Animal Models of Systemic Sclerosis: Using Nailfold Capillaroscopy as a Potential Tool to Evaluate Microcirculation and Microangiopathy: A Narrative Review
Abstract
1. Introduction
2. Methods
3. Results
3.1. Animal Models Induced by Administration of Exogenous Agents
3.2. Natural or Induced Mutant Models Associated with SSc-like Disease
3.3. Genetically Engineered Mice Models
3.4. Animal Models with Manipulation of the Immune System
3.5. Other Genetically Engineered Animal Models That Are Not Fully Characterized
| Model | Target | Function | Mechanism | Fibrosis | Vasculopathy | Inflammation | Autoimmunity | Clinical Evidence | References |
|---|---|---|---|---|---|---|---|---|---|
| Bleomycin-mediated (various mice strains with different susceptibility, C3H/He and B10.A being the most susceptible | Non-specific target | Not applicable | TGF-β, collagen, and ECM synthesis upregulation EC and FB activation IL-4, IL-13, and CCL2 upregulation Oxidative stress and activation of NLRP3 inflammasome resulting in collagen synthesis STAT4 has also been involved | Dermal sclerosis Lung fibrosis | Vascular wall thickening (in deep dermis) has been reported | Present | Topo I, anti-U1 RNP, anti-histone autoantibodies Autoantibody that cross-reacts with gastric mucosa | Case reports of SSc-like syndrome in patients with antitumoral bleomycin | [22,32,37,38,39,63,64,65,66] |
| ROS-induced: Hydroxyl radicals or hypochlorous acid induced (Several strains) Peroxynitrites induced | Involvement of oxidized topo I and oxidative stress | Hydrogen peroxide production by endothelium, monocytes and fibroblasts | Involvement of AOPP, increased synthesis of collagen driven by ROS Activation of ADAM17/NOTCH, PDGFR, and VEGFRs is involved | Skin fibrosis Lung fibrosis Renal involvement | Renal vasculopathy | Present | Topo I | High serum levels of AOPP in dcSSc patients and lung fibrosis vs. controls | [33,34,38,40,63,64,67] |
| TGF-β and CTGF-induced | TGF-β and CTGF signaling | TGF-β, collagen type I, and CCL2 upregulation | TGF-β and CTGF play a role in inducing granulation tissue and fibrosis and increasing synthesis and remodelation of ECM proteins | Skin fibrosis | Not reported | Present | Not reported | See TβRΙΙΔk-fib | [32,43] |
| Angiotensin-II-induced | Angiotensin II receptor signaling activation | Upregulation of CTGF, TGF-β, and endothelial-to-mesenchymal transition MMP-12 has been related to fibrosis and vasculopathy | Administered angiotensin II induces vascular constriction, dermal fibrosis, and inflammation through activation of the TGF-β pathway. Increases fibrocytes and myofibroblasts in skin | Skin fibrosis | Present, including cardiovascular remodeling | Present | Not reported | High levels of Angiotensin II in serum of dcSSc patients. A subgroup of cases presented elevated angiotensin II signaling | [38,40,63,68] |
| Tsk1/+ | Fibrillin-1 gene | Fibrillin is a structural protein of ECM. It has a direct interaction with latent TGF-β binding protein. It is thought that the failure of sequestration of the large latent complex drives an increase in the activation of TGFβ | Hyper-response to IL-4, MCP-3, and TGF-β with increasing synthesis of type I collagen B cell activation resulted from activation of CD19 signaling | Hypodermal fibrosis Emphysema-like disease with little fibrosis | Abnormal vascular tone and cardiomyopathy | Absent | Topo I and fibrillin-1 autoantibodies | Cases of human stiff skin syndrome associated with Fbn1 mutation Association of Fibrillin-1 SNP haplotypes with SSc in Choctaw Indians and Japanese populations | [32,37,38,40,69,70,71,72] |
| Tsk2/+ | Col3α1 | Collagen III synthesis | Gain-of-function mutation that results in increased synthesis of type I and III collagen | Dermal fibrosis | Absent | Present | Antinuclear, topo I/Scl70, anti-centromere, and anti-dsDNA antibodies | [22,32,38,41,73] | |
| UCD-200 and 206 chickens | TGFBR1, IGFBP3, EXOC2/IRF4, CCR8, and SOCS1 | Associated genes play a role in either SSc or autoimmunity | Spontaneously develops vascular damage, fibrosis and inflammation. | Skin fibrosis Lung fibrosis Internal organ fibrosis | Vasculopathy Intimal proliferation with narrowing of arterioles and capillaries and cell infiltration | Present | Polyarthritis Antinuclear antibodies, AECA, aCL antibodies, and rheumatoid factor | Not reported | [5,32,37,38,63] |
| Kinase-deficient TGFβRII model: TβRΙΙΔk-fib | Type II TGF-β receptor | Binding of the ligand to TβRII drives the phosphorylation of serine residues within the type I receptor (TβRI) to initiate TGF-downstream signaling | Paradoxical activation of TGF-β signaling It is postulated that accessory proteins and a nonsignaling type I receptor can modulate TβRII function | Skin fibrosis Pulmonary fibrosis Fibrotic cardiomyopathy | Generalized vascular remodeling | Not present | Not present | Several lines of research support the ling of link between TGF-β and fibrotic disease and systemic sclerosis | [22,32,38,39,40,43,74,75,76] |
| TBRI(CA); Cre-ER mice | Expression of a constitutionally active TGF-β1 type I receptor | TGF-β signaling activation | Increased collagen synthesis | Progressive and generalized dermal fibrosis Myocardial fibrosis Altered aortic dynamics | Thickening of blood vessel walls in small arteries of the lung and kidney Increased levels of von Willebrand factor Altered endothelin-1 signaling | Absent | Not reported | See TβRΙΙΔk-fib | [38,39,63,77] |
| Fbn-1 mutations Knock-in mouse: SSS- associated change W1572C (WC/WC, WC/+) D1545E (DE/+) (Homozygous was lethal) | FBN1 gene | See fibrillarin function in Tsk1 model | Excessive elastin, collagen, and microfibrillar aggregates in dermal TGF-β upregulation | Skin fibrosis | Not reported | Present | Topo I | See Tsk1 clinical evidence | [22,78] |
| Fra-2 Tg mice | Fra-2 overexpression | A member of the Fos family of transcription factors, a downstream mediator of TGF-β and PDGF. Induced by cellular stress and controls apoptosis, inflammation, healing and proliferation. | Upregulation of type I collagen in dermal fibroblasts, EC apoptosis and epithelial-to-mesenchymal transition, PDGF signaling activation | Skin fibrosis Pulmonary fibrosis | Vasculopathy Decrease in the number of capillaries EC apoptosis, pulmonary arterial occlusion | Present | Not present | Fra-2 expression is elevated in SSc dermal fibroblasts, EC, and pneumocyte epithelial cells | [38,40,63] |
| Fli1ΔCTA/ΔCTA | Fli1 | Fli1 has roles in hematopoiesis and vasculogenesis, serves as a transcriptional repressor through its CTA domain, inhibits collagen genes, and is a negative regulator of ECM | Upregulation of dermal fibrillar collagen and Fli1 protein levels | Skin fibrosis | Present. Includes increases vascular permeability, similar to the one observed in SSc | Not reported | Not reported | Fli1 proteins are reduced in dermal fibroblasts, EC, and SMC of SSc patients | [18,38,40] |
| Fli1 ECKO Conditional deletion of Fli1 in EC | Fli1 | Fli1 is a transcription factor expressed in EC and hematopoietic cells. It participates in the regulation of development and differentiation of EC and vasculature | Loss of endothelial integrity Fli-1 depletion has an effect on expression of genes that regulate vascular integrity | Absent | Present, irregular diameter and disorganization of the dermal vascular network | Absent | Not reported | See Fli1 mutated mice | [40,63] |
| Fli1-KLF5-KO | Fli1+KLF5 | Fli1 is a potent repressor of type I collagen gene KLF5 has a potentially synergic effect with Fli1 | Activation of both canonical and non-canonical TGF-β signaling Upregulation of ECM genes and CTGF | Skin fibrosis Lung fibrosis | Obliterative vasculopathy with vascular stenosis, loss and bushy skin capillaries Progressive obliteration of pulmonary arterioles | Present | Antinuclear antibodies | Downregulation of KLF5 in skin samples and SSc fibroblasts Fli1 repression in SSc fibroblast and EC | [20,22] |
| Sirt3-deficient mice | Sirt3 | Class III histone deacetylases that play a key role in maintenance of mitochondrial integrity | Deficiency induces spontaneous multiorgan fibrosis as the mice age, accompanied by oxidative stress and mitochondrial damage | Multiorgan fibrosis including in the lungs and heart | PAH | Not reported | Not reported | Marked suppression of Sirt1 has been found in skin biopsies and explanted fibroblasts from SSc patients in two reports | [22,79] |
| Endothelin-1 Tg | ET-1 overexpression | Potent vasoconstrictor, regulation of blood flow in microvascular beds, sodium and water reabsorption Stimulates proliferation and collagen synthesis in normal fibroblast | Fibrogenic effect including: increased pulmonary matrix synthesis with chronic inflammation, excessive collagen production of SSc fibroblasts enhanced by TGF-β | Renal fibrosis Lung fibrosis (chronic overexpression) | Increased media/lumen ratio of intrarenal arteries | Present | Not reported | Higher serum levels and overproduction of endothelin-1 in SSc patients and in primary RP. Levels were associated with skin score and disease duration Effectiveness of endothelin-1 blockade in pulmonary hypertension and RP treatment | [38,48,49,80,81,82,83] |
| PDGFR-α hyperactivation | PDGFRα conditional overexpression | PDGFs play an important role in the development and maintenance of connective tissue, they are potent mitogens and chemoattractants of mesenchymal cells, they exert their biological effects through binding of PDGFRα/β | Activation of cellular programs that generate connective tissue Connective tissue hyperplasia and increased extracellular matrix deposition | Skin fibrosis Internal organ fibrosis | Not reported | Not reported | Not reported | Increased levels of PDGF and PDGFR in skin and lung samples of SSc patients Presence of stimulatory autoantibodies directed toward PDGFR More common and intense PDGFRβ inmunoreactivity in small vessels of SSc-associated PAH compared to idiopathic PAH | [51,84,85,86,87] |
| Cav-1−/− C57B1/6KO mice | Caveolin-1 | It is an integral membrane protein and a structural component of caveolae. It modulates the activity of caveolae and disrupts TGF-β signaling | Upregulation of TGF-β and ECM proteins ROS, HIF1α y NFκB signaling pathways are activated | Thickening of alveolar septa with uncontrolled hyperproliferation of angioblastic cells and fibrosis Skin fibrosis | Vascular system disfunction Increased accumulation of collagen fibers around blood vessels of the deep dermis | Present | Not reported | Reduced levels of Cav-1 in skin and dermal fibroblasts from SSc patients and in lung samples from ILD-SSc patients Reduction of Cav-1 in lung fibroblasts and lung tissue in bleomycin- induced IPF | [52,53,54,56] |
| Hematopoietic cell transplantation: Scl-GVHD LP/J/ C57BL/6J B10.D2/BALB/c mice RAG-2 KO mice | Non-specific target | TGF-β upregulation, increased type I collage, chemokines, and other growth factors | Fibroblast, T cells, and other leucocytes involved | Skin fibrosis Lung fibrosis (not all models) Involvement of internal organs (kidney, small intestine) (not all models) | Vasculopathy Renal crisis | Present | Autoantibodies | Scl-GVHD after hematopoietic cell transplantation | [32,38,39,61,62,63,72,88] |
| Topo I and CFA-induced SSc | DNA topoisomerase | Relaxation of supercoiled DNA | Upregulation of TGF-β, IL-17 and IL-6 | Skin fibrosis Pulmonary fibrosis | Not reported | Present | Topo I | Several studies reported the presence of autoantibodies including disease-specific autoantibodies | [38,40,63,89] |
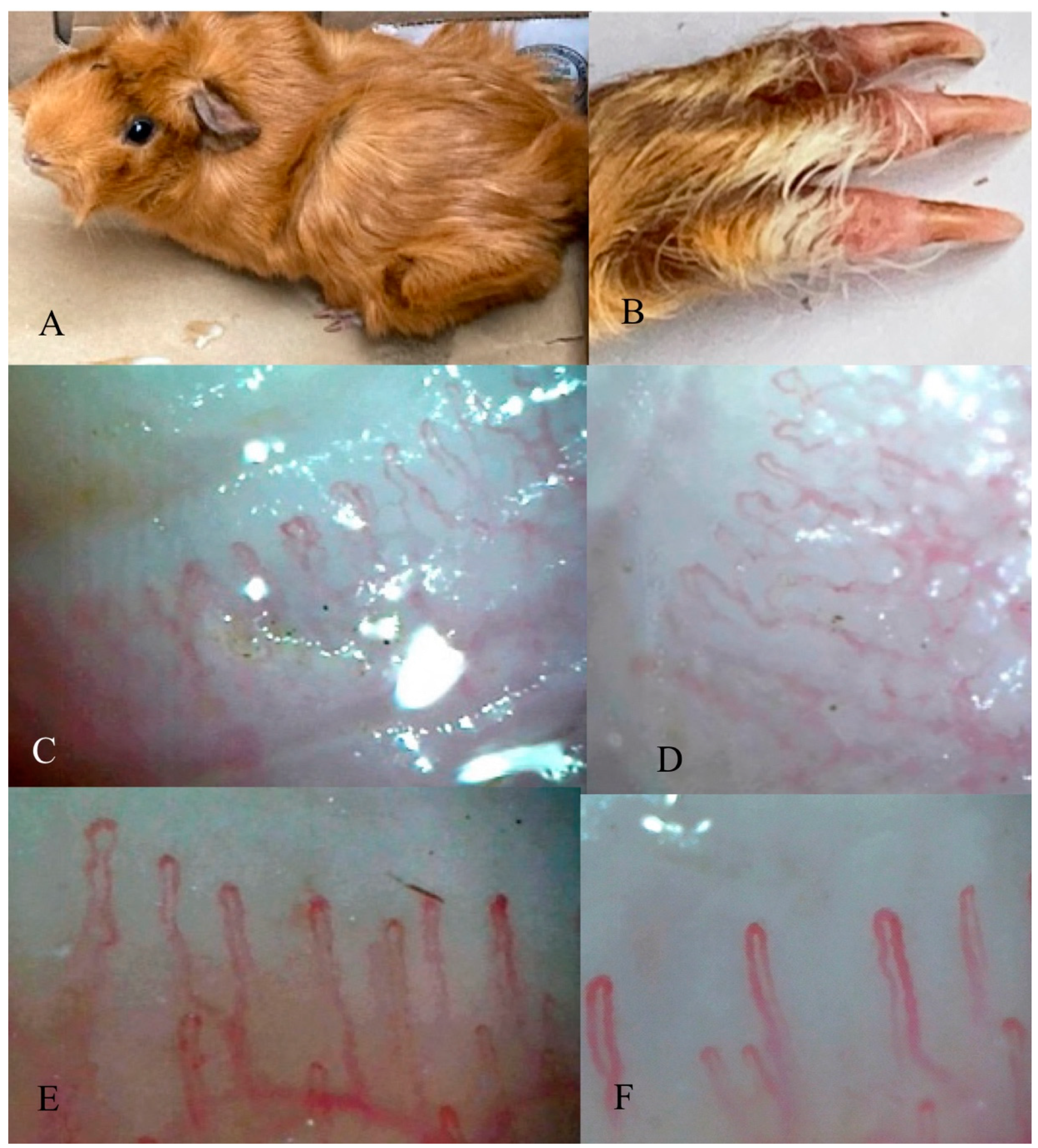
3.6. Nailfold Capillaroscopy in the Guinea Pig
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Varga, J.; Abraham, D. Systemic sclerosis: A prototypic multisystem fibrotic disorder. J. Clin. Investig. 2007, 117, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Gabrielli, A.; Avvedimento, E.V.; Krieg, T. Scleroderma. N. Engl. J. Med. 2009, 360, 1989–2003. [Google Scholar] [CrossRef]
- Hughes, M.; Allanore, Y.; Chung, L.; Pauling, J.D.; Denton, C.P.; Matucci-Cerinic, M. Raynaud phenomenon and digital ulcers in systemic sclerosis. Nat. Rev. Rheumatol. 2020, 16, 208–221. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M.; Soldano, S.; Smith, V. Pathophysiology of systemic sclerosis: Current understanding and new insights. Expert Rev. Clin. Immunol. 2019, 15, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Wick, G.; Andersson, L.; Hala, K.; Gershwin, M.E.; Selmi, C.; Erf, G.F.; Lamont, S.J.; Sgonc, R. Avian models with spontaneous autoimmune diseases. Adv. Immunol. 2006, 92, 71–117. [Google Scholar] [CrossRef]
- Radic, M.; Thomas, J.; McMillan, S.; Frech, T. Does sublingual microscopy correlate with nailfold videocapillaroscopy in systemic sclerosis? Clin. Rheumatol. 2021, 40, 2263–2266. [Google Scholar] [CrossRef]
- Medsger, T.A. Natural history of systemic sclerosis and the assessment of disease activity, severity, functional status, and psychologic well-being. Rheum. Dis. Clin. N. Am. 2003, 29, 255–273. [Google Scholar] [CrossRef]
- Muryoi, T.; Kasturi, K.N.; Kafina, M.J.; Cram, D.S.; Harrison, L.C.; Sasaki, T.; Bona, C.A. Antitopoisomerase I monoclonal autoantibodies from scleroderma patients and tight skin mouse interact with similar epitopes. J. Exp. Med. 1992, 175, 1103–1109. [Google Scholar] [CrossRef]
- Patel, S.; Morrisroe, K.; Proudman, S.; Hansen, D.; Sahhar, J.; Sim, M.R.; Ngian, G.S.; Walker, J.; Strickland, G.; Wilson, M.; et al. Occupational silica exposure in an Australian systemic sclerosis cohort. Rheumatology 2020, 59, 3900–3905. [Google Scholar] [CrossRef]
- Marie, I. Systemic sclerosis and exposure to heavy metals. Autoimmun. Rev. 2019, 18, 62–72. [Google Scholar] [CrossRef]
- Maurer, B.; Stanczyk, J.; Jüngel, A.; Akhmetshina, A.; Trenkmann, M.; Brock, M.; Kowal-Bielecka, O.; Gay, R.E.; Michel, B.A.; Distler, J.H.; et al. MicroRNA-29, a key regulator of collagen expression in systemic sclerosis. Arthritis Rheum. 2010, 62, 1733–1743. [Google Scholar] [CrossRef] [PubMed]
- Bossini-Castillo, L.; Villanueva-Martin, G.; Kerick, M.; Acosta-Herrera, M.; López-Isac, E.; Simeón, C.P.; Ortego-Centeno, N.; Assassi, S.; Hunzelmann, N.; Gabrielli, A.; et al. Genomic Risk Score impact on susceptibility to systemic sclerosis. Ann. Rheum. Dis. 2021, 80, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Feghali-Bostwick, C.; Medsger, T.A.; Wright, T.M. Analysis of systemic sclerosis in twins reveals low concordance for disease and high concordance for the presence of antinuclear antibodies. Arthritis Rheum. 2003, 48, 1956–1963. [Google Scholar] [CrossRef]
- Frech, T.; Khanna, D.; Markewitz, B.; Mineau, G.; Pimentel, R.; Sawitzke, A. Heritability of vasculopathy, autoimmune disease, and fibrosis in systemic sclerosis: A population-based study. Arthritis Rheum. 2010, 62, 2109–2116. [Google Scholar] [CrossRef]
- Arnett, F.C.; Gourh, P.; Shete, S.; Ahn, C.W.; Honey, R.E.; Agarwal, S.K.; Tan, F.K.; McNearney, T.; Fischbach, M.; Fritzler, M.J.; et al. Major histocompatibility complex (MHC) class II alleles, haplotypes and epitopes which confer susceptibility or protection in systemic sclerosis: Analyses in 1300 Caucasian, African-American and Hispanic cases and 1000 controls. Ann. Rheum. Dis. 2010, 69, 822–827. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Bhattacharyya, S.; Lafyatis, R.; Farina, G.; Yu, J.; Thimmapaya, B.; Wei, J.; Varga, J. p300 is elevated in systemic sclerosis and its expression is positively regulated by TGF-Œ≤: Epigenetic feed-forward amplification of fibrosis. J. Investig. Derm. 2013, 133, 1302–1310. [Google Scholar] [CrossRef] [PubMed]
- Altorok, N.; Tsou, P.S.; Coit, P.; Khanna, D.; Sawalha, A.H. Genome-wide DNA methylation analysis in dermal fibroblasts from patients with diffuse and limited systemic sclerosis reveals common and subset-specific DNA methylation aberrancies. Ann. Rheum. Dis. 2015, 74, 1612–1620. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fan, P.S.; Kahaleh, B. Association between enhanced type I collagen expression and epigenetic repression of the FLI1 gene in scleroderma fibroblasts. Arthritis Rheum. 2006, 54, 2271–2279. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Wang, Q.; Sun, X.H.; Liu, R.Z.; Shu, Y.; Kanekura, T.; Huang, J.H.; Li, Y.P.; Wang, J.C.; Zhao, M.; et al. DNA hypermethylation of the forkhead box protein 3 (FOXP3) promoter in CD4+ T cells of patients with systemic sclerosis. Br. J. Derm. 2014, 171, 39–47. [Google Scholar] [CrossRef]
- Noda, S.; Asano, Y.; Nishimura, S.; Taniguchi, T.; Fujiu, K.; Manabe, I.; Nakamura, K.; Yamashita, T.; Saigusa, R.; Akamata, K.; et al. Simultaneous downregulation of KLF5 and Fli1 is a key feature underlying systemic sclerosis. Nat. Commun. 2014, 5, 5797. [Google Scholar] [CrossRef]
- Giacomelli, R.; Liakouli, V.; Berardicurti, O.; Ruscitti, P.; Di Benedetto, P.; Carubbi, F.; Guggino, G.; Di Bartolomeo, S.; Ciccia, F.; Triolo, G.; et al. Interstitial lung disease in systemic sclerosis: Current and future treatment. Rheumatol. Int. 2017, 37, 853–863. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, R.G.; Varga, J.; Tourtellotte, W.G. Animal models of scleroderma: Recent progress. Curr. Opin. Rheumatol. 2016, 28, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Fardoun, M.M.; Nassif, J.; Issa, K.; Baydoun, E.; Eid, A.H. Raynaud’s Phenomenon: A Brief Review of the Underlying Mechanisms. Front. Pharm. 2016, 7, 438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Flanders, K.C.; Phan, S.H. Cellular localization of transforming growth factor-beta expression in bleomycin-induced pulmonary fibrosis. Am. J. Pathol. 1995, 147, 352–361. [Google Scholar] [PubMed]
- Degryse, A.L.; Tanjore, H.; Xu, X.C.; Polosukhin, V.V.; Jones, B.R.; McMahon, F.B.; Gleaves, L.A.; Blackwell, T.S.; Lawson, W.E. Repetitive intratracheal bleomycin models several features of idiopathic pulmonary fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2010, 299, L442–L452. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Takagawa, S.; Katayama, I.; Yamazaki, K.; Hamazaki, Y.; Shinkai, H.; Nishioka, K. Animal model of sclerotic skin. I: Local injections of bleomycin induce sclerotic skin mimicking scleroderma. J. Investig. Derm. 1999, 112, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, A.; Iwata, Y.; Komura, K.; Ogawa, F.; Hara, T.; Muroi, E.; Takenaka, M.; Shimizu, K.; Hasegawa, M.; Fujimoto, M.; et al. CD19 regulates skin and lung fibrosis via Toll-like receptor signaling in a model of bleomycin-induced scleroderma. Am. J. Pathol. 2008, 172, 1650–1663. [Google Scholar] [CrossRef]
- Yamamoto, T.; Nishioka, K. Cellular and molecular mechanisms of bleomycin-induced murine scleroderma: Current update and future perspective. Exp. Derm. 2005, 14, 81–95. [Google Scholar] [CrossRef]
- Adamson, I.Y.; Bowden, D.H. The pathogenesis of bleomycin-induced pulmonary fibrosis in mice. Am. J. Pathol. 1974, 77, 185–197. [Google Scholar]
- Szapiel, S.V.; Elson, N.A.; Fulmer, J.D.; Hunninghake, G.W.; Crystal, R.G. Bleomycin-induced interstitial pulmonary disease in the nude, athymic mouse. Am. Rev. Respir. Dis. 1979, 120, 893–899. [Google Scholar] [CrossRef]
- Belperio, J.A.; Dy, M.; Burdick, M.D.; Xue, Y.Y.; Li, K.; Elias, J.A.; Keane, M.P. Interaction of IL-13 and C10 in the pathogenesis of bleomycin-induced pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2002, 27, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T. Animal model of systemic sclerosis. J. Derm. 2010, 37, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Servettaz, A.; Goulvestre, C.; Kavian, N.; Nicco, C.; Guilpain, P.; Chéreau, C.; Vuiblet, V.; Guillevin, L.; Mouthon, L.; Weill, B.; et al. Selective oxidation of DNA topoisomerase 1 induces systemic sclerosis in the mouse. J. Immunol. 2009, 182, 5855–5864. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.; Tan, J.; Chen, W.; Du, Q.; Xie, B.; Wang, N.; Zhu, H.; Wang, K. The Fibrosis and Immunological Features of Hypochlorous Acid Induced Mouse Model of Systemic Sclerosis. Front. Immunol. 2019, 10, 1861. [Google Scholar] [CrossRef]
- Christner, P.J.; Artlett, C.M.; Conway, R.F.; Jiménez, S.A. Increased numbers of microchimeric cells of fetal origin are associated with dermal fibrosis in mice following injection of vinyl chloride. Arthritis Rheum. 2000, 43, 2598–2605. [Google Scholar] [CrossRef]
- Denton, C.P.; Abraham, D.J. Transgenic analysis of scleroderma: Understanding key pathogenic events in vivo. Autoimmun. Rev. 2004, 3, 285–293. [Google Scholar] [CrossRef]
- Rogai, V.; Lories, R.J.; Guiducci, S.; Luyten, F.P.; Matucci Cerinic, M. Animal models in systemic sclerosis. Clin. Exp. Rheumatol. 2008, 26, 941–946. [Google Scholar]
- Artlett, C.M. Animal models of systemic sclerosis: Their utility and limitations. Open Access Rheumatol. 2014, 6, 65–81. [Google Scholar] [CrossRef]
- Beyer, C.; Schett, G.; Distler, O.; Distler, J.H. Animal models of systemic sclerosis: Prospects and limitations. Arthritis Rheum. 2010, 62, 2831–2844. [Google Scholar] [CrossRef]
- Asano, Y.; Sato, S. Animal models of scleroderma: Current state and recent development. Curr. Rheumatol. Rep. 2013, 15, 382. [Google Scholar] [CrossRef]
- Long, K.B.; Li, Z.; Burgwin, C.M.; Choe, S.G.; Martyanov, V.; Sassi-Gaha, S.; Earl, J.P.; Eutsey, R.A.; Ahmed, A.; Ehrlich, G.D.; et al. The Tsk2/+ mouse fibrotic phenotype is due to a gain-of-function mutation in the PIIINP segment of the Col3a1 gene. J. Investig. Dermatol. 2015, 135, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Barisic-Dujmovic, T.; Boban, I.; Clark, S.H. Regulation of collagen gene expression in the Tsk2 mouse. J. Cell Physiol. 2008, 215, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Denton, C.P.; Zheng, B.; Evans, L.A.; Shi-wen, X.; Ong, V.H.; Fisher, I.; Lazaridis, K.; Abraham, D.J.; Black, C.M.; de Crombrugghe, B. Fibroblast-specific expression of a kinase-deficient type II transforming growth factor beta (TGFbeta) receptor leads to paradoxical activation of TGFbeta signaling pathways with fibrosis in transgenic mice. J. Biol. Chem. 2003, 278, 25109–25119. [Google Scholar] [CrossRef]
- Derrett-Smith, E.C.; Dooley, A.; Gilbane, A.J.; Trinder, S.L.; Khan, K.; Baliga, R.; Holmes, A.M.; Hobbs, A.J.; Abraham, D.; Denton, C.P. Endothelial injury in a transforming growth factor β-dependent mouse model of scleroderma induces pulmonary arterial hypertension. Arthritis Rheum. 2013, 65, 2928–2939. [Google Scholar] [CrossRef] [PubMed]
- Maurer, B.; Distler, J.H.; Distler, O. The Fra-2 transgenic mouse model of systemic sclerosis. Vasc. Pharm. 2013, 58, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Manetti, M.; Rosa, I.; Milia, A.F.; Guiducci, S.; Carmeliet, P.; Ibba-Manneschi, L.; Matucci-Cerinic, M. Inactivation of urokinase-type plasminogen activator receptor (uPAR) gene induces dermal and pulmonary fibrosis and peripheral microvasculopathy in mice: A new model of experimental scleroderma? Ann. Rheum. Dis. 2014, 73, 1700–1709. [Google Scholar] [CrossRef]
- Manetti, M.; Rosa, I.; Fazi, M.; Guiducci, S.; Carmeliet, P.; Ibba-Manneschi, L.; Matucci-Cerinic, M. Systemic sclerosis-like histopathological features in the myocardium of uPAR-deficient mice. Ann. Rheum. Dis. 2016, 75, 474–478. [Google Scholar] [CrossRef]
- Hocher, B.; Thöne-Reineke, C.; Rohmeiss, P.; Schmager, F.; Slowinski, T.; Burst, V.; Siegmund, F.; Quertermous, T.; Bauer, C.; Neumayer, H.H.; et al. Endothelin-1 transgenic mice develop glomerulosclerosis, interstitial fibrosis, and renal cysts but not hypertension. J. Clin. Investig. 1997, 99, 1380–1389. [Google Scholar] [CrossRef]
- Hocher, B.; Schwarz, A.; Fagan, K.A.; Thöne-Reineke, C.; El-Hag, K.; Kusserow, H.; Elitok, S.; Bauer, C.; Neumayer, H.H.; Rodman, D.M.; et al. Pulmonary fibrosis and chronic lung inflammation in ET-1 transgenic mice. Am. J. Respir. Cell Mol. Biol. 2000, 23, 19–26. [Google Scholar] [CrossRef][Green Version]
- Olson, L.E.; Soriano, P. Increased PDGFRalpha activation disrupts connective tissue development and drives systemic fibrosis. Dev. Cell 2009, 16, 303–313. [Google Scholar] [CrossRef]
- Kavian, N.; Servettaz, A.; Marut, W.; Nicco, C.; Chéreau, C.; Weill, B.; Batteux, F. Sunitinib inhibits the phosphorylation of platelet-derived growth factor receptor Œ≤ in the skin of mice with scleroderma-like features and prevents the development of the disease. Arthritis Rheum. 2012, 64, 1990–2000. [Google Scholar] [CrossRef] [PubMed]
- Drab, M.; Verkade, P.; Elger, M.; Kasper, M.; Lohn, M.; Lauterbach, B.; Menne, J.; Lindschau, C.; Mende, F.; Luft, F.C.; et al. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science 2001, 293, 2449–2452. [Google Scholar] [CrossRef] [PubMed]
- Castello-Cros, R.; Whitaker-Menezes, D.; Molchansky, A.; Purkins, G.; Soslowsky, L.J.; Beason, D.P.; Sotgia, F.; Iozzo, R.V.; Lisanti, M.P. Scleroderma-like properties of skin from caveolin-1-deficient mice: Implications for new treatment strategies in patients with fibrosis and systemic sclerosis. Cell Cycle 2011, 10, 2140–2150. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.M.; Zhang, Y.; Kim, H.P.; Zhou, Z.; Feghali-Bostwick, C.A.; Liu, F.; Ifedigbo, E.; Xu, X.; Oury, T.D.; Kaminski, N.; et al. Caveolin-1: A critical regulator of lung fibrosis in idiopathic pulmonary fibrosis. J. Exp. Med. 2006, 203, 2895–2906. [Google Scholar] [CrossRef] [PubMed]
- Del Galdo, F.; Sotgia, F.; de Almeida, C.J.; Jasmin, J.F.; Musick, M.; Lisanti, M.P.; Jiménez, S.A. Decreased expression of caveolin 1 in patients with systemic sclerosis: Crucial role in the pathogenesis of tissue fibrosis. Arthritis Rheum. 2008, 58, 2854–2865. [Google Scholar] [CrossRef] [PubMed]
- Razani, B.; Zhang, X.L.; Bitzer, M.; von Gersdorff, G.; Böttinger, E.P.; Lisanti, M.P. Caveolin-1 regulates transforming growth factor (TGF)-beta/SMAD signaling through an interaction with the TGF-beta type I receptor. J. Biol. Chem. 2001, 276, 6727–6738. [Google Scholar] [CrossRef]
- Le Hir, M.; Martin, M.; Haas, C. A syndrome resembling human systemic sclerosis (scleroderma) in MRL/lpr mice lacking interferon-gamma (IFN-gamma) receptor (MRL/lprgammaR−/−). Clin. Exp. Immunol. 1999, 115, 281–287. [Google Scholar] [CrossRef]
- Sime, P.J.; Xing, Z.; Graham, F.L.; Csaky, K.G.; Gauldie, J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J. Clin. Investig. 1997, 100, 768–776. [Google Scholar] [CrossRef]
- Hamilton, B.L.; Parkman, R. Acute and chronic graft-versus-host disease induced by minor histocompatibility antigens in mice. Transplantation 1983, 36, 150–155. [Google Scholar] [CrossRef]
- Claman, H.N.; Jaffee, B.D.; Huff, J.C.; Clark, R.A. Chronic graft-versus-host disease as a model for scleroderma. II. Mast cell depletion with deposition of immunoglobulins in the skin and fibrosis. Cell Immunol. 1985, 94, 73–84. [Google Scholar] [CrossRef]
- McCormick, L.L.; Zhang, Y.; Tootell, E.; Gilliam, A.C. Anti-TGF-beta treatment prevents skin and lung fibrosis in murine sclerodermatous graft-versus-host disease: A model for human scleroderma. J. Immunol. 1999, 163, 5693–5699. [Google Scholar] [PubMed]
- Ruzek, M.C.; Jha, S.; Ledbetter, S.; Richards, S.M.; Garman, R.D. A modified model of graft-versus-host-induced systemic sclerosis (scleroderma) exhibits all major aspects of the human disease. Arthritis Rheum. 2004, 50, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Morin, F.; Kavian, N.; Batteux, F. Animal models of systemic sclerosis. Curr. Pharm. Des. 2015, 21, 2365–2379. [Google Scholar] [CrossRef] [PubMed]
- Batteux, F.; Kavian, N.; Servettaz, A. New insights on chemically induced animal models of systemic sclerosis. Curr. Opin. Rheumatol. 2011, 23, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Finch, W.R.; Rodnan, G.P.; Buckingham, R.B.; Prince, R.K.; Winkelstein, A. Bleomycin-induced scleroderma. J. Rheumatol. 1980, 7, 651–659. [Google Scholar]
- Kerr, L.D.; Spiera, H. Scleroderma in association with the use of bleomycin: A report of 3 cases. J. Rheumatol. 1992, 19, 294–296. [Google Scholar]
- Artlett, C.M.; Sassi-Gaha, S.; Rieger, J.L.; Boesteanu, A.C.; Feghali-Bostwick, C.A.; Katsikis, P.D. The inflammasome activating caspase 1 mediates fibrosis and myofibroblast differentiation in systemic sclerosis. Arthritis Rheum. 2011, 63, 3563–3574. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Takagi, K.; Hara, M.; Fukasawa, C.; Sugiura, T.; Nishimagi, E.; Harigai, M.; Kamatani, N. Angiotensin II in the lesional skin of systemic sclerosis patients contributes to tissue fibrosis via angiotensin II type 1 receptors. Arthritis Rheum. 2004, 50, 216–226. [Google Scholar] [CrossRef]
- Loeys, B.L.; Gerber, E.E.; Riegert-Johnson, D.; Iqbal, S.; Whiteman, P.; McConnell, V.; Chillakuri, C.R.; Macaya, D.; Coucke, P.J.; De Paepe, A.; et al. Mutations in fibrillin-1 cause congenital scleroderma: Stiff skin syndrome. Sci. Transl. Med. 2010, 2, 23ra20. [Google Scholar] [CrossRef]
- Tan, F.K.; Wang, N.; Kuwana, M.; Chakraborty, R.; Bona, C.A.; Milewicz, D.M.; Arnett, F.C. Association of fibrillin 1 single-nucleotide polymorphism haplotypes with systemic sclerosis in Choctaw and Japanese populations. Arthritis Rheum. 2001, 44, 893–901. [Google Scholar] [CrossRef]
- Ong, V.H.; Evans, L.A.; Shiwen, X.; Fisher, I.B.; Rajkumar, V.; Abraham, D.J.; Black, C.M.; Denton, C.P. Monocyte chemoattractant protein 3 as a mediator of fibrosis: Overexpression in systemic sclerosis and the type 1 tight-skin mouse. Arthritis Rheum. 2003, 48, 1979–1991. [Google Scholar] [CrossRef] [PubMed]
- Christner, P.J.; Jimenez, S.A. Animal models of systemic sclerosis: Insights into systemic sclerosis pathogenesis and potential therapeutic approaches. Curr. Opin. Rheumatol. 2004, 16, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Christner, P.J.; Peters, J.; Hawkins, D.; Siracusa, L.D.; Jiménez, S.A. The tight skin 2 mouse. An animal model of scleroderma displaying cutaneous fibrosis and mononuclear cell infiltration. Arthritis Rheum. 1995, 38, 1791–1798. [Google Scholar] [CrossRef] [PubMed]
- Varga, J.; Bashey, R.I. Regulation of connective tissue synthesis in systemic sclerosis. Int. Rev. Immunol. 1995, 12, 187–199. [Google Scholar] [CrossRef]
- Lafyatis, R. Transforming growth factor β--at the centre of systemic sclerosis. Nat. Rev. Rheumatol. 2014, 10, 706–719. [Google Scholar] [CrossRef]
- Martinović Kaliterna, D.; Petrić, M. Biomarkers of skin and lung fibrosis in systemic sclerosis. Expert Rev. Clin. Immunol. 2019, 15, 1215–1223. [Google Scholar] [CrossRef]
- Sonnylal, S.; Denton, C.P.; Zheng, B.; Keene, D.R.; He, R.; Adams, H.P.; Vanpelt, C.S.; Geng, Y.J.; Deng, J.M.; Behringer, R.R.; et al. Postnatal induction of transforming growth factor beta signaling in fibroblasts of mice recapitulates clinical, histologic, and biochemical features of scleroderma. Arthritis Rheum. 2007, 56, 334–344. [Google Scholar] [CrossRef]
- Gerber, E.E.; Gallo, E.M.; Fontana, S.C.; Davis, E.C.; Wigley, F.M.; Huso, D.L.; Dietz, H.C. Integrin-modulating therapy prevents fibrosis and autoimmunity in mouse models of scleroderma. Nature 2013, 503, 126–130. [Google Scholar] [CrossRef]
- Sundaresan, N.R.; Bindu, S.; Pillai, V.B.; Samant, S.; Pan, Y.; Huang, J.Y.; Gupta, M.; Nagalingam, R.S.; Wolfgeher, D.; Verdin, E.; et al. SIRT3 Blocks Aging-Associated Tissue Fibrosis in Mice by Deacetylating and Activating Glycogen Synthase Kinase 3β. Mol. Cell Biol. 2015, 36, 678–692. [Google Scholar] [CrossRef]
- Vancheeswaran, R.; Magoulas, T.; Efrat, G.; Wheeler-Jones, C.; Olsen, I.; Penny, R.; Black, C.M. Circulating endothelin-1 levels in systemic sclerosis subsets--A marker of fibrosis or vascular dysfunction? J. Rheumatol. 1994, 21, 1838–1844. [Google Scholar]
- Stochmal, A.; Czuwara, J.; Zaremba, M.; Rudnicka, L. Altered serum level of metabolic and endothelial factors in patients with systemic sclerosis. Arch. Dermatol. Res. 2020, 312, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Herrick, A.L. Evidence-based management of Raynaud’s phenomenon. Ther. Adv. Musculoskelet. Dis. 2017, 9, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, Y.; Suzuki, K.; Hara, M.; Hidaka, T.; Ishizuka, T.; Kawagoe, M.; Nakamura, H. Increased endothelin-1 production in fibroblasts derived from patients with systemic sclerosis. Ann. Rheum. Dis. 1994, 53, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Gay, S.; Jones, R.E.; Huang, G.Q.; Gay, R.E. Immunohistologic demonstration of platelet-derived growth factor (PDGF) and sis-oncogene expression in scleroderma. J. Investig. Dermatol. 1989, 92, 301–303. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.Y.; Zhang, J.Z.; Tu, P.; Ma, S.Q. Expression of platelet-derived growth factor B-chain and platelet-derived growth factor beta-receptor in fibroblasts of scleroderma. J. Dermatol. Sci. 1998, 18, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Ludwicka, A.; Ohba, T.; Trojanowska, M.; Yamakage, A.; Strange, C.; Smith, E.A.; Leroy, E.C.; Sutherland, S.; Silver, R.M. Elevated levels of platelet derived growth factor and transforming growth factor-beta 1 in bronchoalveolar lavage fluid from patients with scleroderma. J. Rheumatol. 1995, 22, 1876–1883. [Google Scholar]
- Soria, A.; Cario-André, M.; Lepreux, S.; Rezvani, H.R.; Pasquet, J.M.; Pain, C.; Schaeverbeke, T.; Mahon, F.X.; Taïeb, A. The effect of imatinib (Glivec) on scleroderma and normal dermal fibroblasts: A preclinical study. Dermatology 2008, 216, 109–117. [Google Scholar] [CrossRef]
- Yang, H.; Li, Z.T.; Lin, R.; Fan, Z.P.; Huang, F.; Jiang, Q.L.; Zhou, H.S.; Liu, Q.F.; Sun, J. Sclerodermatous chronic graft-versus-host disease after hematopoietic stem cell transplantation: Incidence, clinical characteristics and risk factors. Nan Fang Yi Ke Da Xue Xue Bao 2016, 36, 807–813. [Google Scholar]
- Yang, C.; Tang, S.; Zhu, D.; Ding, Y.; Qiao, J. Classical Disease-Specific Autoantibodies in Systemic Sclerosis: Clinical Features, Gene Susceptibility, and Disease Stratification. Front. Med. 2020, 7, 587773. [Google Scholar] [CrossRef]
- Allen, J. Capillaroscopy in Healthy Subjects of Different Ages. In Atlas of Capillaroscopy in Rheumatic Disease, 2011st ed.; Cutolo, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 49–53. [Google Scholar]
- Bakirci, S.; Celik, E.; Acikgoz, S.B.; Erturk, Z.; Tocoglu, A.G.; Imga, N.N.; Kaya, M.; Tamer, A. The evaluation of nailfold videocapillaroscopy findings in patients with type 2 diabetes with and without diabetic retinopathy. North Clin. Istanb. 2019, 6, 146–150. [Google Scholar] [CrossRef]
- Hsu, P.C.; Liao, P.Y.; Chang, H.H.; Chiang, J.Y.; Huang, Y.C.; Lo, L.C. Nailfold capillary abnormalities are associated with type 2 diabetes progression and correlated with peripheral neuropathy. Medicine 2016, 95, e5714. [Google Scholar] [CrossRef] [PubMed]
- Kuryliszyn-Moskal, A.; Zarzycki, W.; Dubicki, A.; Moskal, D.; Kosztyła-Hojna, B.; Hryniewicz, A. Clinical usefulness of videocapillaroscopy and selected endothelial cell activation markers in people with Type 1 diabetes mellitus complicated by microangiopathy. Adv. Med. Sci. 2017, 62, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, G.; Guerrero, R.; Paredes, C.; Ríos, C. Nailfold capillaroscopy in diabetes mellitus. Microvasc. Res. 2017, 112, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Gallucci, F.; Russo, R.; Buono, R.; Acampora, R.; Madrid, E.; Uomo, G. Indications and results of videocapillaroscopy in clinical practice. Adv. Med. Sci. 2008, 53, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M.; Pizzorni, C.; Secchi, M.E.; Sulli, A. Capillaroscopy. Best Pract. Res. Clin. Rheumatol. 2008, 22, 1093–1108. [Google Scholar] [CrossRef]
- Pavan, T.R.; Bredemeier, M.; Hax, V.; Capobianco, K.G.; da Silva Mendonça Chakr, R.; Xavier, R.M. Capillary loss on nailfold capillary microscopy is associated with mortality in systemic sclerosis. Clin. Rheumatol. 2018, 37, 475–481. [Google Scholar] [CrossRef]
- Kowal-Bielecka, O.; Distler, O.; Neidhart, M.; Künzler, P.; Rethage, J.; Nawrath, M.; Carossino, A.; Pap, T.; Müller-Ladner, U.; Michel, B.A.; et al. Evidence of 5-lipoxygenase overexpression in the skin of patients with systemic sclerosis: A newly identified pathway to skin inflammation in systemic sclerosis. Arthritis Rheum. 2001, 44, 1865–1875. [Google Scholar] [CrossRef]
- Chwieśko-Minarowska, S.; Kowal, K.; Bielecki, M.; Kowal-Bielecka, O. The role of leukotrienes in the pathogenesis of systemic sclerosis. Folia Histochem. Cytobiol. 2012, 50, 180–185. [Google Scholar] [CrossRef][Green Version]
- Xiao, R.; Yoshida, N.; Higashi, Y.; Lu, Q.J.; Fukushige, T.; Kanzaki, T.; Kanekura, T. Retinoic acids exhibit anti-fibrotic activity through the inhibition of 5-lipoxygenase expression in scleroderma fibroblasts. J. Dermatol. 2011, 38, 345–353. [Google Scholar] [CrossRef]
- Liang, M.; Lv, J.; Jiang, Z.; He, H.; Chen, C.; Xiong, Y.; Zhu, X.; Xue, Y.; Yu, Y.; Yang, S.; et al. Promotion of Myofibroblast Differentiation and Tissue Fibrosis by the Leukotriene B. Arthritis Rheumatol. 2020, 72, 1013–1025. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandujano, A.; Golubov, M. Animal Models of Systemic Sclerosis: Using Nailfold Capillaroscopy as a Potential Tool to Evaluate Microcirculation and Microangiopathy: A Narrative Review. Life 2022, 12, 703. https://doi.org/10.3390/life12050703
Mandujano A, Golubov M. Animal Models of Systemic Sclerosis: Using Nailfold Capillaroscopy as a Potential Tool to Evaluate Microcirculation and Microangiopathy: A Narrative Review. Life. 2022; 12(5):703. https://doi.org/10.3390/life12050703
Chicago/Turabian StyleMandujano, Angélica, and Melissa Golubov. 2022. "Animal Models of Systemic Sclerosis: Using Nailfold Capillaroscopy as a Potential Tool to Evaluate Microcirculation and Microangiopathy: A Narrative Review" Life 12, no. 5: 703. https://doi.org/10.3390/life12050703
APA StyleMandujano, A., & Golubov, M. (2022). Animal Models of Systemic Sclerosis: Using Nailfold Capillaroscopy as a Potential Tool to Evaluate Microcirculation and Microangiopathy: A Narrative Review. Life, 12(5), 703. https://doi.org/10.3390/life12050703






