglesias S, Rodríguez-Reguero J, Duarte-Herrera ID, Pérez-Oliveira S, Junco-Vicente A, Lago CG, et al. Association of the Genetic Variation in the Long Non-Coding RNA FENDRR with the Risk of Developing Hypertrophic Cardiomyopathy. Life. 2022; 12(6):818. https://doi.org/10.3390/life12060818
Chicago/Turabian StyleCuesta-Llavona, Elías, Rebeca Lorca, Valeria Rolle, Belén Alonso, Sara Iglesias, Julian Rodríguez-Reguero, Israel David Duarte-Herrera, Sergio Pérez-Oliveira, Alejandro Junco-Vicente, Claudia García Lago, and et al. 2022. "Association of the Genetic Variation in the Long Non-Coding RNA FENDRR with the Risk of Developing Hypertrophic Cardiomyopathy" Life 12, no. 6: 818. https://doi.org/10.3390/life12060818
APA StyleCuesta-Llavona, E., Lorca, R., Rolle, V., Alonso, B., Iglesias, S., Rodríguez-Reguero, J., Duarte-Herrera, I. D., Pérez-Oliveira, S., Junco-Vicente, A., Lago, C. G., Coto, E., & Gómez, J. (2022). Association of the Genetic Variation in the Long Non-Coding RNA FENDRR with the Risk of Developing Hypertrophic Cardiomyopathy. Life, 12(6), 818. https://doi.org/10.3390/life12060818







