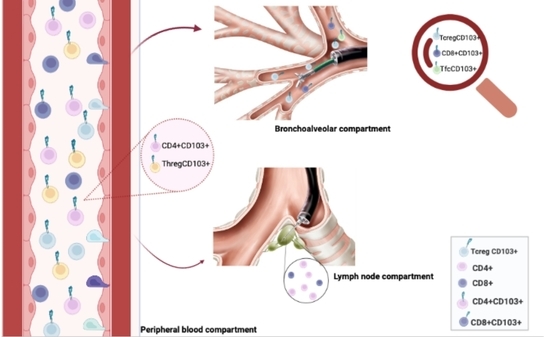
CD103 Expression on Regulatory and Follicular T Cells in Lymph Nodes, Bronchoalveolar Lavage Fluid and Peripheral Blood of Sarcoidosis Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Gating Strategy
2.3. Statistical Analysis
3. Results
3.1. Study Population
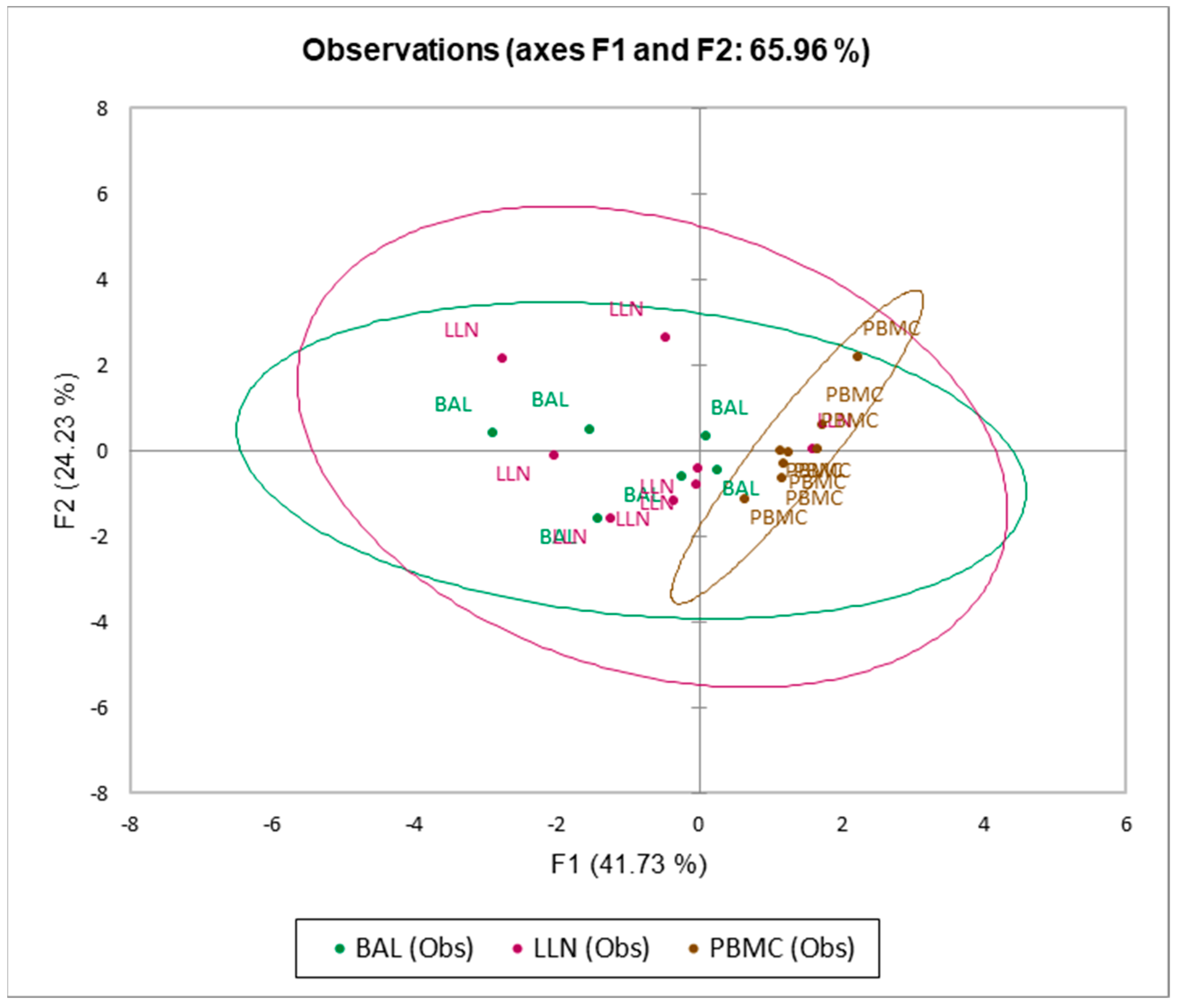
3.2. CD4, CD8, CD19 and CD103 Expression in LLN, BAL and PBMC Samples
3.3. T Regulatory Cells and CD103 Expression in LLN, BAL and PBMC Samples
3.4. T Follicular Cells and CD103 Expression in LLN, BAL and PBMC Samples
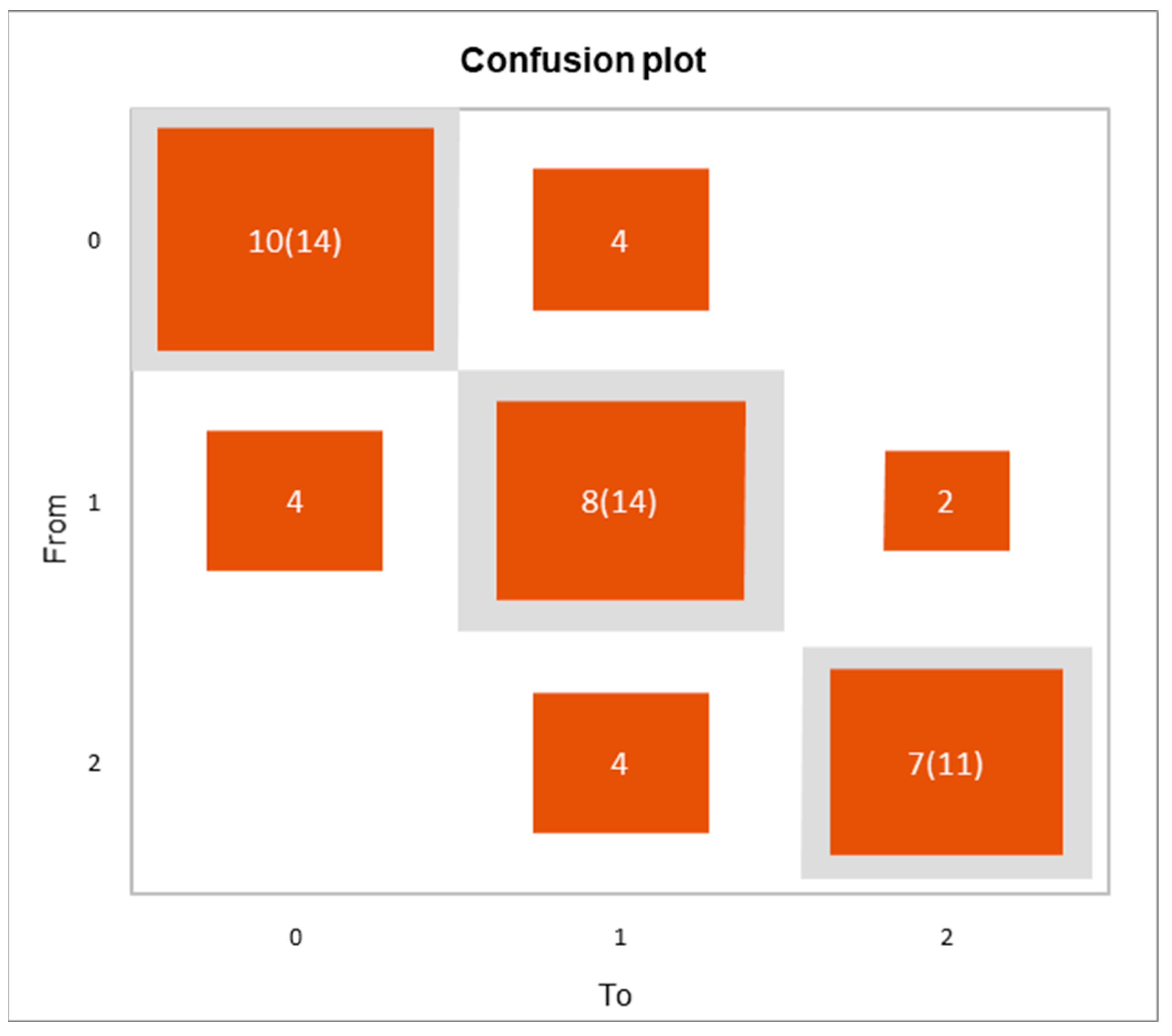
3.5. Multivariate Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Costabel, U.; Hunninghake, G.W. ATS/ERS/WASOG Statement on Sarcoidosis. Sarcoidosis Statement Committee. American Thoracic Society. European Respiratory Society. World Association for Sarcoidosis and Other Granulomatous Disorders. Eur. Respir. J. 1999, 14, 735–737. [Google Scholar] [CrossRef] [PubMed]
- Müller-Quernheim, J.; Prasse, A.; Zissel, G. Pathogenesis of Sarcoidosis. Presse Med. 2012, 41, e275–e287. [Google Scholar] [CrossRef] [PubMed]
- Agostini, C.; Meneghin, A.; Semenzato, G. T-Lymphocytes and Cytokines in Sarcoidosis. Curr. Opin. Pulm. Med. 2002, 8, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Baughman, R.P.; Lower, E.E.; Gibson, K. Pulmonary Manifestations of Sarcoidosis. Presse Med. 2012, 41, e289–e302. [Google Scholar] [CrossRef]
- Capelozzi, V.L.; Faludi, E.P.; Balthazar, A.B.; Fernezlian, S.d.M.; Filho, J.V.B.; Parra, E.R. Bronchoalveolar Lavage Improves Diagnostic Accuracy in Patients with Diffuse Lung Disease. Diagn. Cytopathol. 2013, 41, 1–8. [Google Scholar] [CrossRef]
- d’Alessandro, M.; Carleo, A.; Cameli, P.; Bergantini, L.; Perrone, A.; Vietri, L.; Lanzarone, N.; Vagaggini, C.; Sestini, P.; Bargagli, E. BAL Biomarkers’ Panel for Differential Diagnosis of Interstitial Lung Diseases. Clin. Exp. Med. 2020, 20, 207–216. [Google Scholar] [CrossRef]
- Katchar, K.; Wahlström, J.; Eklund, A.; Grunewald, J. Highly Activated T-Cell Receptor AV2S3(+) CD4(+) Lung T-Cell Expansions in Pulmonary Sarcoidosis. Am. J. Respir. Crit. Care Med. 2001, 163, 1540–1545. [Google Scholar] [CrossRef]
- Jean, C.; Gravelle, P.; Fournie, J.-J.; Laurent, G. Influence of Stress on Extracellular Matrix and Integrin Biology. Oncogene 2011, 30, 2697–2706. [Google Scholar] [CrossRef]
- Shj, K.; Da, H. Integrin Cross-Talk Modulates Stiffness-Independent Motility of CD4+ T Lymphocytes. Mol. Biol. Cell 2021, 32, 18. [Google Scholar] [CrossRef]
- Kinashi, T. Intracellular Signalling Controlling Integrin Activation in Lymphocytes. Nat. Rev. Immunol. 2005, 5, 546–559. [Google Scholar] [CrossRef]
- Sheridan, B.S.; Lefrançois, L. Regional and Mucosal Memory T Cells. Nat. Immunol. 2011, 12, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Aleksonienė, R.; Besusparis, J.; Gruslys, V.; Jurgauskienė, L.; Laurinavičienė, A.; Laurinavičius, A.; Malickaitė, R.; Norkūnienė, J.; Zablockis, R.; Žurauskas, E.; et al. CD31+, CD38+, CD44+, and CD103+ Lymphocytes in Peripheral Blood, Bronchoalveolar Lavage Fluid and Lung Biopsy Tissue in Sarcoid Patients and Controls. J. Thorac. Dis. 2021, 13, 2300–2318. [Google Scholar] [CrossRef] [PubMed]
- Hyldgaard, C.; Kaae, S.; Riddervold, M.; Hoffmann, H.J.; Hilberg, O. Value of S-ACE, BAL Lymphocytosis, and CD4+/CD8+ and CD103+CD4+/CD4+ T-Cell Ratios in Diagnosis of Sarcoidosis. Eur. Respir. J. 2012, 39, 1037–1039. [Google Scholar] [CrossRef] [PubMed]
- Heron, M.; Slieker, W.A.T.; Zanen, P.; van Lochem, E.G.; Hooijkaas, H.; van den Bosch, J.M.M.; van Velzen-Blad, H. Evaluation of CD103 as a Cellular Marker for the Diagnosis of Pulmonary Sarcoidosis. Clin. Immunol. 2008, 126, 338–344. [Google Scholar] [CrossRef]
- Qiu, Z.; Chu, T.H.; Sheridan, B.S. TGF-β: Many Paths to CD103+ CD8 T Cell Residency. Cells 2021, 10, 989. [Google Scholar] [CrossRef]
- Peters, C.; Meyer, A.; Kouakanou, L.; Feder, J.; Schricker, T.; Lettau, M.; Janssen, O.; Wesch, D.; Kabelitz, D. TGF-β Enhances the Cytotoxic Activity of Vδ2 T Cells. Oncoimmunology 2019, 8, e1522471. [Google Scholar] [CrossRef]
- Nembrini, S.; König, I.R.; Wright, M.N. The Revival of the Gini Importance? Bioinformatics 2018, 34, 3711–3718. [Google Scholar] [CrossRef]
- Sweiss, N.J.; Salloum, R.; Gandhi, S.; Ghandi, S.; Alegre, M.-L.; Sawaqed, R.; Badaracco, M.; Pursell, K.; Pitrak, D.; Baughman, R.P.; et al. Significant CD4, CD8, and CD19 Lymphopenia in Peripheral Blood of Sarcoidosis Patients Correlates with Severe Disease Manifestations. PLoS ONE 2010, 5, e9088. [Google Scholar] [CrossRef]
- Grunewald, J.; Eklund, A. Role of CD4+ T Cells in Sarcoidosis. Proc. Am. Thorac. Soc. 2007, 4, 461–464. [Google Scholar] [CrossRef]
- Grunewald, J.; Eklund, A.; Wahlström, J. CD4+ T Cells in Sarcoidosis: Targets and Tools. Expert Rev. Clin. Immunol. 2006, 2, 877–886. [Google Scholar] [CrossRef]
- Danila, E.; Norkūniene, J.; Jurgauskiene, L.; Malickaite, R. Diagnostic Role of BAL Fluid CD4/CD8 Ratio in Different Radiographic and Clinical Forms of Pulmonary Sarcoidosis. Clin. Respir. J. 2009, 3, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Oda, K.; Ishimoto, H.; Yatera, K.; Yamada, S.; Nakao, H.; Ogoshi, T.; Noguchi, S.; Yamasaki, K.; Kawanami, T.; Mukae, H. Relationship between the Ratios of CD4/CD8 T-Lymphocytes in the Bronchoalveolar Lavage Fluid and Lymph Nodes in Patients with Sarcoidosis. Respir. Investig. 2014, 52, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Salah, S.; Abad, S.; Monnet, D.; Brézin, A.P. Sarcoidosis. J. Fr. Ophtalmol. 2018, 41, e451–e467. [Google Scholar] [CrossRef] [PubMed]
- Cepek, K.L.; Shaw, S.K.; Parker, C.M.; Russell, G.J.; Morrow, J.S.; Rimm, D.L.; Brenner, M.B. Adhesion between Epithelial Cells and T Lymphocytes Mediated by E-Cadherin and the Alpha E Beta 7 Integrin. Nature 1994, 372, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Cameli, P.; Gonnelli, S.; Bargagli, E.; d’Alessandro, M.; Bergantini, L.; Favetta, V.; Tomai Pitinca, M.D.; Lisi, E.; Refini, R.M.; Pieroni, M.; et al. The Role of Urinary Calcium and Chitotriosidase in a Cohort of Chronic Sarcoidosis Patients. Respiration 2020, 99, 207–212. [Google Scholar] [CrossRef]
- Bennett, D.; Cameli, P.; Lanzarone, N.; Carobene, L.; Bianchi, N.; Fui, A.; Rizzi, L.; Bergantini, L.; Cillis, G.; d’Alessandro, M.; et al. Chitotriosidase: A biomarker of activity and severity in patients with sarcoidosis. Respir. Res. 2020, 21, 6, Erratum in: Respir. Res. 2020, 21, 34. [Google Scholar] [CrossRef]
- Couto, M.; Palmares, C.; Beltrão, M.; Neves, S.; Mota, P.; Morais, A.; Delgado, L. Integrin α E β 7 (CD103) Expression in Bronchoalveolar Lymphocytes of Patients with Hypersensitivity Pneumonitis. Int. Arch. Occup. Environ. Health 2015, 88, 167–173. [Google Scholar] [CrossRef]
- Teoh, C.M.; Tan, S.S.L.; Tran, T. Integrins as Therapeutic Targets for Respiratory Diseases. Curr. Mol. Med. 2015, 15, 714–734. [Google Scholar] [CrossRef]
- Mota, P.C.; Morais, A.; Palmares, C.; Beltrão, M.; Melo, N.; Santos, A.C.; Delgado, L. Diagnostic Value of CD103 Expression in Bronchoalveolar Lymphocytes in Sarcoidosis. Respir. Med. 2012, 106, 1014–1020. [Google Scholar] [CrossRef][Green Version]
- Kolopp-Sarda, M.-N.; Kohler, C.; De March, A.K.; Béné, M.-C.; Faure, G. Discriminative Immunophenotype of Bronchoalveolar Lavage CD4 Lymphocytes in Sarcoidosis. Lab. Investig. 2000, 80, 1065–1069. [Google Scholar] [CrossRef]
- Sathaliyawala, T.; Kubota, M.; Yudanin, N.; Turner, D.; Camp, P.; Thome, J.J.C.; Bickham, K.L.; Lerner, H.; Goldstein, M.; Sykes, M.; et al. Distribution and Compartmentalization of Human Circulating and Tissue-Resident Memory T Cell Subsets. Immunity 2013, 38, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Crouser, E.D. Role of Imbalance between Th17 and Regulatory T-Cells in Sarcoidosis. Curr. Opin. Pulm. Med. 2018, 24, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Lu, Z.; Jiang, C.; Liu, J.; Wang, Y.; Xu, Z. Imbalance between Th17 and Regulatory T-Cells in Sarcoidosis. Int. J. Mol. Sci. 2013, 14, 21463–21473. [Google Scholar] [CrossRef]
- d’Alessandro, M.; Bergantini, L.; Cameli, P.; Mezzasalma, F.; Refini, R.M.; Pieroni, M.; Sestini, P.; Bargagli, E. Adaptive Immune System in Pulmonary Sarcoidosis-Comparison of Peripheral and Alveolar Biomarkers. Clin. Exp. Immunol. 2021, 205, 406–416. [Google Scholar] [CrossRef]
- Corthay, A. How Do Regulatory T Cells Work? Scand. J. Immunol. 2009, 70, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Kudryavtsev, I.; Serebriakova, M.; Starshinova, A.; Zinchenko, Y.; Basantsova, N.; Malkova, A.; Soprun, L.; Churilov, L.P.; Toubi, E.; Yablonskiy, P.; et al. Imbalance in B Cell and T Follicular Helper Cell Subsets in Pulmonary Sarcoidosis. Sci. Rep. 2020, 10, 1059. [Google Scholar] [CrossRef] [PubMed]
- Ly, N.T.M.; Ueda-Hayakawa, I.; Nguyen, C.T.H.; Okamoto, H. Exploring the Imbalance of Circulating Follicular Helper CD4+ T Cells in Sarcoidosis Patients. J. Dermatol. Sci. 2020, 97, 216–224. [Google Scholar] [CrossRef]
- Huang, Q.; Hu, J.; Tang, J.; Xu, L.; Ye, L. Molecular Basis of the Differentiation and Function of Virus Specific Follicular Helper CD4+ T Cells. Front. Immunol. 2019, 10, 249. [Google Scholar] [CrossRef]
- Van den Bossche, J.; Laoui, D.; Naessens, T.; Smits, H.H.; Hokke, C.H.; Stijlemans, B.; Grooten, J.; De Baetselier, P.; Van Ginderachter, J.A. E-Cadherin Expression in Macrophages Dampens Their Inflammatory Responsiveness in Vitro, but Does Not Modulate M2-Regulated Pathologies in Vivo. Sci. Rep. 2015, 5, 12599. [Google Scholar] [CrossRef]
- Shah, K.K.; Pritt, B.S.; Alexander, M.P. Histopathologic Review of Granulomatous Inflammation. J. Clin. Tuberc. Mycobact. Dis. 2017, 7, 1–12. [Google Scholar] [CrossRef]


| Parameters Mean ± SD | LLN | BAL | PBMC |
|---|---|---|---|
| CD4 (Th) | 67.1 ± 25.5 | 66.3 ± 16.3 | 55.1 ± 15.4 |
| CD4+CD103+ | 36.4 ± 22.6 | 37.0 ± 20.2 | 64.5 ± 25.1 |
| CD4+CXCR5+ (Tfh) | 0.755 ± 0.853 | 0.681 ± 0.724 | 0.302 ± 0.360 |
| Tfh CD103+ | 3.59 ± 3.77 | 5.80 ± 6.00 | 3.27 ± 2.29 |
| CD4+CD127+CD25+ (Th Effector) | 3.26 ± 3.41 | 1.08 ± 1.02 | 3.67 ± 3.44 |
| CD4+CD127+CD25− (Th Naive) | 43.8 ± 21.3 | 23.2 ± 21.1 | 51.5 ± 21.7 |
| CD4+CD25+CD127− (Th Reg) | 7.01 ± 3.97 | 7.01 ± 5.15 | 4.78 ± 2.92 |
| ThReg CD103+ | 34.2 ± 31.0 | 29.4 ± 28.8 | 60.8 ± 21.5 |
| CD8 (Tc) | 14.4 ± 12.6 | 21.8 ± 15.6 | 30.7 ± 12.8 |
| CD8+CD103+ | 0.751 ± 0.964 | 0.183 ± 0.449 | 0.910 ± 1.11 |
| CD8+CXCR5+ (Tfc) | 0.0817 ± 0.200 | 0.0390 ± 0.123 | 0.216 ± 0.285 |
| Tfc CD103+ | 0.0357 ± 0.0945 | 0.823 ± 1.15 | 0.134 ± 0.228 |
| CD8+CD127+CD25+ (Tc Effector) | 4.44 ± 4.39 | 1.32 ± 3.25 | 0.747 ± 0.865 |
| CD8+CD127+CD25− (Tc Naive) | 21.4 ± 12.9 | 10.4 ± 10.8 | 16.7 ± 11.4 |
| CD8+CD25+CD127− (Tc Reg) | 0.503 ± 0.649 | 1.01 ± 1.38 | 0.480 ± 0.565 |
| TcReg CD103+ | 5.00 ± 11.2 | 47.7 ± 43.5 | 6.03 ± 13.6 |
| CD4/CD8 ratio | 4.85 ± 4.33 | 3.10 ± 3.60 | 1.80 ± 1.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
d’Alessandro, M.; Gangi, S.; Cavallaro, D.; Bergantini, L.; Mezzasalma, F.; Cattelan, S.; Baglioni, S.; Abbritti, M.; Cameli, P.; Bargagli, E. CD103 Expression on Regulatory and Follicular T Cells in Lymph Nodes, Bronchoalveolar Lavage Fluid and Peripheral Blood of Sarcoidosis Patients. Life 2022, 12, 762. https://doi.org/10.3390/life12050762
d’Alessandro M, Gangi S, Cavallaro D, Bergantini L, Mezzasalma F, Cattelan S, Baglioni S, Abbritti M, Cameli P, Bargagli E. CD103 Expression on Regulatory and Follicular T Cells in Lymph Nodes, Bronchoalveolar Lavage Fluid and Peripheral Blood of Sarcoidosis Patients. Life. 2022; 12(5):762. https://doi.org/10.3390/life12050762
Chicago/Turabian Styled’Alessandro, Miriana, Sara Gangi, Dalila Cavallaro, Laura Bergantini, Fabrizio Mezzasalma, Stefano Cattelan, Stefano Baglioni, Marta Abbritti, Paolo Cameli, and Elena Bargagli. 2022. "CD103 Expression on Regulatory and Follicular T Cells in Lymph Nodes, Bronchoalveolar Lavage Fluid and Peripheral Blood of Sarcoidosis Patients" Life 12, no. 5: 762. https://doi.org/10.3390/life12050762
APA Styled’Alessandro, M., Gangi, S., Cavallaro, D., Bergantini, L., Mezzasalma, F., Cattelan, S., Baglioni, S., Abbritti, M., Cameli, P., & Bargagli, E. (2022). CD103 Expression on Regulatory and Follicular T Cells in Lymph Nodes, Bronchoalveolar Lavage Fluid and Peripheral Blood of Sarcoidosis Patients. Life, 12(5), 762. https://doi.org/10.3390/life12050762