Growth Parameters and Mortality Rates Estimated for Seven Data-Deficient Fishes from the Azores Based on Length-Frequency Data
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Growth Parameters
2.3. Mortality Rates
3. Results
3.1. Length Distribution
3.2. Growth Parameters
3.3. Mortality Rates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sparre, P.; Venema, S.C. Introduction to Tropical Fish Stock Assessment. Pt. 1: Manual. Pt. 2: Exercises; FAO: Rome, Italy, 1998; ISBN 0429-9345. [Google Scholar]
- Cadima, E.L. Fish Stock Assessment Manual; FAO: Rome, Italy, 2003; ISBN 9251045054. [Google Scholar]
- Cochrane, K.L. A Fishery Manager’s Guidebook: Management Measures and Their Application; FAO: Rome, Italy, 2002. [Google Scholar]
- ICES. Guide to ICES Advisory Framework and Principles. In Report of the ICES Advisory Committee. ICES Advice; ICES: Copenhagen, Denmark, 2020; pp. 1–8. [Google Scholar]
- Pauly, D. Some Simple Methods for the Assessment of Tropical Fish Stocks; FAO: Rome, Italy, 1983. [Google Scholar]
- Then, A.Y.; Hoenig, J.M.; Huynh, Q.C. Estimating Fishing and Natural Mortality Rates, and Catchability Coefficient, from a Series of Observations on Mean Length and Fishing Effort. ICES J. Mar. Sci. 2017, 75, 610–620. [Google Scholar] [CrossRef]
- Von Bertalanffy, L. A Quantitative Theory of Organic Growth (Inquires on Growth Laws. II). Hum. Biol. 1938, 10, 181–213. [Google Scholar]
- Gedamke, T.; Hoenig, J.M. Estimating Mortality from Mean Length Data in Nonequilibrium Situations, with Application to the Assessment of Goosefish. Trans. Am. Fish. Soc. 2006, 135, 476–487. [Google Scholar] [CrossRef]
- Quinn, T.J.; Deriso, R.B. Quantitative Fish Dynamics; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Santos, R.; Medeiros-Leal, W.; Pinho, M. Synopsis of Biological, Ecological and Fisheries-Related Information on Priority Marine Species in the Azores Region. Arquipel. Life Mar. Sci. 2020, 1, 1–138. [Google Scholar]
- Vasconcelos, J.; Alves, A.; Gouveia, E.; Faria, G. Age and Growth of the Blue Jack Mackerel, Trachurus picturatus Bowdich, 1825 (Pisces: Teleostei) off Madeira Archipelago. Arquipél. Life Mar. Sci. 2006, 23A, 47–57. [Google Scholar]
- Carvalho, N.; Perrotta, R.G.; Isidro, E. Age, Growth and Maturity in the Chub Mackerel (Scomber japonicus Houttuyn, 1782) from the Azores. Arquipel. Life Mar. Sci. 2002, 19A, 93–99. [Google Scholar]
- Daley, T.T.; Leaf, R.T. Age and Growth of Atlantic Chub Mackerel (Scomber colias) in the Northwest Atlantic. J. Northwest Atl. Fish. Sci. 2019, 50, 1–12. [Google Scholar] [CrossRef]
- Delgado, J.; Reis, S.; González, J.A.; Isidro, E.; Biscoito, M.; Freitas, M.; Tuset, V.M. Reproduction and Growth of Aphanopus carbo and A. intermedius (Teleostei: Trichiuridae) in the Northeastern Atlantic. J. Appl. Ichthyol. 2013, 29, 1008–1014. [Google Scholar] [CrossRef]
- Vasconcelos, J.; Sousa, R.; Riera, R.; Delgado, J.; Faria, G.; Serrano Gordo, L. Stock Assessment of the Blue Jack Mackerel, Trachurus picturatus, in the North-Eastern Atlantic. Fish. Manag. Ecol. 2018, 25, 233–239. [Google Scholar] [CrossRef]
- Jurado-Ruzafa, A.; Hernández, E.; Santamaría, T.G. Age, Growth and Natural Mortality of Atlantic Chub Mackerel Scomber colias Gmelin 1789 (Perciformes: Scombridae), from Mauritania (NW Africa). Vieraea 2017, 45, 53–64. [Google Scholar] [CrossRef]
- Garcia, A.; Canha, Â.; Reis, D.; Diogo, H. Others Life History Parameters of Blue Jack Mackerel Trachurus picturatus (Teleostei: Carangidae) from North-East Atlantic. J. Mar. Biol. Assoc. U. K. 2015, 95, 401–410. [Google Scholar] [CrossRef]
- Kožul, V.; Skaramuca, B.; Kraljević, M.; Dulčić, J.; Glamuzina, B. Age, Growth and Mortality of the Mediterranean Amberjack Seriola dumerili (Risso 1810) from the South-Eastern Adriatic Sea. J. Appl. Ichthyol. 2001, 17, 134–141. [Google Scholar] [CrossRef]
- Morales-Nin, B.; Sena-Carvalho, D. Age and Growth of the Black Scabbard Fish (Aphanopus carbo) off Madeira. Fish. Res. 1996, 25, 239–251. [Google Scholar] [CrossRef]
- Pajuelo, J.G.; González, J.A.; Santana, J.I.; Lorenzo, J.M.; García-Mederos, A.; Tuset, V. Biological Parameters of the Bathyal Fish Black Scabbardfish (Aphanopus carbo Lowe, 1839) off the Canary Islands, Central-East Atlantic. Fish. Res. 2008, 92, 140–147. [Google Scholar] [CrossRef]
- Thompson, B.A.; Beasley, M.; Wilson, C.A. Age Distribution and Growth of Greater Amberjack, Seriola dumerili, from the North-Central Gulf of Mexico. Fish. Bull. 1999, 97, 362–371. [Google Scholar]
- Velasco, E.M.; Del Arbol, J.; Baro, J.; Sobrino, I. Age and Growth of the Spanish Chub Mackerel Scomber Colias off Southern Spain: A Comparison between Samples from the NE Atlantic and the SW Mediterranean. Rev. Biol. Mar. Oceanogr. 2011, 46, 27–34. [Google Scholar] [CrossRef]
- Harris, P.J.; Wyanski, D.M.; White, D.B.; Mikell, P.P.; Eyo, P.B. Age, Growth, and Reproduction of Greater Amberjack off the Southeastern U.S. Atlantic Coast. Trans. Am. Fish. Soc. 2007, 136, 1534–1545. [Google Scholar] [CrossRef]
- Navarro, M.R.; Landa, J.; Villamor, B.; Domínguez-Petit, R. First Approach to the Growth and Age Corroboration of Northeast Atlantic Chub Mackerel (Scomber colias) in Northern Iberian Waters. Estuar. Coast. Shelf Sci. 2021, 259, 107433. [Google Scholar] [CrossRef]
- Manooch, C.S., III; Potts, J.C. Age, Growth and Mortality of Greater Amberjack from the Southeastern United States. Fish. Res. 1997, 30, 229–240. [Google Scholar] [CrossRef]
- Manooch, C.S., III; Potts, J.C. Age, Growth, and Mortality of Greater Amberjack, Seriola dumerili, from the U.S. Gulf of Mexico Headboat Fishery. Bull. Mar. Sci. 1997, 61, 671–683. [Google Scholar]
- Petrakis, G.; Papaconstantinou, C. Biology of Sparisoma cretense in the Dodecanese (Greece). J. Appl. Ichthyol. 1990, 6, 14–23. [Google Scholar] [CrossRef]
- Jurado-Ruzafa, A.; Santamaría, M.T.G. Age, Growth and Natural Mortality of Blue Jack Mackerel Trachurus picturatus (Carangidae) from the Canary Islands, Spain (NW Africa). Afr. J. Mar. Sci. 2018, 40, 451–460. [Google Scholar] [CrossRef]
- Čikeš Keč, V.; Zorica, B. Length–Weight Relationship, Age, Growth and Mortality of Atlantic Chub Mackerel Scomber colias in the Adriatic Sea. J. Mar. Biol. Assoc. U. K. 2013, 93, 341–349. [Google Scholar] [CrossRef]
- Amorim, P.; Sousa, P.; Menezes, G.M. Sustainability Status of the Grouper Fishery in the Azores Archipelago: A Length-Based Approach. Mar. Policy 2021, 130, 104562. [Google Scholar] [CrossRef]
- Tuset, V.M.; González, J.A.; Lozano, I.J.; García-Díaz, M.M. Age and Growth of the Blacktail Comber, Serranus atricauda (Serranidae), off the Canary Islands (Central-Eastern Atlantic). Bull. Mar. Sci. 2004, 74, 53–68. [Google Scholar]
- Santos, R.; Medeiros-Leal, W.; Pinho, M. Stock Assessment Prioritization in the Azores: Procedures, Current Challenges and Recommendations. Arquipel. Life Mar. Sci. 2020, 37, 20–45. [Google Scholar]
- Isidro, H.A. Age and Growth of Trachurus picturatus (Bowdich, 1825) (Teleostei: Carangidae) from the Azores. Arquipel. Life Mar. Sci. 1990, 8, 45–54. [Google Scholar]
- Neves, A.; Vieira, A.R.; Sequeira, V.; Silva, E.; Silva, F.; Duarte, A.M.; Mendes, S.; Ganhão, R.; Assis, C.; Rebelo, R.; et al. Modelling Fish Growth with Imperfect Data: The Case of Trachurus picturatus. Fishes 2022, 7, 52. [Google Scholar] [CrossRef]
- Shahrani, S.; Shakman, E. Biological Aspects of Scorpaena scrofa Linnaeus, 1758 in the Western Libyan Coast. Libyan J. Mar. Sci. 2015, 14, 29–42. [Google Scholar]
- Vieira, A.R.; Farias, I.; Figueiredo, I.; Neves, A.; Morales-Nin, B.; Sequeira, V.; Martins, M.R.; Gordo, L.S. Age and Growth of Black Scabbardfish (Aphanopus carbo Lowe, 1839) in the Southern NE Atlantic. Sci. Mar. 2009, 73, 33–46. [Google Scholar] [CrossRef] [Green Version]
- EU. Council Regulation (EC) No 199/2008 of 25 February 2008 Concerning the Establishment of a Community Framework for the Collection, Management and Use of Data in the Fisheries Sector and Support for Scientific Advice Regarding the Common Fisheries Policy. Off. J. Eur. Union L 2008, 60, 1–12. [Google Scholar]
- DGRM. Work Plan for Data Collection in the Fisheries and Aquaculture Sectors; DGRM: Lisbon, Portugal, 2016. [Google Scholar]
- Schwamborn, R.; Mildenberger, T.K.; Taylor, M.H. Assessing Sources of Uncertainty in Length-Based Estimates of Body Growth in Populations of Fishes and Macroinvertebrates with Bootstrapped ELEFAN. Ecol. Modell. 2019, 393, 37–51. [Google Scholar] [CrossRef] [Green Version]
- Mildenberger, T.K.; Taylor, M.H.; Wolff, M. TropFishR: An R Package for Fisheries Analysis with Length-Frequency Data. Methods Ecol. Evol. 2017, 8, 1520–1527. [Google Scholar] [CrossRef] [Green Version]
- Taylor, M.H.; Mildenberger, T.K. Extending Electronic Length Frequency Analysis in R. Fish. Manag. Ecol. 2017, 24, 230–238. [Google Scholar] [CrossRef]
- Schwamborn, R.; Mildenberger, T.K.; Taylor, M.H. Fishboot: Bootstrap-Based Methods for the Study of Fish Stocks and Aquatic Populations; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Ricker, W.E. Computation and Interpretation of Biological Statistics of Fish Populations. Bull. Fish. Res. Bd. Can. 1975, 191, 1–382. [Google Scholar]
- Beverton, R.J.H.; Holt, S.J. A Review of the Lifespans and Mortality Rates of Fish in Nature, and Their Relation to Growth and Other Physiological Characteristics. In Proceedings of the CIBA Foundation Colloquia on Ageing, Boston, MA, USA, 1 January 1959; Volume 5, pp. 142–180. [Google Scholar]
- Taylor, C.C. Temperature, Growth, and Mortality—The Pacific Cockle. ICES J. Mar. Sci. 1960, 26, 117–124. [Google Scholar] [CrossRef]
- Cubillos, L.A.; Alarcón, R.; Brante, A. Empirical Estimates of Natural Mortality for the Chilean Hake (Merluccius gayi): Evaluation of Precision. Fish. Res. 1999, 42, 147–153. [Google Scholar] [CrossRef]
- Frisk, M.G.; Miller, T.J.; Fogarty, M.J. Estimation and Analysis of Biological Parameters in Elasmobranch Fishes: A Comparative Life History Study. Can. J. Fish. Aquat. Sci. 2001, 58, 969–981. [Google Scholar] [CrossRef]
- Hewitt, D.A.; Hoenig, J.M. Comparison of Two Approaches for Estimating Natural Mortality Based on Longevity. Fish. Bull. 2005, 103, 433. [Google Scholar]
- Tanaka, S. Studies on the Dynamics and the Management of Fish Populations. Bull. Tokai Fish. Res. Lab. 1960, 28, 1–200. [Google Scholar]
- Alverson, D.L.; Carney, M.J. A Graphic Review of the Growth and Decay of Population Cohorts. ICES J. Mar. Sci. 1975, 36, 133–143. [Google Scholar] [CrossRef]
- Pauly, D. On the Interrelationships between Natural Mortality, Growth Parameters, and Mean Environmental Temperature in 175 Fish Stocks. ICES J. Mar. Sci. 1980, 39, 175–192. [Google Scholar] [CrossRef]
- Hoenig, J.M. Empirical Use of Longevity Data to Estimate Mortality Rates. Fish. Bull. 1983, 82, 898–903. [Google Scholar]
- Alagaraja, K. Simple Methods for Estimation of Parameters for Assessing Exploited Fish Stocks. Indian J. Fish. 1984, 31, 177–208. [Google Scholar]
- Djabali, F.; Mehailia, A.; Koudil, M.; Brahmi, B. Empirical Equations for the Estimation of Natural Mortality in Mediterranean Teleosts. Naga ICLARM Q. 1993, 16, 35–37. [Google Scholar]
- Pauly, D.; Binohlan, C. FishBase and AUXIM as Tools for Comparing Life-History Patterns, Growth and Natural Mortality of Fish: Applications to Snappers and Groupers; International Center for Living Aquatic Resources Management (ICLARM): Bayan Lepas, Malaysia, 1996; pp. 218–243. [Google Scholar]
- Jensen, A.L. Beverton and Holt Life History Invariants Result from Optimal Trade-off of Reproduction and Survival. Can. J. Fish. Aquat. Sci. 1996, 53, 820–822. [Google Scholar] [CrossRef]
- Lafon, V.; Martins, A.; Figueiredo, M.; Rodrigues, M.; Bashmachnikov, I.; Mendonça, A.; Macedo, L.; Goulart, N. Sea Surface Temperature Distribution in the Azores Region. Part I: AVHRR Imagery and in Situ Data Processing. Arquipel. Life Mar. Sci. 2004, 21A, 1–18. [Google Scholar]
- Martins, A.; Bashmachnikov, I.; Mendonça, A. Multi-Sensor (SeaWiFS/MODIS/AVHRR) Surface Signature of the Azores Current. Geophys. Res. Abstr. 2008, 10, EGU2008-A-11379. [Google Scholar]
- ICES. Azores Ecoregion—Ecosystem Overview. In Report of the ICES Advisory Committee, 2020. ICES Advice 2020; ICES: Copenhagen, Denmark, 2020. [Google Scholar]
- Froese, R.; Binohlan, C. Empirical Relationships to Estimate Asymptotic Length, Length at First Maturity and Length at Maximum Yield per Recruit in Fishes, with a Simple Method to Evaluate Length Frequency Data. J. Fish Biol. 2000, 56, 758–773. [Google Scholar] [CrossRef]
- Rosa, A.; Menezes, G.; Melo, O.; Pinho, M.R. Weight–Length Relationships of 33 Demersal Fish Species from Azores Archipelago. Fish. Res. 2006, 80, 329–332. [Google Scholar] [CrossRef]
- Afonso, P.; Morato, T.; Santos, R.S. Spatial Patterns in Reproductive Traits of the Temperate Parrotfish Sparisoma cretense. Fish. Res. 2008, 90, 92–99. [Google Scholar] [CrossRef]
- Navarro, M.R.; Villamor, B.; Myklevoll, S.; Gil, J.; Abaunza, P.; Canoura, J. Maximum Size of Atlantic Mackerel (Scomber scombrus) and Atlantic Chub Mackerel (Scomber colias) in the Northeast Atlantic. Cybium 2012, 36, 406–408. [Google Scholar]
- Kelly, C.J.; Connolly, P.L.; Clarke, M.W. The Deep Water Fisheries of the Rockall Trough: Some Insights Gleaned from Irish Survey Data. In ICES CM Documents; International Council for the Exploration of the Sea (ICES): Copenhangen, Denmark, 1998; Volume O:40, p. 22. [Google Scholar]
- ICES. Black Scabbardfish (Aphanopus carbo) in Subareas 1, 2, 4–8, 10, and 14, and Divisions 3.a, 9.a, and 12.b (Northeast Atlantic and Arctic Ocean). In ICES Advice on Fishing Opportunities, Catch, and Effort; ICES: Copenhagen, Denmark, 2020. [Google Scholar]
- Moreira, C.; Froufe, E.; Sial, A.N.; Caeiro, A.; Vaz-Pires, P.; Correia, A.T. Population Structure of the Blue Jack Mackerel (Trachurus picturatus) in the NE Atlantic Inferred from Otolith Microchemistry. Fish. Res. 2018, 197, 113–122. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, C.; Sun, M.; Xu, B.; Ji, Y.; Xue, Y.; Ren, Y. Fishing Pressure and Lifespan Affect the Estimation of Growth Parameters Using ELEFAN. Fish. Res. 2021, 238, 105903. [Google Scholar] [CrossRef]
- Tu, C.-Y.; Chen, K.-T.; Hsieh, C. Fishing and Temperature Effects on the Size Structure of Exploited Fish Stocks. Sci. Rep. 2018, 8, 7132. [Google Scholar] [CrossRef] [PubMed]
- Pauly, D. Gill Size and Temperature as Governing Factors in Fish Growth: A Generalization of von Bertalanffy’s Growth Formula; Institut für Meereskunde: Hamburg, Germany, 1979. [Google Scholar]
- Pauly, D.; Munro, J.L. Once More on the Comparison of Growth in Fish and Invertebrates. Fishbyte 1984, 2, 1–21. [Google Scholar]
- Vetter, E.F. Estimation of Natural Mortality in Fish Stocks: A Review. Fish. Bull. 1988, 86, 25–43. [Google Scholar]
- Gulland, J.A. Fish Stock Assessment. A Manual of Basic Method; FAO/Wiley Series on Food and Agriculture; FAO: Rome, Italy, 1983. [Google Scholar]
- Bonfil, R. The Purpose of Stock Assessment and the Objectives of Fisheries. In Management Techniques for Elasmobranch Fisheries; Musick, J.A., Bonfil, R., Eds.; Food & Agriculture Organization: Rome, Italy, 2005; pp. 6–14. [Google Scholar]
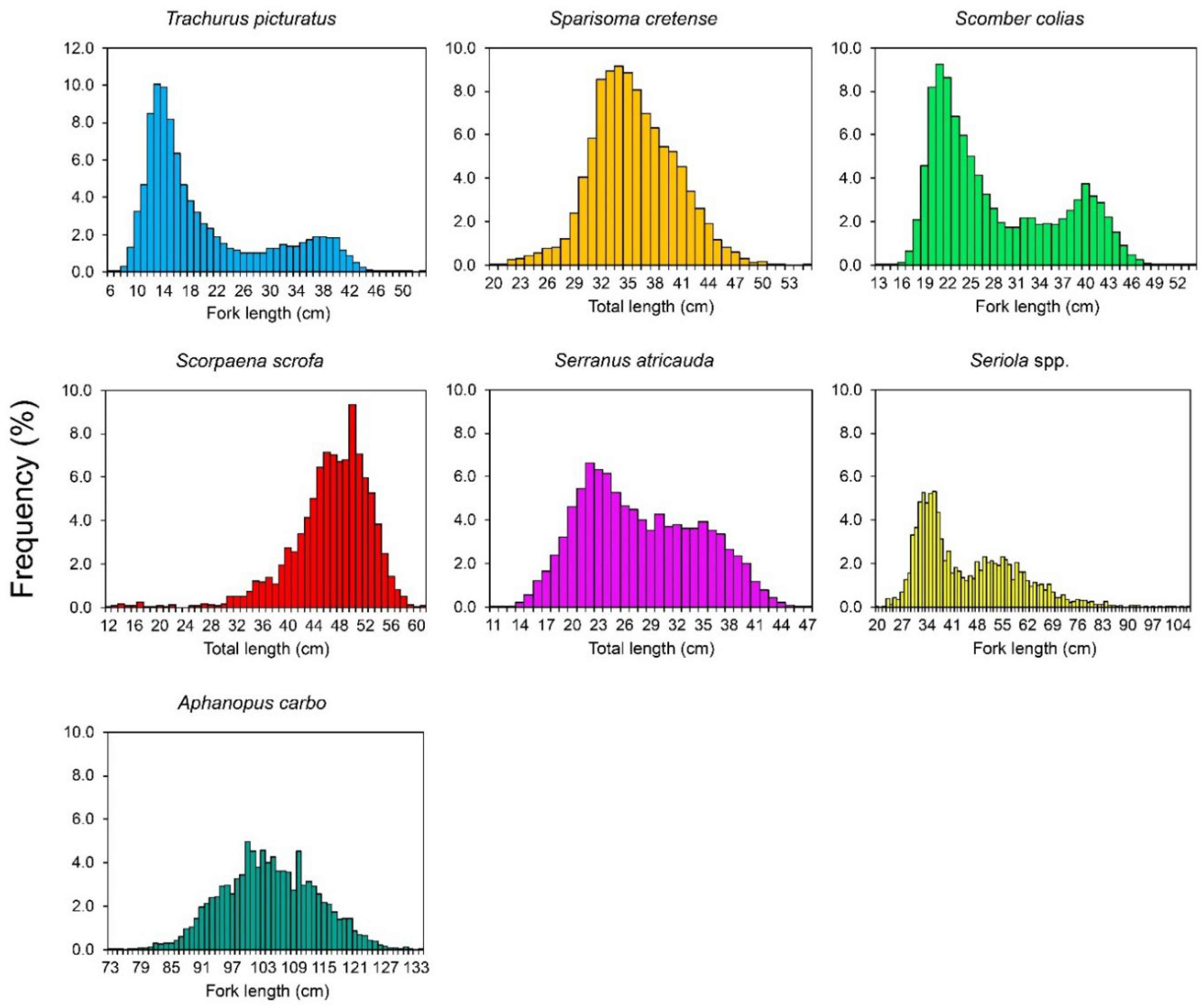
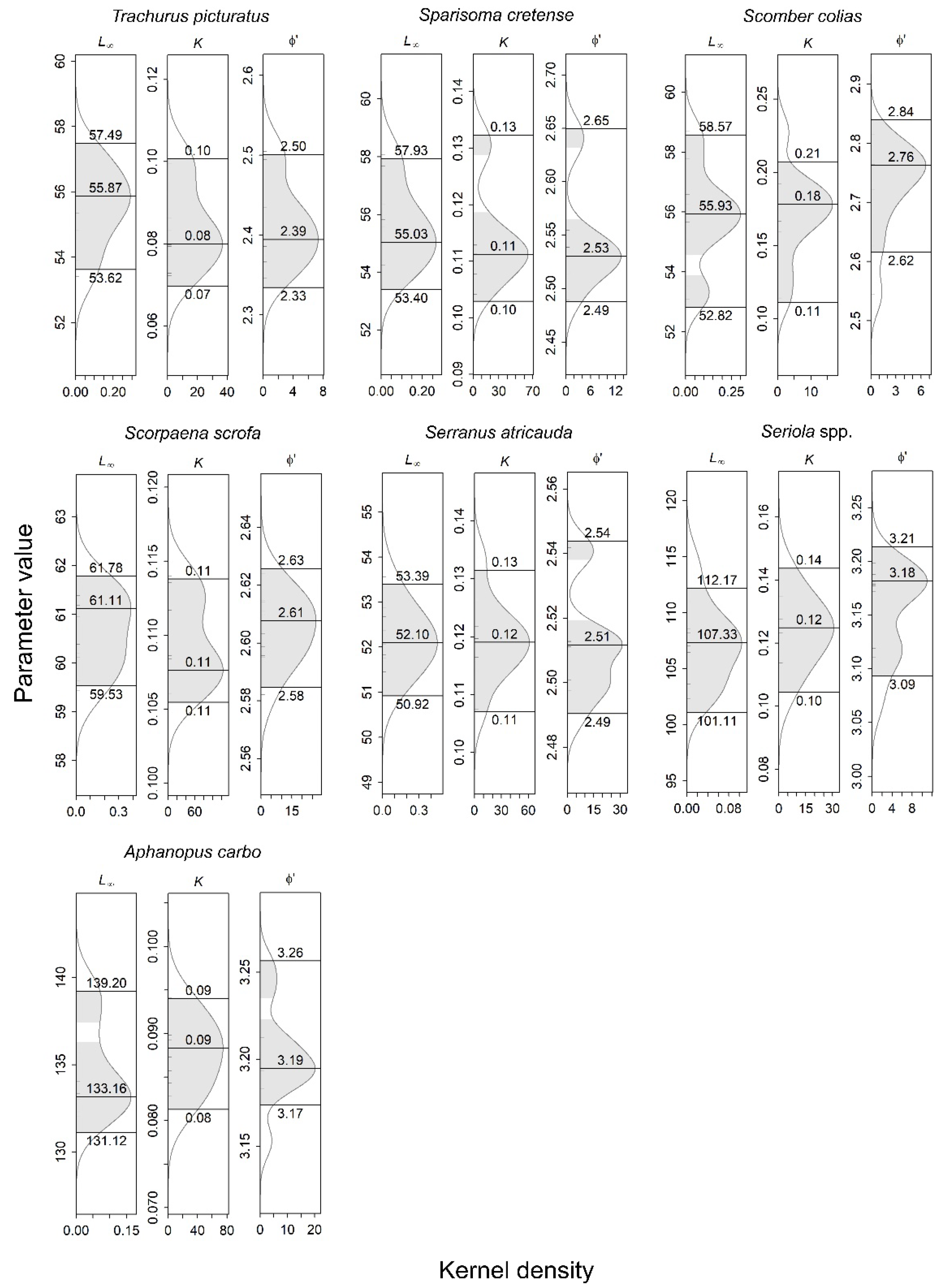
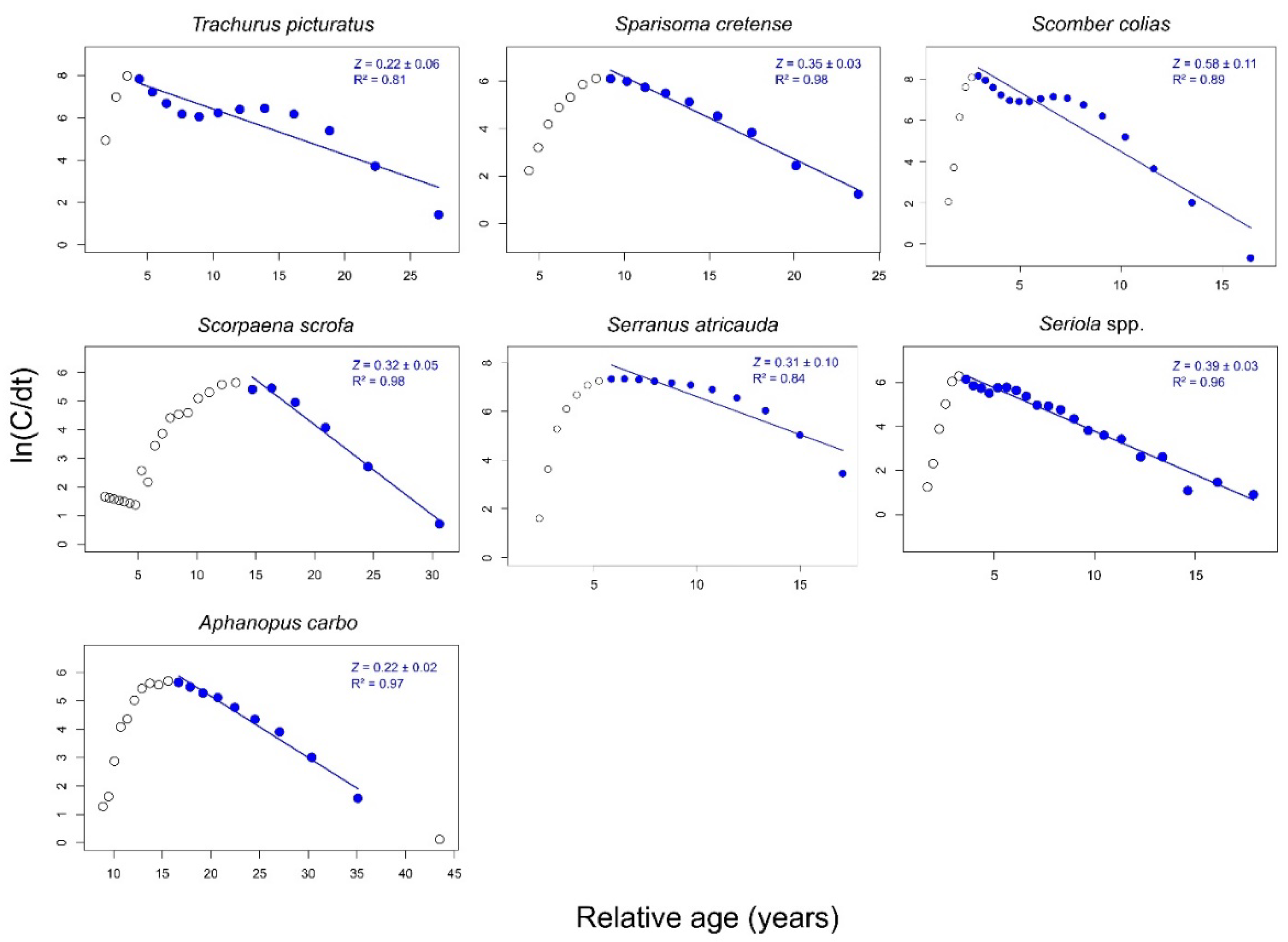
| Area | Method | n | Size | Growth Parameter | Mortality Rate | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| L∞ | k | ϕ′ | Z | M | F | |||||
| T. picturatus | ||||||||||
| Isidro [33] | Azores | DR | 516 | LF | 52.9 | 0.20 | 2.75 | |||
| Vasconcelos et al. [11] | Madeira | DR | 578 | LT | 42.3 | 0.16 | 2.46 | |||
| Vasconcelos et al. [11] | Madeira | BC | 229 | LT | 48.3 | 0.13 | 2.50 | |||
| Garcia et al. [17] | Azores | DR | 1420 | LF | 58.3 | 0.09 | 2.48 | |||
| Garcia et al. [17] | Azores | BC | 796 | LF | 52.9 | 0.11 | 2.49 | |||
| Jurado-Ruzafa and Santamaria [28] | Canaries | DR | 913 | LT | 34.9 | 0.21 | 2.42 | 0.40 | ||
| Vasconcelos et al. [15] | Madeira | IM | 10,713 | LT | 0.16 | 0.75–1.64 | 0.33 | |||
| Neves et al. [34] | Mainland Portugal | BC | 376 | LT | 46.7 | 0.15 | 2.51 | |||
| Neves et al. [34] | Mainland Portugal | DR | 575 | LT | 63.3 | 0.07 | 2.45 | |||
| S. cretense | ||||||||||
| Petrakis and Papaconstantinou [27] | Greece | DR | 399 | LT | 38.9 | 0.16 | 2.38 | 0.81 | 0.38 | 0.43 |
| S. colias | ||||||||||
| Carvalho et al. [12] | Azores | DR | 349 | LT | 57.5 | 0.20 | 2.82 | 0.19 | ||
| Velasco et al. [22] | Gulf of Cádiz | BC | 121 | LT | 43.0 | 0.25 | 2.7 | |||
| Velasco et al. [22] | SW Mediterranean | BC | 98 | LT | 40.0 | 0.37 | 2.77 | |||
| Čikeš Keč and Zorica [29] | Adriatic Sea | DR | 280 | LF | 45.3 | 0.18 | 2.57 | 0.91 | 0.35 | 0.56 |
| Jurado-Ruzafa et al. [16] | Mauritania | BC | 163 | LT | 48.4 | 0.25 | 2.76 | 0.47 | ||
| Daley and Leaf [13] | Gulf of Mexico | DR | 60 | LT | 33.6 | 1.75 | 3.29 | |||
| S. scrofa | ||||||||||
| Shahrani and Shakman [35] | Libya | DR | 94 | LT | 116.0 | 0.05 | 2.82 | |||
| S. atricauda | ||||||||||
| Tuset et al. [31] | Canaries | DR | 406 | LT | 43.9 | 0.16 | 2.49 | |||
| Tuset et al. [31] | Canaries | BC | 15 | LT | 52.3 | 0.09 | 2.39 | |||
| Tuset et al. [31] | Canaries | MX | 490 | LT | 49.5 | 0.11 | 2.43 | |||
| Amorim et al. [30] | Azores | EF | - | LT | 41.4 | 0.26 | 2.65 | |||
| Seriolaspp. | ||||||||||
| Manooch and Potts [25] | SE U.S.A. | BC | 190 | LF | 151.4 | 0.12 | 3.44 | 0.60–0.65 | ||
| Manooch and Potts [26] | Gulf of Mexico | BC | 291 | LF | 110.9 | 0.23 | 3.45 | 0.68–0.70 | ||
| Thompson et al. [21] | Gulf of Mexico | DR | 552 | LF | 138.9 | 0.25 | 3.68 | |||
| Kožul et al. [18] | Adriatic Sea | DR | 298 | LT | 174.6 | 0.19 | 3.76 | 0.41 | 0.3 | 0.11 |
| Harris et al. [23] | SE U.S.A. | DR | 1985 | LF | 124.2 | 0.28 | 3.64 | |||
| A. carbo | ||||||||||
| Morales-Nin and Sena-Carvalho [19] | Madeira | DR | 649 | LT | 138.6 | 0.25 | 3.68 | |||
| Pajuelo et al. [20] | Canaries | DR | 298 | LT | 147.7 | 0.20 | 3.64 | |||
| Vieira et al. [36] | Madeira | BC | 436 | LT | 146.1–158.6 | 0.12–0.15 | 3.48–3.49 | |||
| Vieira et al. [36] | Mainland Portugal | BC | 1075 | LT | 124.0–135.4 | 0.17–0.21 | 3.49–3.50 | |||
| Delgado et al. [14] | Madeira | BC | 587 | LT | 131.9–136.2 | 0.15–0.17 | 3.45–3.46 | |||
| Scientific Name | Common Name | Sampling Period | n | Size Measurement |
|---|---|---|---|---|
| Trachurus picturatus | Blue jack mackerel | 2010–2017 | 104,299 | LF |
| Sparisoma cretense | Parrotfish | 2010–2017 | 10,217 | LT |
| Scomber colias | Atlantic chub mackerel | 2010–2017 | 57,723 | LF |
| Scorpaena scrofa | Red scorpionfish | 2010–2017 | 2983 | LT |
| Serranus atricauda | Blacktail comber | 2010–2017 | 31,045 | LT |
| Seriola spp. | Amberjacks nei | 1990–2017 | 3998 | LF |
| Aphanopus carbo | Black scabbardfish | 2011–2014 | 6029 | LF |
| Empirical Formula | Source | Species | ||||||
|---|---|---|---|---|---|---|---|---|
| Trachurus picturatus | Sparisoma cretense | Scomber colias | Scorpaena scrofa | Serranus atricauda | Seriola spp. | Aphanopus carbo | ||
| M = 5/tmax | [45] | 0.13 | 0.18 | 0.30 | 0.18 | 0.20 | 0.20 | 0.15 |
| M = 2.996/tmax | [46] | 0.08 | 0.11 | 0.18 | 0.11 | 0.12 | 0.12 | 0.09 |
| M = 3/tmax | [50] | 0.08 | 0.11 | 0.18 | 0.11 | 0.12 | 0.12 | 0.09 |
| M = 3 k/(exp(0.38 tmax × k − 1)) | [51] | 0.21 | 0.29 | 0.47 | 0.29 | 0.31 | 0.31 | 0.24 |
| M = exp(− 0.0066 − 0.279 log(L∞) + 0.6543 log(k) + 0.4634 log(T)) | [52] | 0.53 | 0.58 | 0.67 | 0.58 | 0.60 | 0.55 | 0.50 |
| M = 3/tmax | [53] | 0.08 | 0.11 | 0.18 | 0.11 | 0.12 | 0.12 | 0.09 |
| M = 4.6/tmax | [54] | 0.12 | 0.17 | 0.28 | 0.17 | 0.18 | 0.18 | 0.14 |
| M = 1.0661 L∞− 0.1172 × k 0.5092 | [55] | 0.18 | 0.22 | 0.28 | 0.21 | 0.23 | 0.21 | 0.18 |
| M = − 0.1778 + 3.1687 k | [56] | 0.08 | 0.17 | 0.39 | 0.17 | 0.20 | 0.20 | 0.11 |
| M = 1.6 k | [57] | 0.13 | 0.18 | 0.29 | 0.18 | 0.19 | 0.19 | 0.14 |
| M = 1.5 k | [57] | 0.12 | 0.17 | 0.27 | 0.17 | 0.18 | 0.18 | 0.14 |
| M = 1.4 k | [47] | 0.11 | 0.15 | 0.25 | 0.15 | 0.17 | 0.17 | 0.13 |
| M = 4.22/tmax | [48] | 0.11 | 0.15 | 0.25 | 0.15 | 0.17 | 0.17 | 0.13 |
| M = 4.22/tmax | [49] | 0.11 | 0.15 | 0.25 | 0.15 | 0.17 | 0.17 | 0.13 |
| Mean value | 0.15 | 0.20 | 0.30 | 0.20 | 0.21 | 0.21 | 0.16 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, R.; Peixoto, U.I.; Medeiros-Leal, W.; Novoa-Pabon, A.; Pinho, M. Growth Parameters and Mortality Rates Estimated for Seven Data-Deficient Fishes from the Azores Based on Length-Frequency Data. Life 2022, 12, 778. https://doi.org/10.3390/life12060778
Santos R, Peixoto UI, Medeiros-Leal W, Novoa-Pabon A, Pinho M. Growth Parameters and Mortality Rates Estimated for Seven Data-Deficient Fishes from the Azores Based on Length-Frequency Data. Life. 2022; 12(6):778. https://doi.org/10.3390/life12060778
Chicago/Turabian StyleSantos, Régis, Ualerson Iran Peixoto, Wendell Medeiros-Leal, Ana Novoa-Pabon, and Mário Pinho. 2022. "Growth Parameters and Mortality Rates Estimated for Seven Data-Deficient Fishes from the Azores Based on Length-Frequency Data" Life 12, no. 6: 778. https://doi.org/10.3390/life12060778
APA StyleSantos, R., Peixoto, U. I., Medeiros-Leal, W., Novoa-Pabon, A., & Pinho, M. (2022). Growth Parameters and Mortality Rates Estimated for Seven Data-Deficient Fishes from the Azores Based on Length-Frequency Data. Life, 12(6), 778. https://doi.org/10.3390/life12060778







