Dapagliflozin, Liraglutide, and Their Combination Attenuate Diabetes Mellitus-Associated Hepato-Renal Injury—Insight into Oxidative Injury/Inflammation/Apoptosis Modulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Assessement of Liver and Kidney Function
2.3. Assessment of Oxidative Stress
2.4. Real Time Polymerase Chain Reaction (RT-PCR)
2.5. Histological Investigation
2.6. Immunohistochemistry
2.7. Statistical Analyses
3. Results
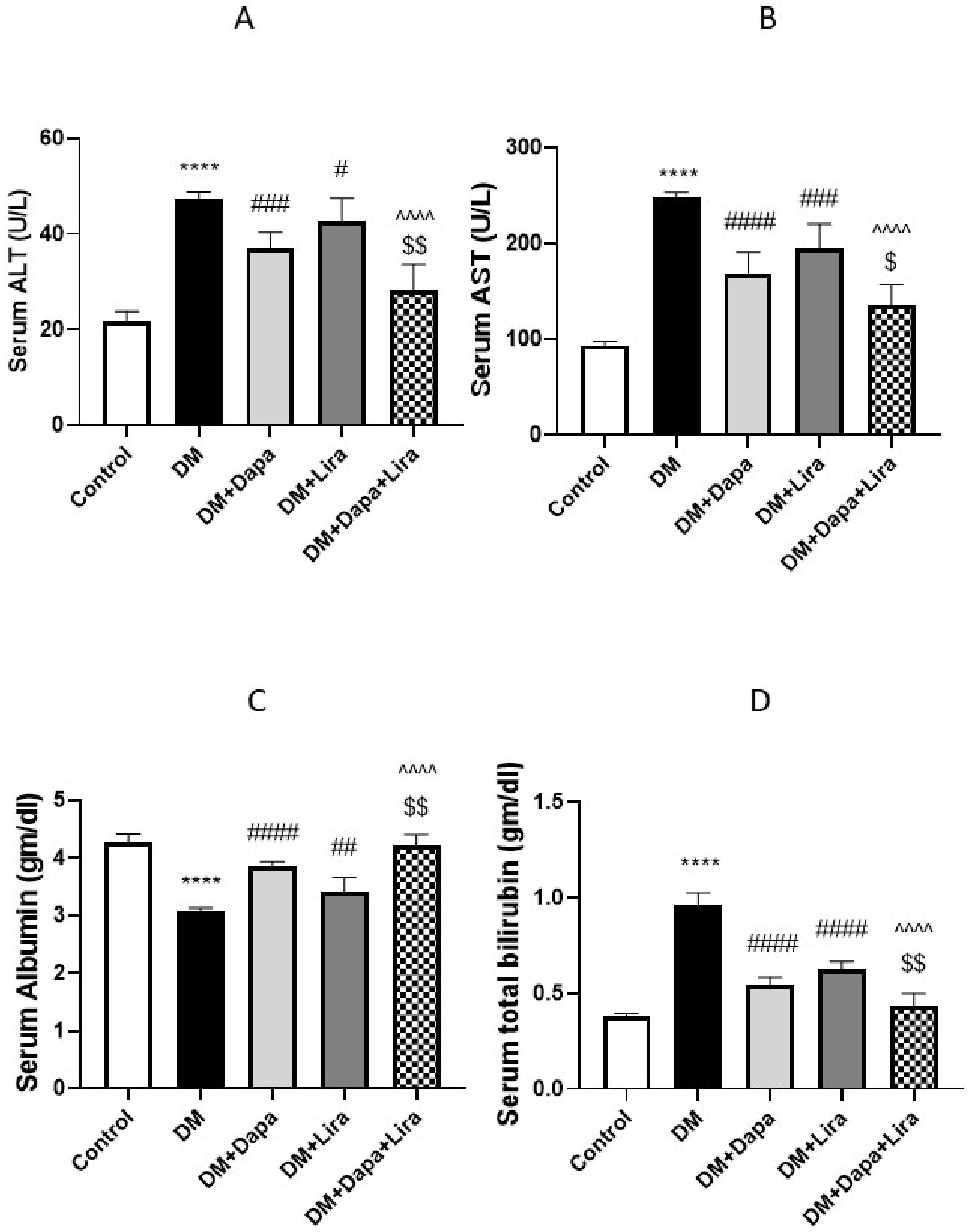
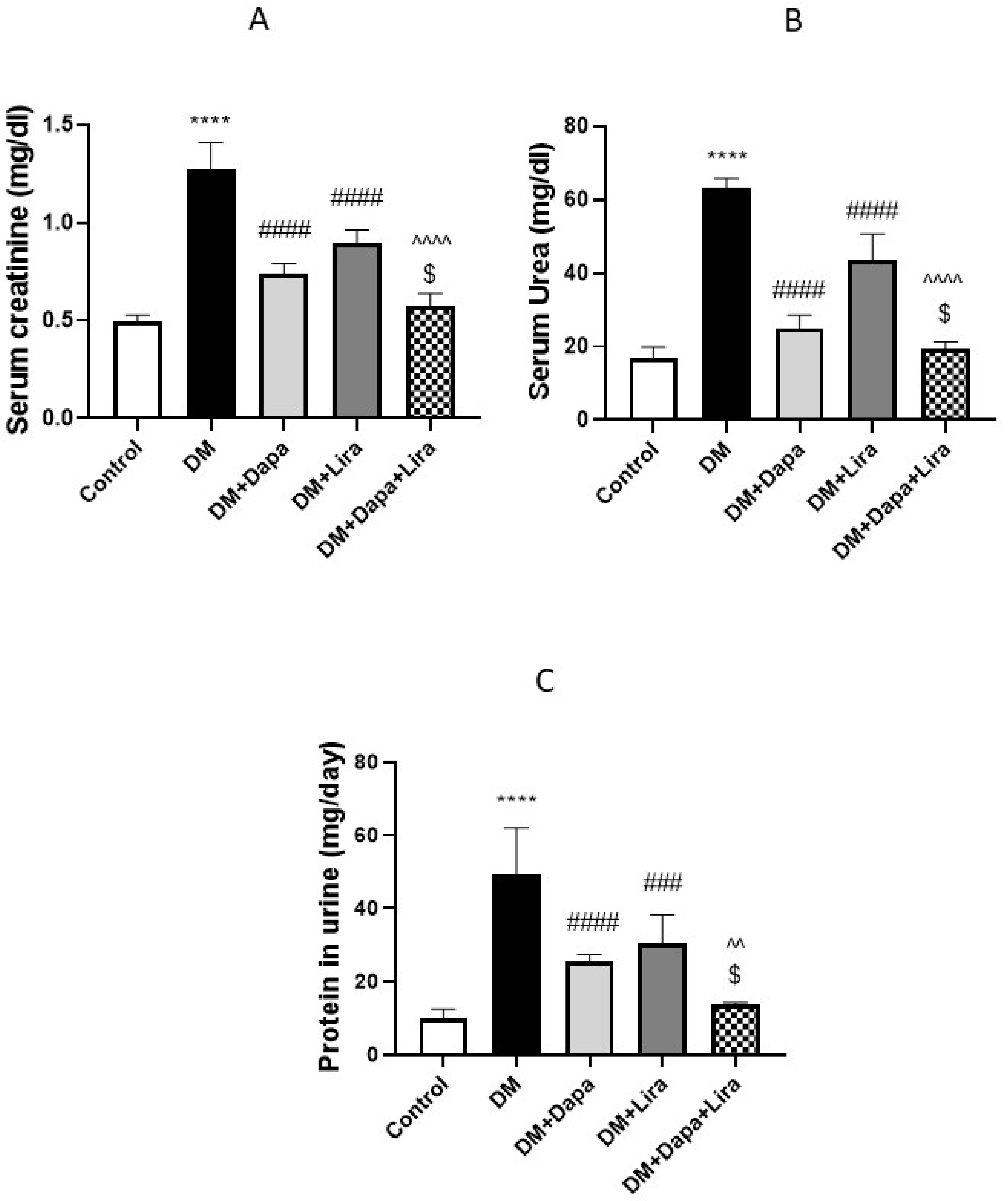
3.1. Dapa and/or Lira Treatment Improved Hepatic and Renal Function in Diabetic Rats
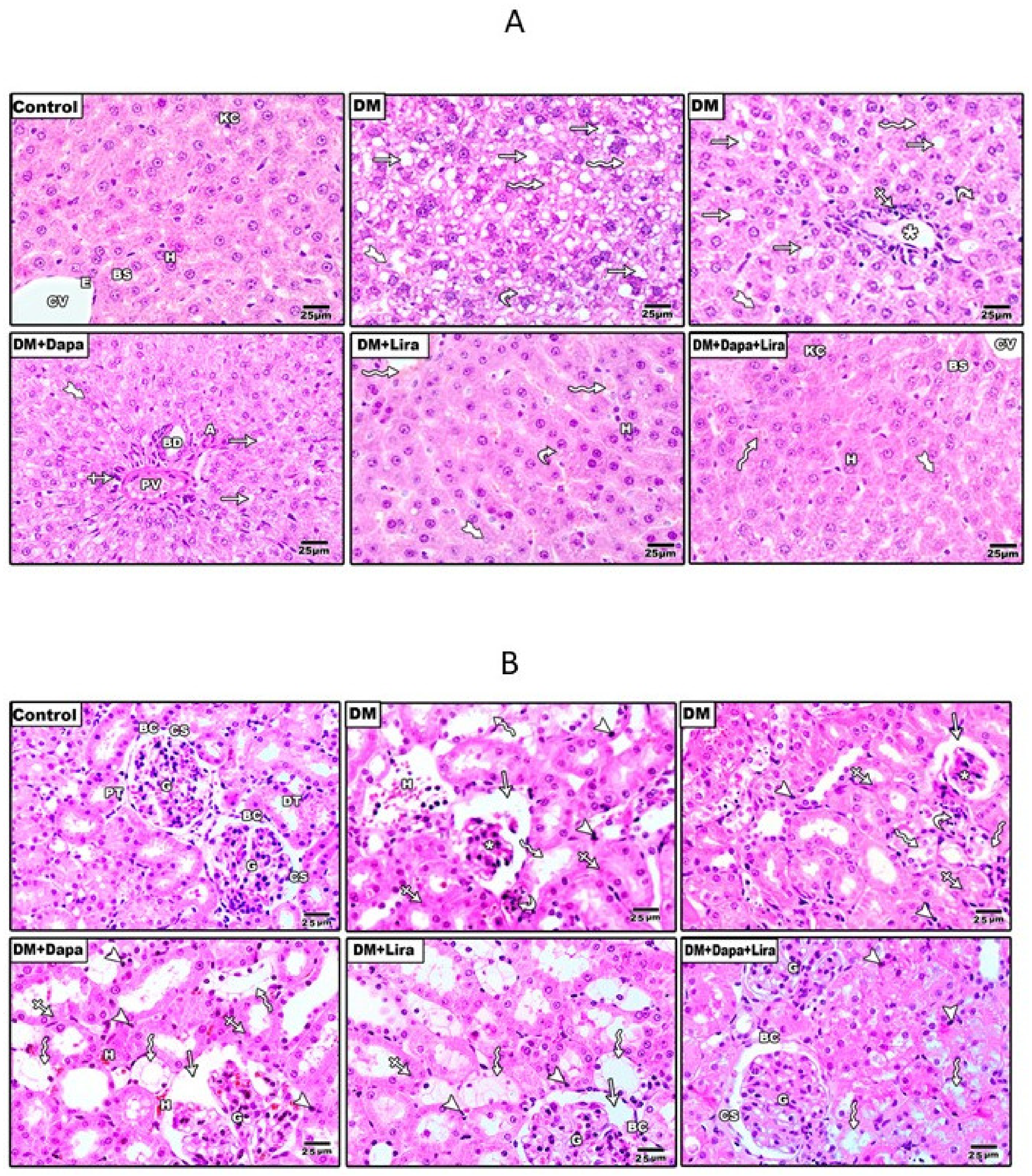
3.2. Dapa and/or Lira Treatment Attenuated Diabetes-Induced Hepatic and Renal Cellular Injury
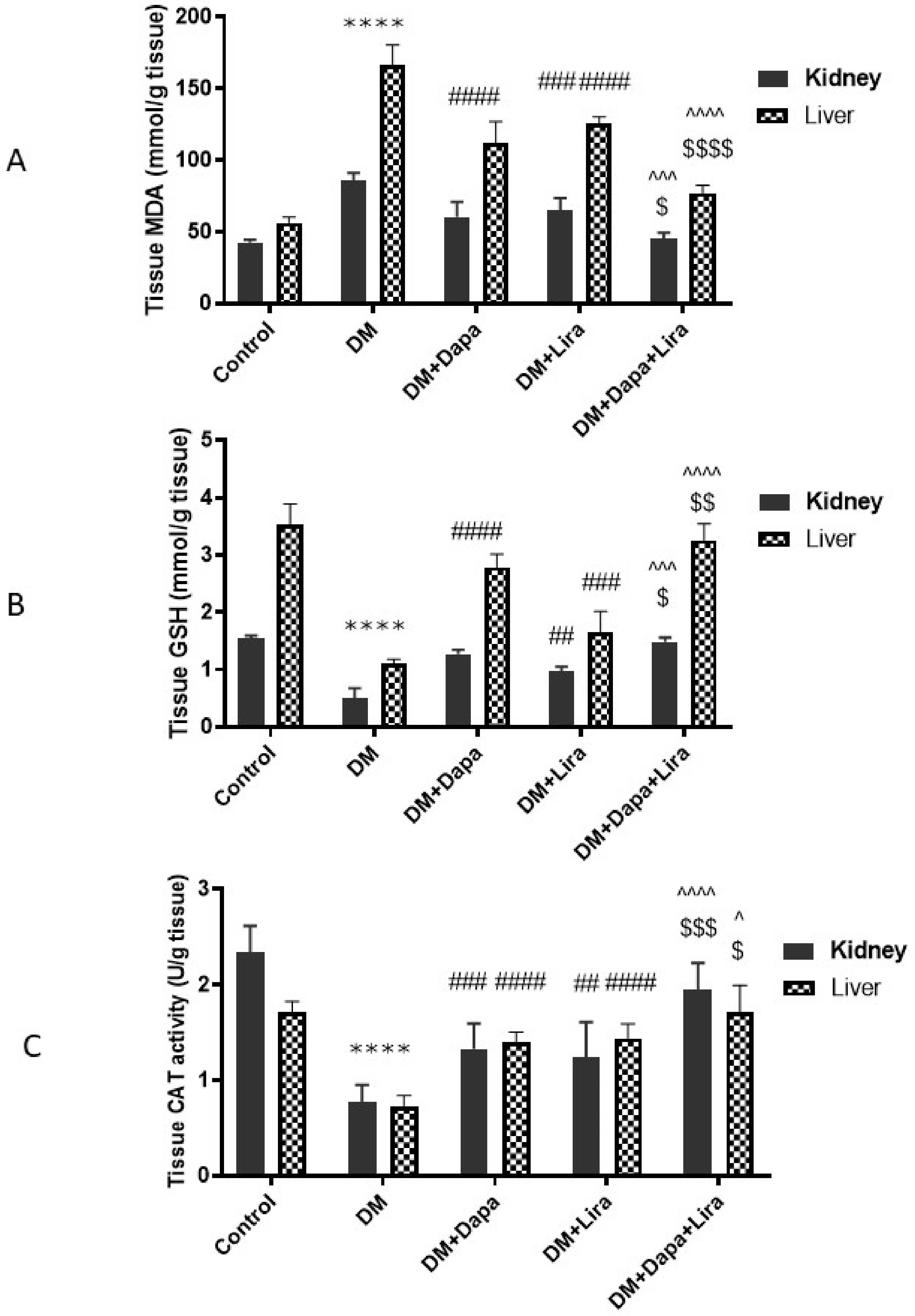
3.3. Dapa and/or Lira Treatment Combated Oxidative Stress in Hepatic and Renal Tissue of Diabetic Rats
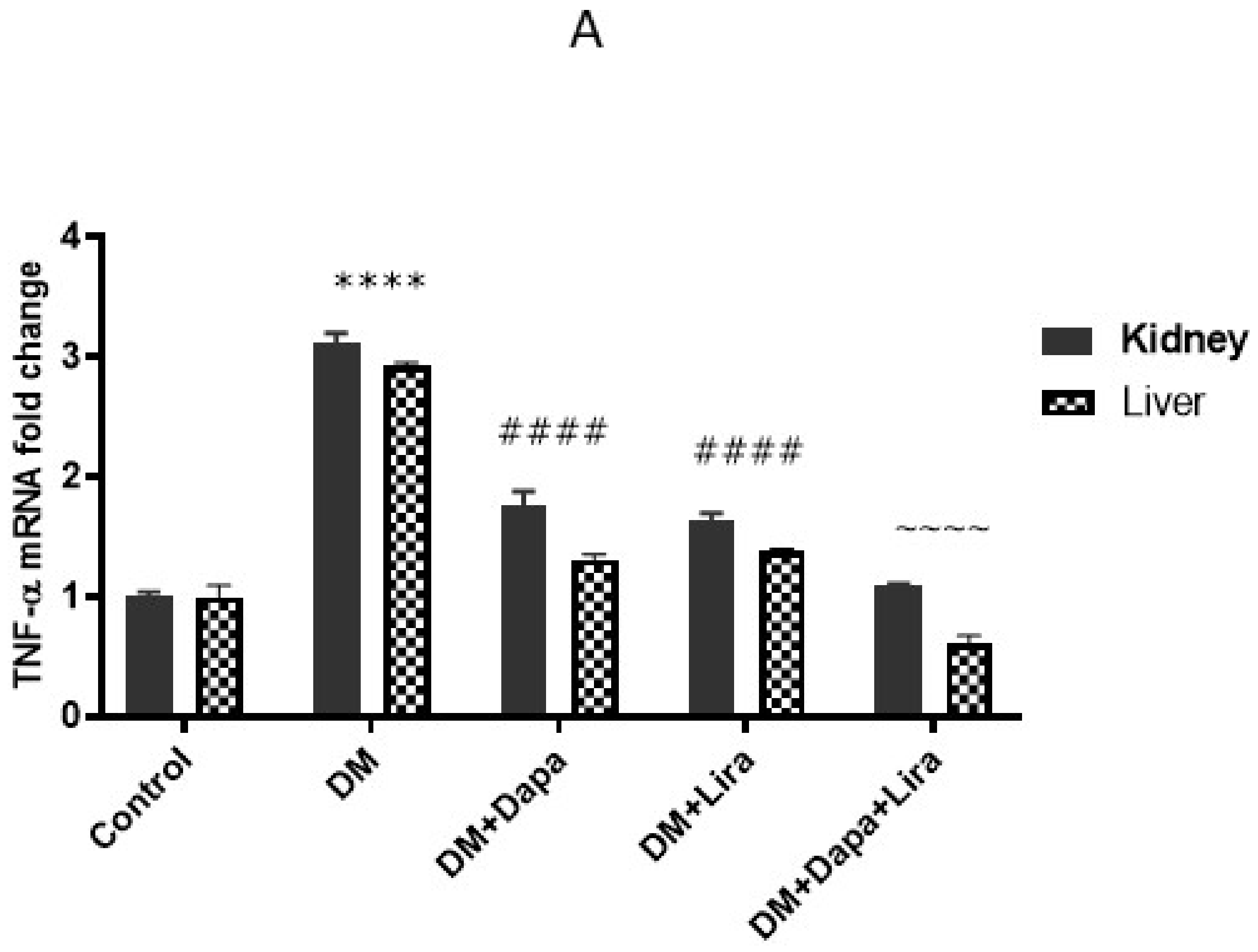
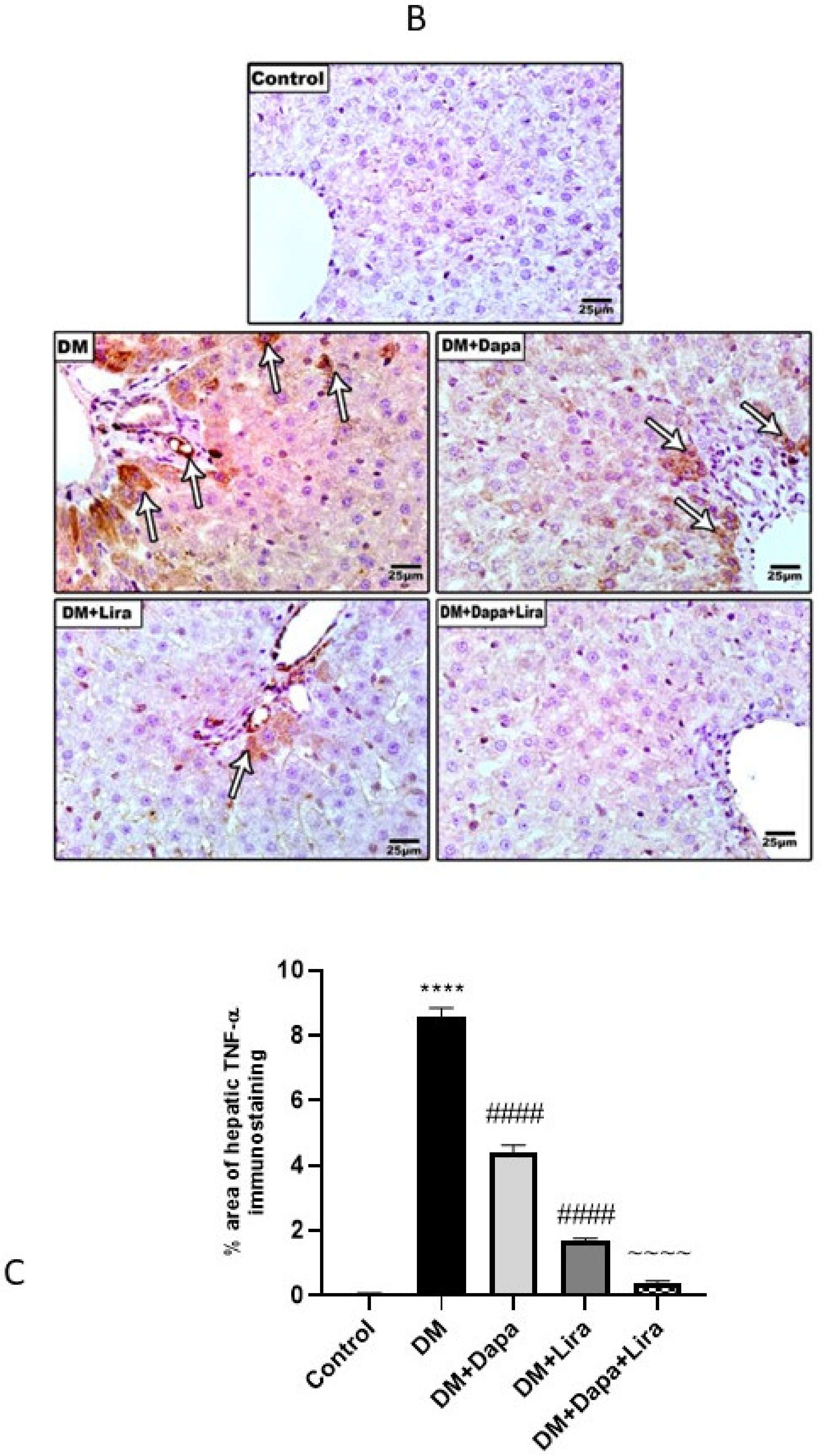
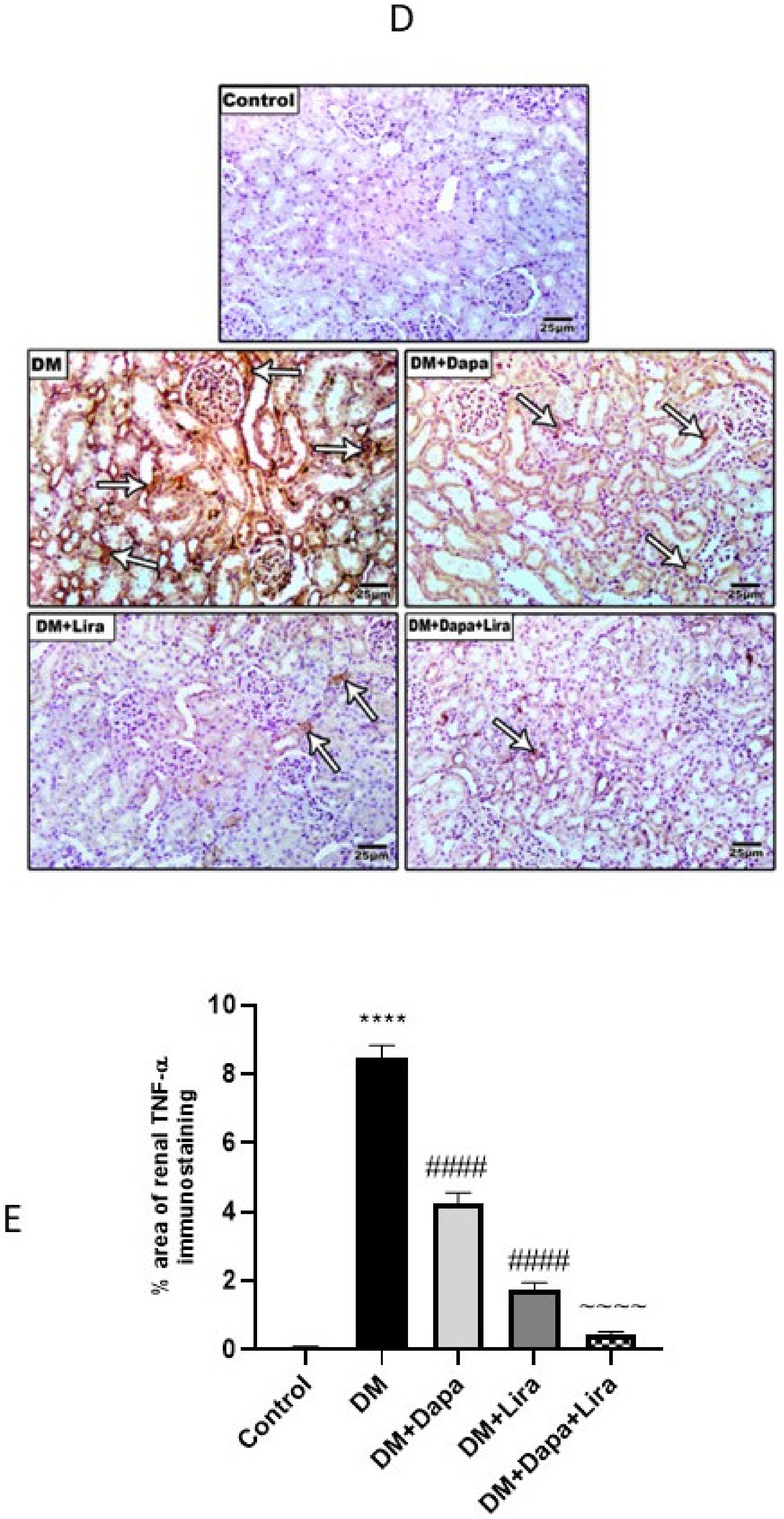
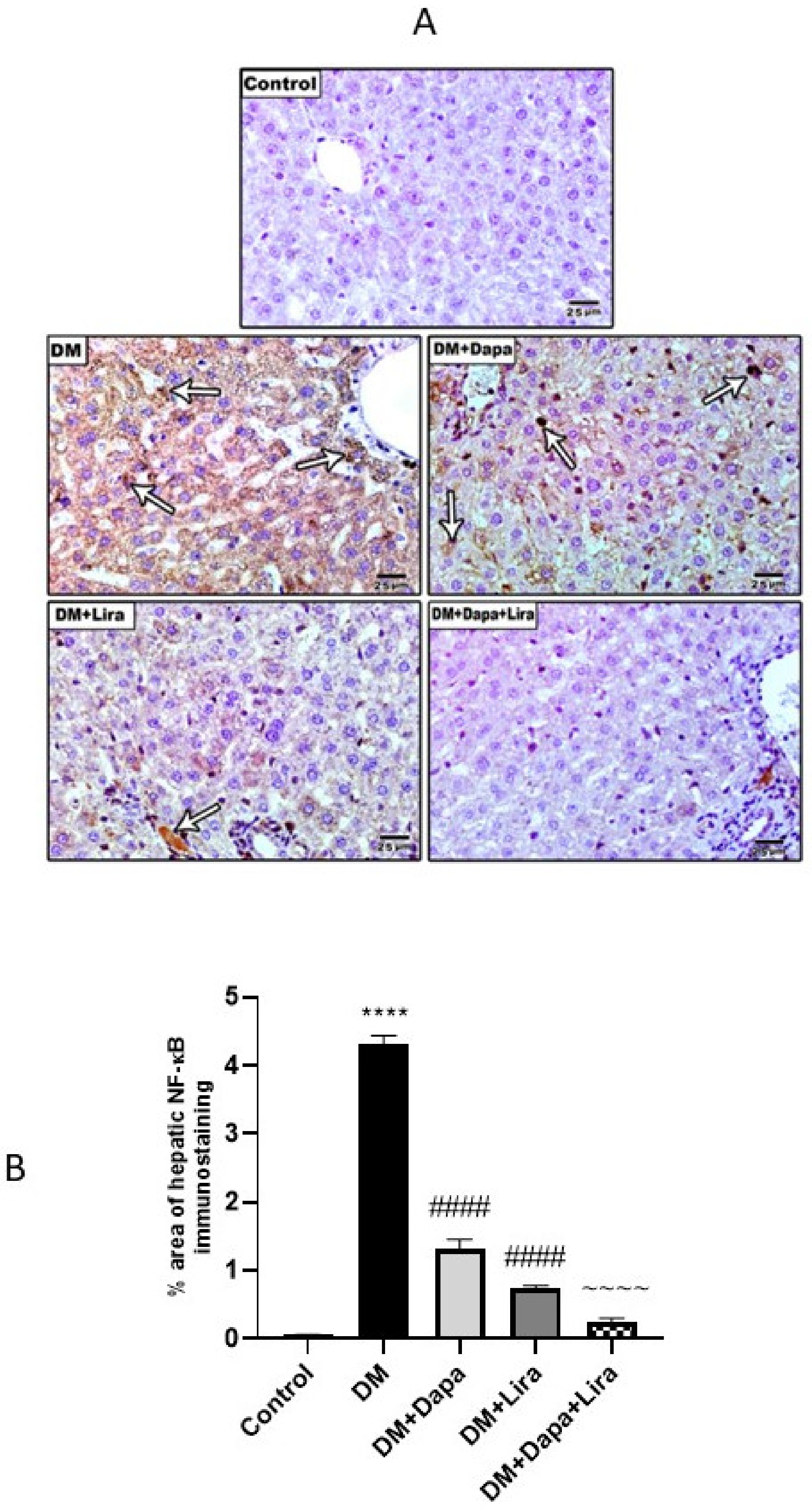
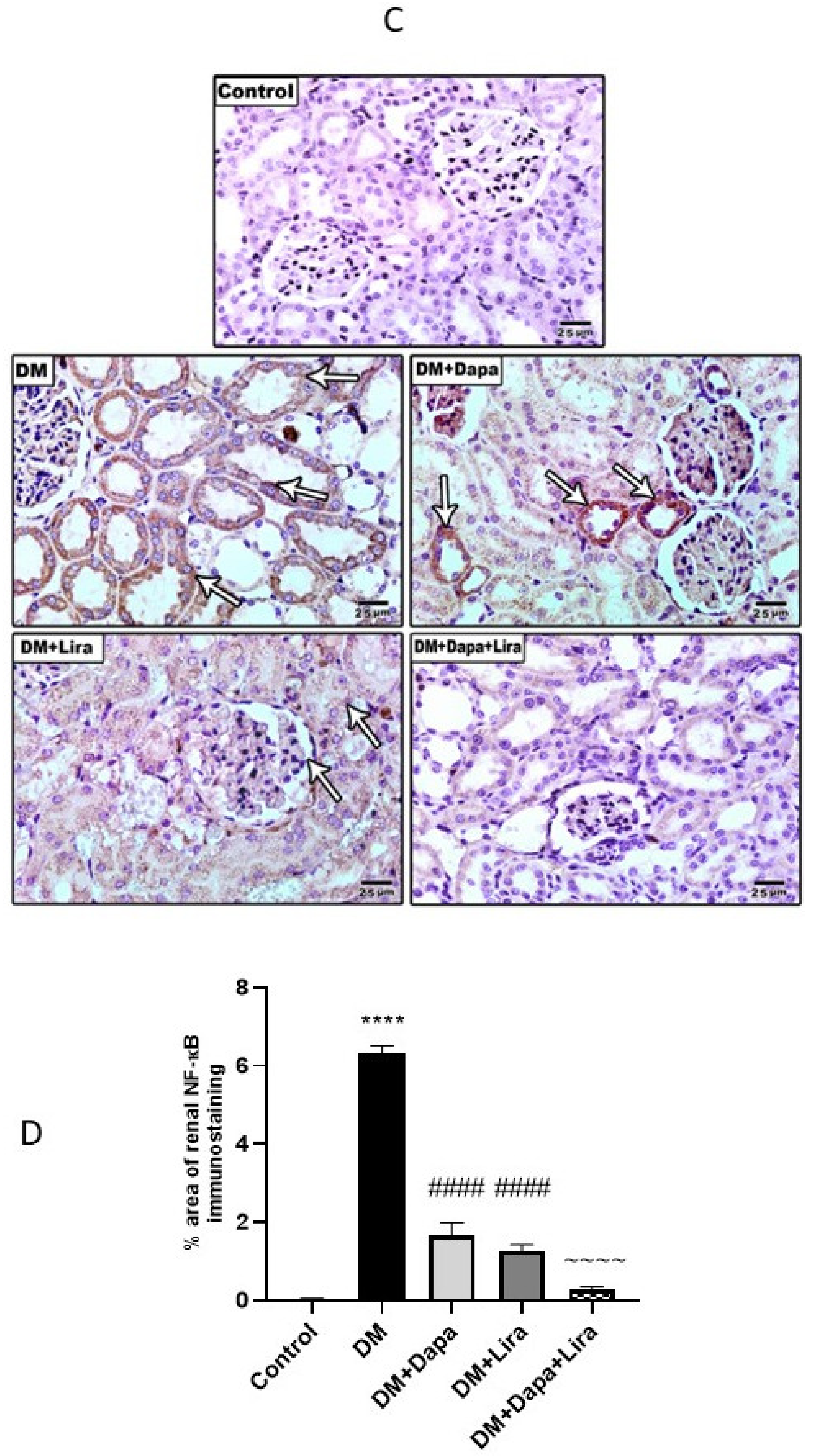
3.4. Dapa and/or Lira Treatment Attenuated Inflammation in Hepatic and Renal Tissue of Diabetic Rats
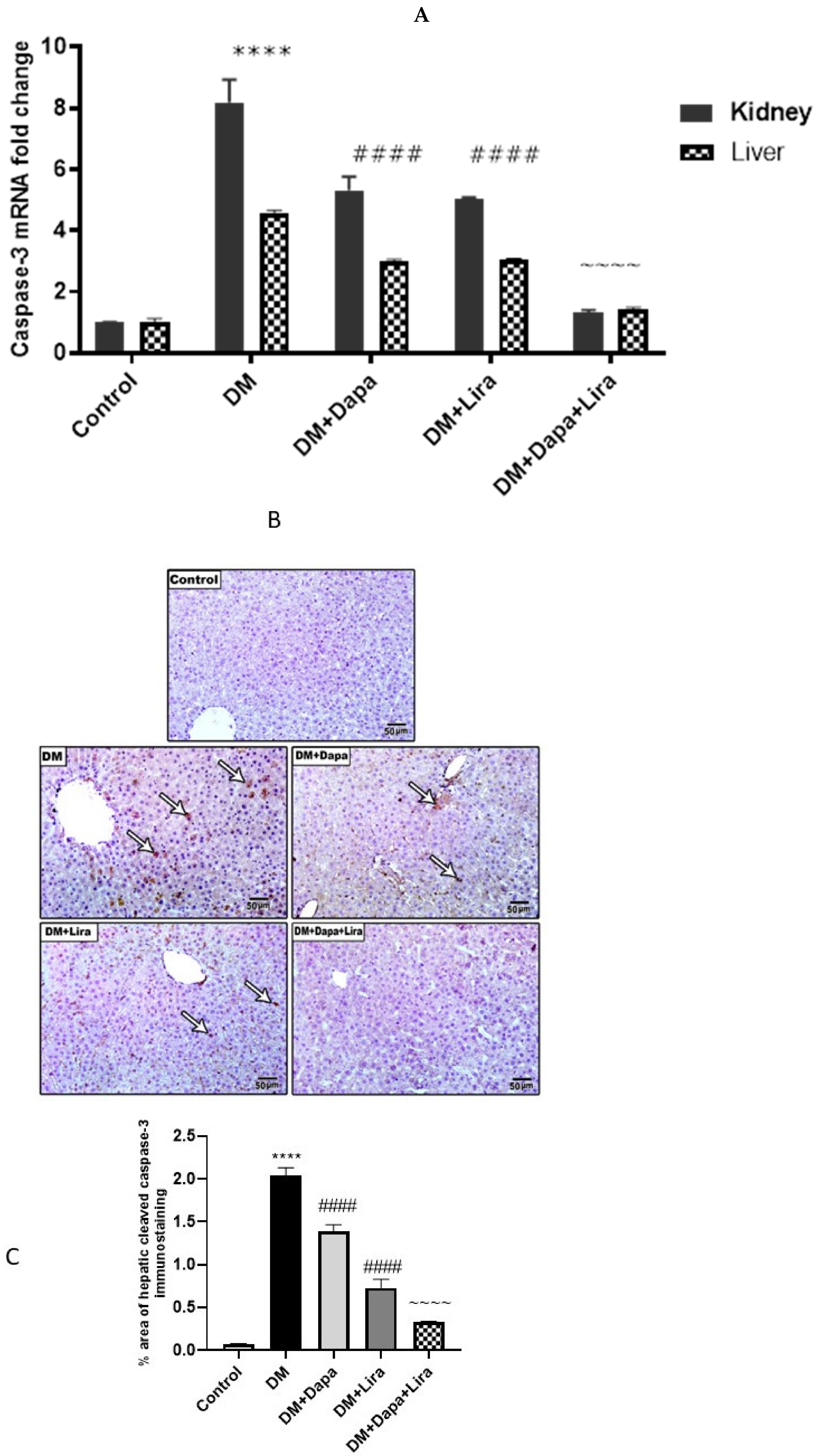
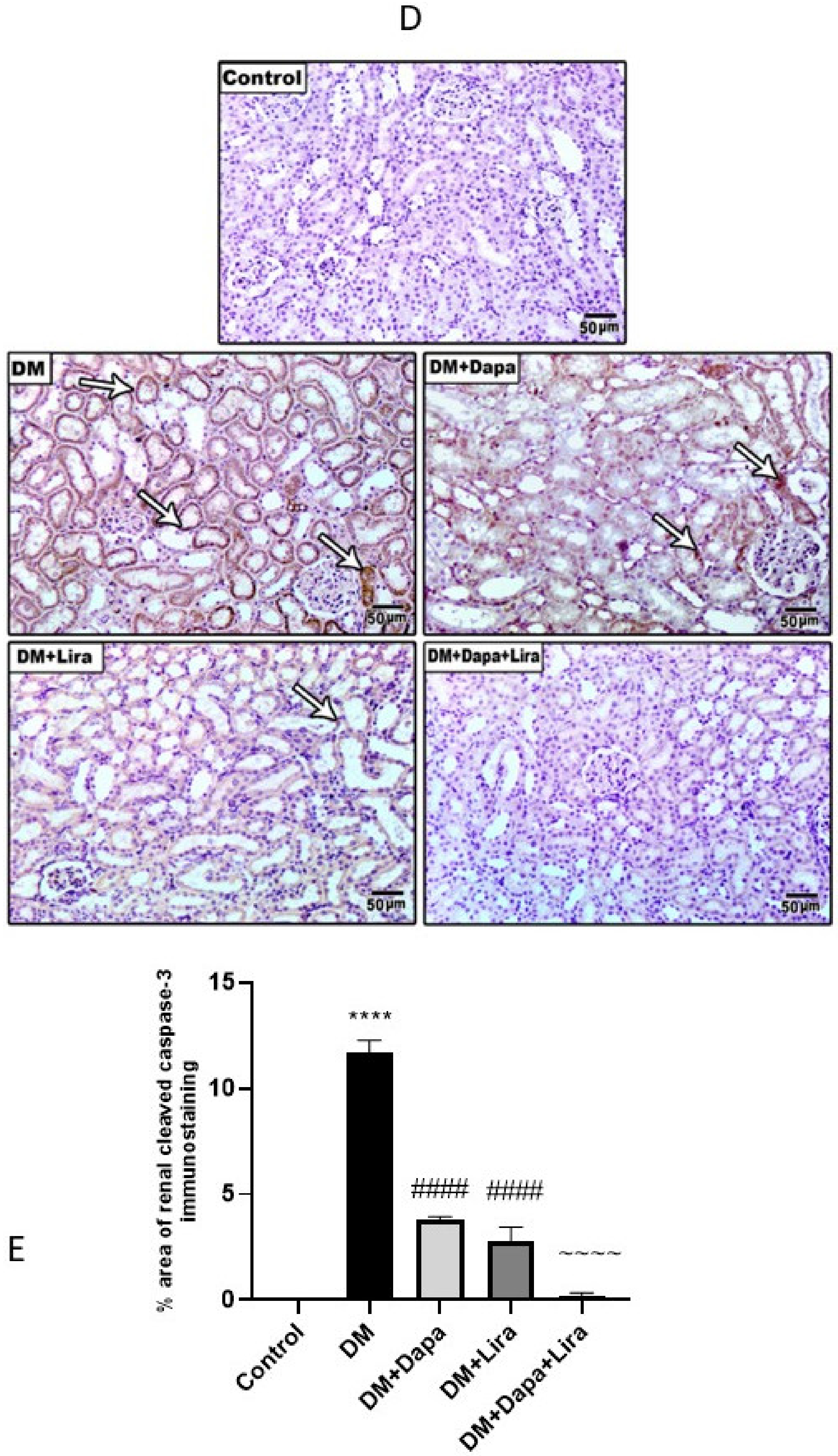
3.5. Dapa and/or Lira Treatment Attenuated Apoptosis in the Hepatic and Renal Tissue of Diabetic Rats
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Eisa, N.H.; Khodir, A.E.; El-Sherbiny, M.; Elsherbiny, N.M.; Said, E. Phenethyl isothiocyanate attenuates diabetic nephropathy via modulation of glycative/oxidative/inflammatory signaling in diabetic rats. Biomed. Pharmacother. 2021, 142, 111666. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Yang, Y.; Liu, Y.-T.; Zhu, J. Liraglutide Regulates the Kidney and Liver in Diabetic Nephropathy Rats through the miR-34a/SIRT1 Pathway. J. Diabetes Res. 2021, 2021, 8873956. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B.; Tran, T.; Everhart, J.E. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology 2004, 126, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lu, X.; Tan, Y.; Li, B.; Miao, X.; Jin, L.; Shi, X.; Zhang, X.; Miao, L.; Li, X.; et al. Diabetes-Induced Hepatic Pathogenic Damage, Inflammation, Oxidative Stress, and Insulin Resistance Was Exacerbated in Zinc Deficient Mouse Model. PLoS ONE 2012, 7, e49257. [Google Scholar] [CrossRef] [Green Version]
- Al-Attar, A.M.; Alsalmi, F.A. Influence of olive leaves extract on hepatorenal injury in streptozotocin diabetic rats. Saudi J. Biol. Sci. 2019, 26, 1865–1874. [Google Scholar] [CrossRef]
- Mohamed, J.; Nazratun Nafizah, A.H.; Zariyantey, A.H.; Budin, S.B. Mechanisms of Diabetes-Induced Liver Damage: The role of oxidative stress and inflammation. Sultan. Qaboos. Univ. Med. J. 2016, 16, e132–e141. [Google Scholar] [CrossRef]
- Yazdi, H.B.; Hojati, V.; Shiravi, A.; Hosseinian, S.; Vaezi, G.; Hadjzadeh, A.M.-A. Liver Dysfunction and Oxidative Stress in Streptozotocin-Induced Diabetic Rats: Protective Role of Artemisia Turanica. J. Pharmacopunct. 2019, 22, 109–114. [Google Scholar] [CrossRef]
- Wei, J.; Tian, J.; Tang, C.; Fang, X.; Miao, R.; Wu, H.; Wang, X.; Tong, X. The Influence of Different Types of Diabetes on Vascular Complications. J. Diabetes Res. 2022, 2022, 3448618. [Google Scholar] [CrossRef]
- Elsherbiny, N.M.; Zaitone, S.A.; Mohammad, H.M.F.; El-Sherbiny, M. Renoprotective effect of nifuroxazide in diabetes-induced nephropathy: Impact on NFκB, oxidative stress, and apoptosis. Toxicol. Mech. Methods 2018, 28, 467–473. [Google Scholar] [CrossRef]
- Said, E.; Zaitone, S.A.; Eldosoky, M.; Elsherbiny, N. Nifuroxazide, a STAT3 inhibitor, mitigates inflammatory burden and protects against diabetes-induced nephropathy in rats. Chem. Interactions 2018, 281, 111–120. [Google Scholar] [CrossRef]
- Pugh, D.; Gallacher, P.J.; Dhaun, N. Management of Hypertension in Chronic Kidney Disease. Drugs 2019, 79, 365–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moneim, A.E.A.; Al-Quraishy, S.; Dkhil, M.A. Anti-hyperglycemic activity of selenium nanoparticles in streptozotocin-induced diabetic rats. Int. J. Nanomed. 2015, 10, 6741–6756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhillon, S. Dapagliflozin: A Review in Type 2 Diabetes. Drugs 2019, 79, 1135–1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, A.; Marso, S.P.; Neeland, I.J. Liraglutide for weight management: A critical review of the evidence. Obes. Sci. Pract. 2016, 3, 3–14. [Google Scholar] [CrossRef]
- Nna, V.U.; Abu Bakar, A.B.; Ahmad, A.; Eleazu, C.O.; Mohamed, M. Oxidative Stress, NF-κB-Mediated Inflammation and Apoptosis in the Testes of Streptozotocin-Induced Diabetic Rats: Combined Protective Effects of Malaysian Propolis and Metformin. Antioxidants 2019, 8, 465. [Google Scholar] [CrossRef] [Green Version]
- Alzahrani, S.; Ajwah, S.M.; Alsharif, S.Y.; Said, E.; El-Sherbiny, M.; Zaitone, S.A.; Al-Shabrawey, M.; Elsherbiny, N.M. Isoliquiritigenin downregulates miR-195 and attenuates oxidative stress and inflammation in STZ-induced retinal injury. Naunyn-Schmiedebergs Arch. Exp. Pathol. Pharmakol. 2020, 393, 2375–2385. [Google Scholar] [CrossRef]
- Alzahrani, S.; Said, E.; Ajwah, S.M.; Alsharif, S.Y.; El-Bayoumi, K.S.; Zaitone, S.A.; Qushawy, M.; Elsherbiny, N.M. Isoliquiritigenin attenuates inflammation and modulates Nrf2/caspase-3 signalling in STZ-induced aortic injury. J. Pharm. Pharmacol. 2021, 73, 193–205. [Google Scholar] [CrossRef]
- Zeng, Z.; Yuan, Q.; Yu, R.; Zhang, J.; Ma, H.; Chen, S. Ameliorative Effects of Probiotic Lactobacillus paracasei NL41 on Insulin Sensitivity, Oxidative Stress, and Beta-Cell Function in a Type 2 Diabetes Mellitus Rat Model. Mol. Nutr. Food Res. 2019, 63, e1900457. [Google Scholar] [CrossRef]
- Nethengwe, M.; Okaiyeto, K.; Oguntibeju, O.O.; Brooks, N.L. Ameliorative effects of Anchomanes difformis aqueous extract against oxidative stress in the testes and epididymis of streptozotocin-induced diabetic male Wistar rats. Saudi J. Biol. Sci. 2022, 29, 3122–3132. [Google Scholar] [CrossRef]
- Tsai, K.-L.; Hsieh, P.-L.; Chou, W.-C.; Cheng, H.-C.; Huang, Y.-T.; Chan, S.-H. Dapagliflozin attenuates hypoxia/reoxygenation-caused cardiac dysfunction and oxidative damage through modulation of AMPK. Cell Biosci. 2021, 11, 44. [Google Scholar] [CrossRef]
- Deger, M.; Kaya, B.; Akdogan, N.; Kaplan, H.M.; Bagir, E.; Izol, V.; Aridogan, I.A. Protective effect of dapagliflozin against cyclosporine A-induced nephrotoxicity. Drug Chem. Toxicol. 2021, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zaibi, N.; Li, P.; Xu, S.-Z. Protective effects of dapagliflozin against oxidative stress-induced cell injury in human proximal tubular cells. PLoS ONE 2021, 16, e0247234. [Google Scholar] [CrossRef] [PubMed]
- El-Shafey, M.; El-Agawy, M.S.E.-D.; Eldosoky, M.; Ebrahim, H.A.; Elsherbini, D.M.A.; El-Sherbiny, M.; Asseri, S.M.; Elsherbiny, N.M. Role of Dapagliflozin and Liraglutide on Diabetes-Induced Cardiomyopathy in Rats: Implication of Oxidative Stress, Inflammation, and Apoptosis. Front. Endocrinol. 2022, 13, 862394. [Google Scholar] [CrossRef]
- Le, Y.; Wei, R.; Yang, K.; Lang, S.; Gu, L.; Liu, J.; Hong, T.; Yang, J. Liraglutide ameliorates palmitate-induced oxidative injury in islet microvascular endothelial cells through GLP-1 receptor/PKA and GTPCH1/eNOS signaling pathways. Peptides 2020, 124, 170212. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.-G.; Luo, Y.; Wang, H.; Li, J.-Y.; Yang, J.; Liu, Y.-X.; Qu, H.-Q.; Wang, B.-L.; Zhu, M. Liraglutide Ameliorates Lipotoxicity-Induced Oxidative Stress by Activating the NRF2 Pathway in HepG2 Cells. Horm. Metab. Res. 2020, 52, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Ma, D.; Gao, X.; Wang, J.; Li, R.; Liu, Z.; Wang, T.; Wang, S.; Liu, J.; Liu, X. Liraglutide Ameliorates Erectile Dysfunction via Regulating Oxidative Stress, the RhoA/ROCK Pathway and Autophagy in Diabetes Mellitus. Front. Pharmacol. 2020, 11, 1257. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [Green Version]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [Green Version]
- Gaspari, T.; Spizzo, I.; Liu, H.; Hu, Y.; Simpson, R.W.; Widdop, R.E.; Dear, A.E. Dapagliflozin attenuates human vascular endothelial cell activation and induces vasorelaxation: A potential mechanism for inhibition of atherogenesis. Diabetes Vasc. Dis. Res. 2017, 15, 64–73. [Google Scholar] [CrossRef] [Green Version]
- Di Tomo, P.; Lanuti, P.; Di Pietro, N.; Baldassarre, M.P.A.; Marchisio, M.; Pandolfi, A.; Consoli, A.; Formoso, G. Liraglutide mitigates TNF-α induced pro-atherogenic changes and microvesicle release in HUVEC from diabetic women. Diabetes Metab. Res. Rev. 2017, 33, e2925. [Google Scholar] [CrossRef]
- Savchenko, L.G.; Digtiar, N.I.; Selikhova, L.G.; Kaidasheva, E.I.; Shlykova, O.A.; Vesnina, L.E.; Kaidashev, I. Liraglutide exerts an anti-inflammatory action in obese patients with type 2 diabetes. Romanian J. Intern. Med. 2019, 57, 233–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Nishitoh, H.; Ichijo, H.; Kyriakis, J.M.; Guardavaccaro, D.; Corrente, G.; Covone, F.; Micheli, L.; D’Agnano, I.; Starace, G.; et al. Activation of Apoptosis Signal-Regulating Kinase 1 (ASK1) by Tumor Necrosis Factor Receptor-Associated Factor 2 Requires Prior Dissociation of the ASK1 Inhibitor Thioredoxin. Mol. Cell. Biol. 2000, 20, 1797–1815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hettmann, T.; DiDonato, J.; Karin, M.; Leiden, J.M. An essential role for nuclear factor kappaB in promoting double positive thymocyte apoptosis. J. Exp. Med. 1999, 189, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-T.; Lin, Y.-W.; Ho, C.-H.; Chen, Z.-C.; Liu, P.-Y.; Shih, J.-Y. Dapagliflozin suppresses ER stress and protects doxorubicin-induced cardiotoxicity in breast cancer patients. Arch. Toxicol. 2020, 95, 659–671. [Google Scholar] [CrossRef]
- Lahnwong, S.; Palee, S.; Apaijai, N.; Sriwichaiin, S.; Kerdphoo, S.; Jaiwongkam, T.; Chattipakorn, S.C.; Chattipakorn, N. Acute dapagliflozin administration exerts cardioprotective effects in rats with cardiac ischemia/reperfusion injury. Cardiovasc. Diabetol. 2020, 19, 91. [Google Scholar] [CrossRef]
- Arab, H.H.; Al-Shorbagy, M.Y.; Saad, M.A. Activation of autophagy and suppression of apoptosis by dapagliflozin attenuates experimental inflammatory bowel disease in rats: Targeting AMPK/mTOR, HMGB1/RAGE and Nrf2/HO-1 pathways. Chem. Interact. 2021, 335, 109368. [Google Scholar] [CrossRef]
- Zhang, L.; Li, C.; Zhu, Q.; Li, N.; Zhou, H. Liraglutide, a glucagon-like peptide-1 analog, inhibits high glucose-induced oxidative stress and apoptosis in neonatal rat cardiomyocytes. Exp. Ther. Med. 2019, 17, 3734–3740. [Google Scholar] [CrossRef]
- Ding, M.; Fang, Q.-H.; Cui, Y.-T.; Shen, Q.-L.; Liu, Q.; Wang, P.-H.; Yu, D.-M.; Li, C.-J. Liraglutide prevents β-cell apoptosis via inactivation of NOX2 and its related signaling pathway. J. Diabetes Its Complicat. 2019, 33, 267–277. [Google Scholar] [CrossRef]
| Gene | Sequence | |
|---|---|---|
| TNF-α | Forward | 5′-TAC TGA ACT TCG GGG TGA TTG GTC C-3′ |
| Reverse | 5′-CAG CCT TCT CCC TTG AAG AGA ACC-3′ | |
| Caspase-3 | Forward | 5′-ATGGACAACAACGAAACCTC-3′ |
| Reverse | 5′-TTAGTGATAAAAGTACAGTTCTT-3′ | |
| β-actin | Forward | 5′-CTAAGGCCAACCGTGAAAAG-3′ |
| Reverse | 5′-GCCTGGATGGCTACGTACA-3′ | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Sherbiny, M.; El-Shafey, M.; Said, E.; Shaker, G.A.; El-Dosoky, M.; Ebrahim, H.A.; Abed, S.Y.; Ibraheem, K.M.; Mohsen Faheem, A.; AlMutawa, M.; et al. Dapagliflozin, Liraglutide, and Their Combination Attenuate Diabetes Mellitus-Associated Hepato-Renal Injury—Insight into Oxidative Injury/Inflammation/Apoptosis Modulation. Life 2022, 12, 764. https://doi.org/10.3390/life12050764
El-Sherbiny M, El-Shafey M, Said E, Shaker GA, El-Dosoky M, Ebrahim HA, Abed SY, Ibraheem KM, Mohsen Faheem A, AlMutawa M, et al. Dapagliflozin, Liraglutide, and Their Combination Attenuate Diabetes Mellitus-Associated Hepato-Renal Injury—Insight into Oxidative Injury/Inflammation/Apoptosis Modulation. Life. 2022; 12(5):764. https://doi.org/10.3390/life12050764
Chicago/Turabian StyleEl-Sherbiny, Mohamed, Mohamed El-Shafey, Eman Said, Gehan Ahmed Shaker, Mohamed El-Dosoky, Hasnaa Ali Ebrahim, Sally Yussef Abed, Khalid M. Ibraheem, Ahmed Mohsen Faheem, Muntazar AlMutawa, and et al. 2022. "Dapagliflozin, Liraglutide, and Their Combination Attenuate Diabetes Mellitus-Associated Hepato-Renal Injury—Insight into Oxidative Injury/Inflammation/Apoptosis Modulation" Life 12, no. 5: 764. https://doi.org/10.3390/life12050764
APA StyleEl-Sherbiny, M., El-Shafey, M., Said, E., Shaker, G. A., El-Dosoky, M., Ebrahim, H. A., Abed, S. Y., Ibraheem, K. M., Mohsen Faheem, A., AlMutawa, M., Alatawi, B., & Elsherbiny, N. M. (2022). Dapagliflozin, Liraglutide, and Their Combination Attenuate Diabetes Mellitus-Associated Hepato-Renal Injury—Insight into Oxidative Injury/Inflammation/Apoptosis Modulation. Life, 12(5), 764. https://doi.org/10.3390/life12050764








