Photobiomodulation Therapy Combined with Static Magnetic Field Reduces Pain in Patients with Chronic Nonspecific Neck and/or Shoulder Pain: A Randomized, Triple-Blinded, Placebo-Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Aspects
2.2. Eligibility Criteria
2.3. Randomization and Blinding Procedures
2.4. Interventions
2.5. Outcomes
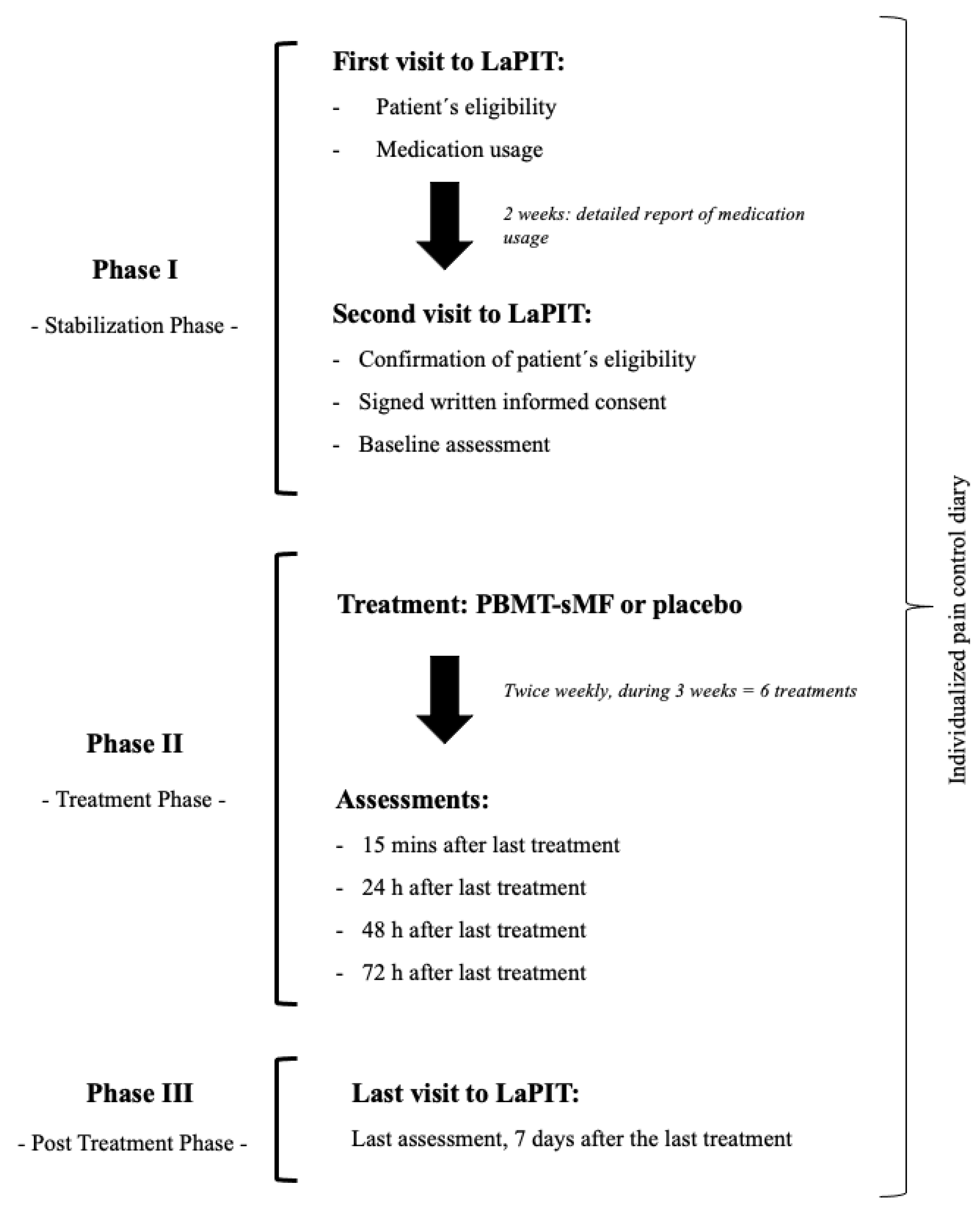
2.6. Procedures
2.7. Sample Size
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fejer, R.; Kyvik, K.O.; Hartvigsen, J. The prevalence of neck pain in the world population: A systematic critical review of the literature. Eur. Spine J. 2006, 15, 834–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safiri, S.; Kolahi, A.A.; Hoy, D.; Buchbinder, R.; Mansournia, M.A.; Bettampadi, D.; Ashrafi-Asgarabad, A.; Almasi-Hashiani, A.; Smith, E.; Sepidarkish, M.; et al. Global, regional, and national burden of neck pain in the general population, 1990–2017: Systematic analysis of the Global Burden of Disease Study 2017. BMJ 2020, 368, m791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarquis, L.M.M.; Coggon, D.; Ntani, G.; Walker-Bone, K.; Palmer, K.T.; Felli, V.E.; Harari, R.; Barrero, L.H.; Felknor, S.A.; Gimeno, D.; et al. Classification of neck/shoulder pain in epidemiological research: A comparison of personal and occupational characteristics, disability, and prognosis among 12,195 workers from 18 countries. Pain 2016, 157, 1028–1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Côté, P.; van der Velde, G.; Cassidy, J.D.; Carroll, L.J.; Hogg-Johnson, S.; Holm, L.W.; Carragee, E.J.; Haldeman, S.; Nordin, M.; Hurwitz, E.L.; et al. The burden and determinants of neck pain in workers: Results of the Bone and Joint Decade 2000-2010 Task Force on Neck Pain and Its Associated Disorders. Spine (Phila Pa 1976) 2008, 33 (Suppl. 4), S60–S74. [Google Scholar] [CrossRef] [PubMed]
- GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1545–1602, Erratum in Lancet 2017, 389, e1. [Google Scholar]
- Hogg-Johnson, S.; van der Velde, G.; Carroll, L.J.; Holm, L.W.; Cassidy, J.D.; Guzman, J.; Côté, P.; Haldeman, S.; Ammendolia, C.; Carragee, E.; et al. The burden and determinants of neck pain in the general population: Results of the Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders. Spine (Phila Pa 1976) 2008, 33 (Suppl. 4), S39–S51. [Google Scholar] [CrossRef]
- Cohen, S.P.; Hooten, W.M. Advances in the diagnosis and management of neck pain. BMJ 2017, 358, j3221. [Google Scholar] [CrossRef]
- Borghouts, J.A.J.; Koes, B.W.; Bouter, L.M. The clinical course and prognostic factors of non-specific neck pain: A systematic review. Pain 1998, 77, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Vos, C.J.; Verhagen, A.P.; Passchier, J.; Koes, B.W. Clinical course and prognostic factors in acute neck pain: An inception cohort study in general practice. Pain Med. 2008, 9, 572–580. [Google Scholar] [CrossRef]
- Bier, J.D.; Scholten-Peeters, G.G.M.; Staal, J.B.; Pool, J.; van Tulder, M.; Beekman, E.; Meerhoff, G.M.; Knoop, J.; Verhagen, A.P. KNGF Guideline Neck Pain; Royal Dutch Society for Physical Therapy: Amersfoort, The Netherlands, 2016; Available online: https://www.kngf2.nl/kennisplatform/richtlijnen/nekpijn (accessed on 29 March 2022).
- Anders, J.J.; Lanzafame, R.J.; Arany, P.R. Low-level light/laser therapy versus photobiomodulation therapy. Photomed. Laser Surg. 2015, 33, 183–184. [Google Scholar] [CrossRef] [Green Version]
- Albuquerque-Pontes, G.M.; Vieira, R.P.; Tomazoni, S.S.; Caires, C.O.; Nemeth, V.; Vanin, A.A.; Santos, L.A.; Pinto, H.D.; Marcos, R.L.; Bjordal, J.M.; et al. Effect of pre-irradiation with different doses, wavelengths, and application intervals of low-level laser therapy on cytochrome c oxidase activity in intact skeletal muscle of rats. Lasers Med. Sci. 2015, 30, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Farivar, S.; Malekshahabi, T.; Shiari, R. Biological effects of low level laser therapy. J. Lasers Med. Sci. 2014, 5, 58–62. [Google Scholar] [PubMed]
- Okano, H. Effects of static magnetic fields in biology: Role of free radicals. Front. Biosci. 2008, 13, 6106–6125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedmann, H.; Lipovsky, A.; Nitzan, Y.; Lubart, R. Combined magnetic and pulsed laser fields produce synergistic acceleration of cellular electron transfer. Laser Ther. 2009, 18, 137–141. [Google Scholar] [CrossRef] [Green Version]
- Leal-Junior, E.C.; Johnson, D.S.; Saltmarche, A.; Demchak, T. Adjunctive use of combination of super-pulsed laser and light-emitting diodes phototherapy on nonspecific knee pain: Double-blinded randomized placebo-controlled trial. Lasers Med. Sci. 2014, 29, 1839–1847. [Google Scholar] [CrossRef]
- Langella, L.G.; Casalechi, H.L.; Tomazoni, S.S.; Johnson, D.S.; Albertini, R.; Pallotta, R.C.; Marcos, R.L.; de Carvalho, P.T.C.; Leal-Junior, E.C.P. Photobiomodulation therapy (PBMT) on acute pain and inflammation in patients who underwent total hip arthroplasty-a randomized, triple-blind, placebo-controlled clinical trial. Lasers Med. Sci. 2018, 33, 1933–1940. [Google Scholar] [CrossRef]
- da Silva, M.M.; Albertini, R.; de Tarso Camillo de Carvalho, P.; Leal-Junior, E.C.P.; Bussadori, S.K.; Vieira, S.S.; Bocalini, D.S.; de Oliveira, L.V.F.; Grandinetti, V.; Silva, J.A., Jr.; et al. Randomized, blinded, controlled trial on effectiveness of photobiomodulation therapy and exercise training in the fibromyalgia treatment. Lasers Med. Sci. 2018, 33, 343–351. [Google Scholar] [CrossRef]
- Herpich, C.M.; Leal-Junior, E.C.P.; Politti, F.; de Paula Gomes, C.A.F.; Dos Santos Glória, I.P.; de Souza Amaral, M.F.R.; Herpich, G.; de Azevedo, L.M.A.; de Oliveira Gonzalez, T.; Biasotto-Gonzalez, D.A. Intraoral photobiomodulation diminishes pain and improves functioning in women with temporomandibular disorder: A randomized, sham-controlled, double-blind clinical trial: Intraoral photobiomodulation diminishes pain in women with temporomandibular disorder. Lasers Med. Sci. 2020, 35, 439–445. [Google Scholar]
- de Paula Gomes, C.A.F.; Politti, F.; de Souza Bacelar Pereira, C.; da Silva, A.C.B.; Dibai-Filho, A.V.; de Oliveira, A.R.; Biasotto-Gonzalez, D.A. Exercise program combined with electrophysical modalities in subjects with knee osteoarthritis: A randomised, placebo-controlled clinical trial. BMC Musculoskelet. Disord. 2020, 21, 258. [Google Scholar] [CrossRef]
- Guimarães, L.S.; Costa, L.D.C.M.; Araujo, A.C.; Nascimento, D.P.; Medeiros, F.C.; Avanzi, M.A.; Leal-Junior, E.C.P.; Costa, L.O.P.; Tomazoni, S.S. Photobiomodulation therapy is not better than placebo in patients with chronic nonspecific low back pain: A randomised placebo-controlled trial. Pain 2021, 162, 1612–1620. [Google Scholar] [CrossRef]
- Chow, R.T.; Johnson, M.I.; Lopes-Martins, R.A.; Bjordal, J.M. Efficacy of low-level laser therapy in the management of neck pain: A systematic review and meta-analysis of randomised placebo or active-treatment controlled trials. Lancet 2009, 374, 1897–1908. [Google Scholar] [CrossRef]
- Gross, A.R.; Dziengo, S.; Boers, O.; Goldsmith, C.H.; Graham, N.; Lilge, L.; Burnie, S.; White, R. Low Level Laser Therapy (LLLT) for Neck Pain: A Systematic Review and Meta-Regression. Open Orthop. J. 2013, 7, 396–419. [Google Scholar] [CrossRef] [PubMed]
- Blanpied, P.R.; Gross, A.R.; Elliott, J.M.; Devaney, L.L.; Clewley, D.; Walton, D.M.; Sparks, C.; Robertson, E.K. Neck Pain: Revision 2017. J. Orthop. Sports Phys. Ther. 2017, 47, A1–A83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grandinétti Vdos, S.; Miranda, E.F.; Johnson, D.S.; de Paiva, P.R.; Tomazoni, S.S.; Vanin, A.A.; Albuquerque-Pontes, G.M.; Frigo, L.; Marcos, R.L.; de Carvalho, P.T.; et al. The thermal impact of phototherapy with concurrent super-pulsed lasers and red and infrared LEDs on human skin. Lasers Med. Sci 2015, 30, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Rosner, B. Hypothesis Testing: Categorical Data—Estimation of Sample Size and Power for Comparing Two Binomial Proportions: Fundamentals of Biostatistics, 4th ed.; Brooks/Cole: Boston, MA, USA, 2010. [Google Scholar]
- Elkins, M.R.; Moseley, A.M. Intention-to-treat analysis. J. Physiother. 2015, 61, 165–167. [Google Scholar] [CrossRef] [Green Version]
- Gur, A.; Sarac, A.J.; Cevik, R.; Altindag, O.; Sarac, S. Efficacy of 904 nm gallium arsenide low level laser therapy in the management of chronic myofascial pain in the neck: A double-blind and randomize-controlled trial. Lasers Surg. Med. 2004, 35, 229–235. [Google Scholar] [CrossRef]
- Hakgüder, A.; Birtane, M.; Gürcan, S.; Kokino, S.; Turan, F.N. Efficacy of low level laser therapy in myofascial pain syndrome: An algometric and thermographic evaluation. Lasers Surg. Med. 2003, 33, 339–343. [Google Scholar] [CrossRef]
- Dundar, U.; Evcik, D.; Samli, F.; Pusak, H.; Kavuncu, V. The effect of gallium arsenide aluminum laser therapy in the management of cervical myofascial pain syndrome: A double blind, placebo-controlled study. Clin. Rheumatol. 2007, 26, 930–934. [Google Scholar] [CrossRef]
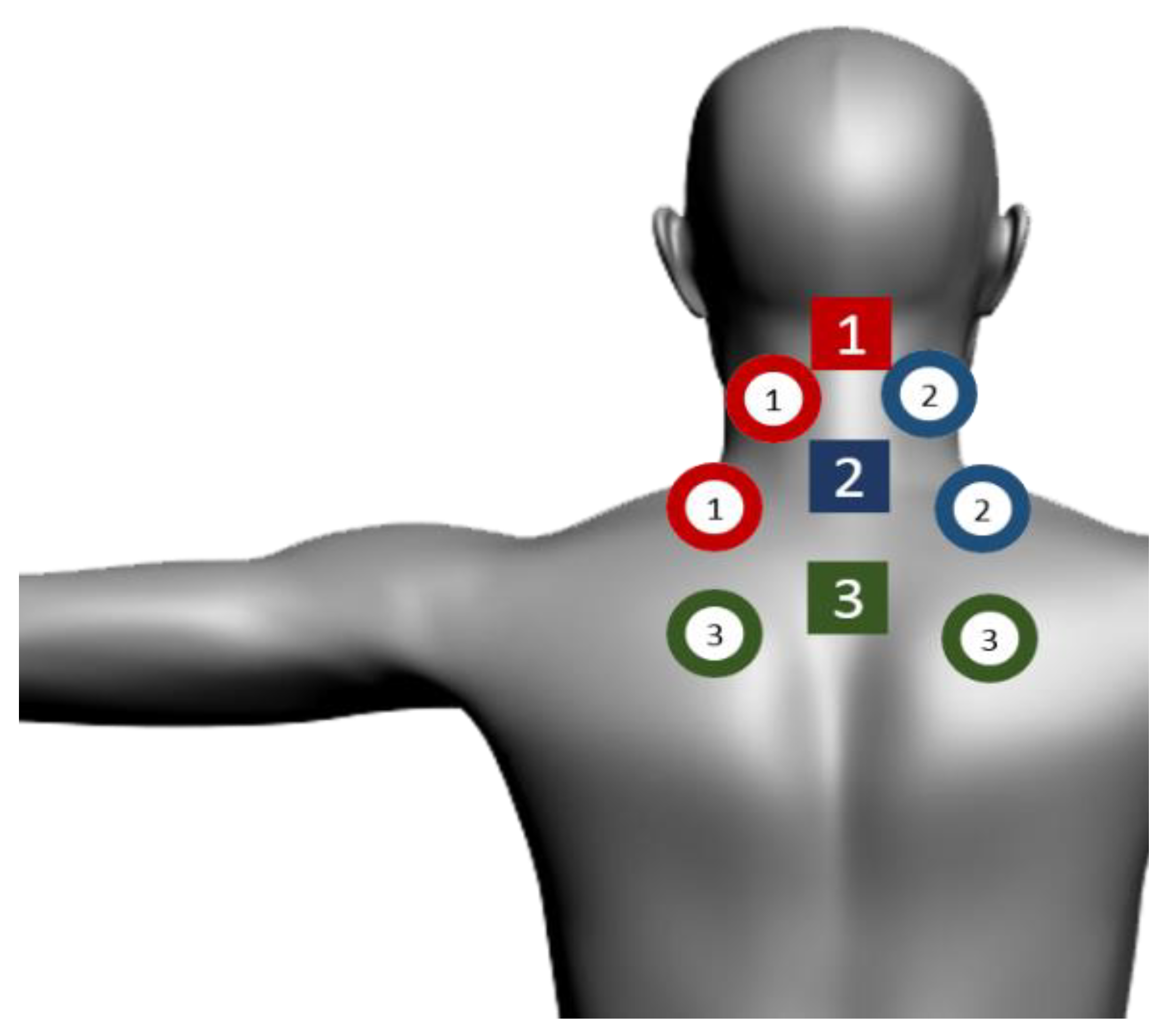

| SE25 | LaserShower | |
|---|---|---|
| Number of lasers | 1 Super-pulsed infrared | 4 Super-pulsed infrared |
| Wavelength (nm) | 905 (±1) | 905 (±1) |
| Frequency (Hz) | 3000 | 3000 |
| Peak power (W)-each | 25 | 12.50 |
| Average mean optical output (mW)-each | 7.5 | 3.75 |
| Power density (mW/cm2)-each | 17.05 | 8.52 |
| Energy density (J/cm2)-each | 3.07 | 1.53 |
| Dose (J)-each | 1.35 | 0.675 |
| Spot size of laser (cm2)-each | 0.44 | 0.44 |
| Number of red LEDs | 4 Red | 4 Red |
| Wavelength of red LEDs (nm) | 640 (±10) | 640 (±10) |
| Frequency (Hz) | 2 | 2 |
| Average optical output (mW)-each | 15 | 15 |
| Power density (mW/cm2)-each | 16.67 | 16.67 |
| Energy density (J/cm2)-each | 3 | 3 |
| Dose (J)-each | 2.7 | 2.7 |
| Spot size of red LED (cm2)-each | 0.9 | 0.9 |
| Number of infrared LEDs | 4 Infrared | 4 Infrared |
| Wavelength of infrared LEDs (nm) | 875 (±10) | 875 (±10) |
| Frequency (Hz) | 16 | 16 |
| Average optical output (mW)-each | 17.5 | 17.5 |
| Power density (mW/cm2)-each | 19.44 | 19.44 |
| Energy density (J/cm2)-each | 3.5 | 3.5 |
| Dose (J)-each | 3.15 | 3.15 |
| Spot Size of LED (cm2)-each | 0.9 | 0.9 |
| Number of magnets | 1 | 1 |
| Magnetic Field (mT) | 35 | 35 |
| Irradiation time per site (sec) | 180 | 180 |
| Total dose per site (J) | 24.75 | 26.10 |
| Aperture of device (cm2) | 4 | 20 |
| Total dose applied per treatment session (J) | 230.85 J | |
| Application mode | Cluster probe held stationary in skin contact with a 90-degree angle and slight pressure | Cluster probe held stationary in skin contact with a 90-degree angle and slight pressure |
| Variables | Active PBMT-sMF (n = 36) | Placebo PBMT-sMF (n = 36) |
|---|---|---|
| Age (y) | 32.78 (9.99) | 31.39 (9.90) |
| Gender (%) | ||
| Female | 24 (66.7) | 23 (63.9) |
| Male | 12 (33.3) | 13 (36.1) |
| Fitzpatrick skin type (%) | ||
| Lighter skinned | 22 (61.1) | 22 (61.1) |
| Darker skinned | 14 (38.9) | 14 (38.9) |
| Weight (kg) | 71.92 (14.19) | 76.35 (16.02) |
| Height (cm) | 167.6 (8.59) | 167.1 (10.35) |
| Individual subject success criteria (%) | 36 (100) | 22 (61) |
| Pain intensity (0–100) | 68.42 (11.31) | 70.31 (13.44) |
| ROM (°) | ||
| Neck | ||
| Flexion | 54.03 (15.20) | 52.81 (10.78) |
| Extension | 46.67 (12.54) | 51.03 (11.10) |
| Lateral (right) | 40.69 (11.45) | 39.50 (9.72) |
| Lateral (left) | 41.89 (11.29) | 38.00 (7.86) |
| Rotation (right) | 57.64 (11.05) | 63.42 (11.79) |
| Rotation (left) | 63.28 (8.92) | 67.36 (9.75) |
| Shoulder | ||
| Flexion (right) | 159.58 (17.97) | 164.25 (19.23) |
| Flexion (left) | 160.17 (19.52) | 166.58 (14.27) |
| Extension (right) | 50.08 (9.45) | 48.75 (11.67) |
| Extension (left) | 52.08 (12.78) | 50.36 (13.84) |
| External rotation (right) | 76.11 (11.66) | 73.69 (10.86) |
| External rotation (left) | 71.81 (13.07) | 74.36 (12.35) |
| Internal rotation (right) | 76.81 (12.91) | 76.64 (11.79) |
| Internal rotation (left) | 78.25 (8.91) | 77.92 (11.55) |
| Abduction (right) | 147.47 (26.48) | 156.86 (26.48) |
| Abduction (left) | 149.39 (22.62) | 162.44 (20.88) |
| Pain Intensity (VAS) | Active PBMT-sMF (n = 36) | Placebo PBMT-sMF (n = 36) |
|---|---|---|
| Baseline | 68.42 (64.59 to −72.24) | 70.31 (65.76 to −74.85) |
| 15 min post-intervention | 16.19 (12.59 to −19.78) | 44.29 (39.03 to −49.54) |
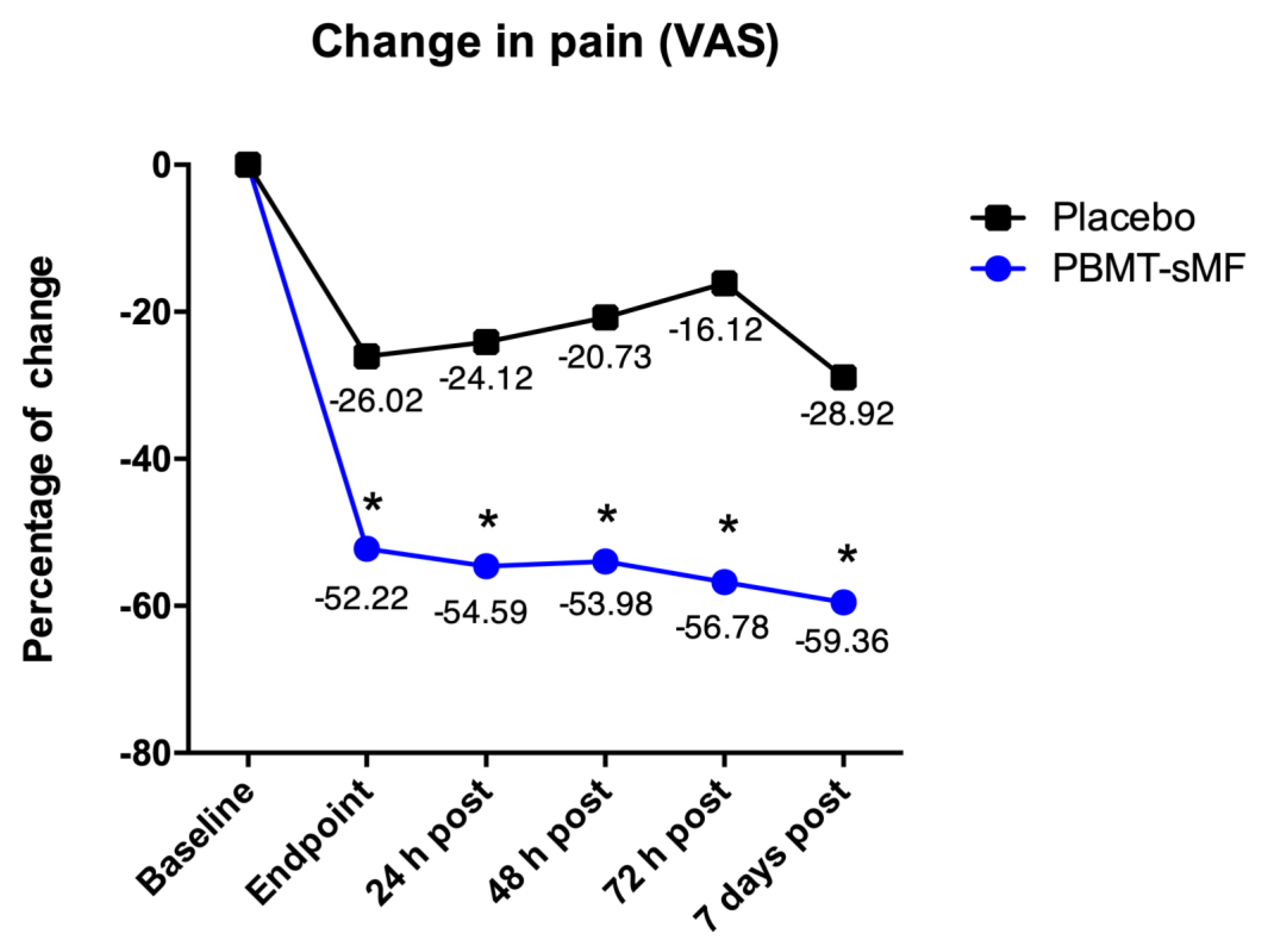
| Change | −52.23 (−56.79 to −47.60) | −26.02 (−31.50 to −20.53) |
| Time Points | Active PBMT/sMF (n = 36) | Placebo PBMT-sMF (n = 36) |
|---|---|---|
| Baseline | 68.42 (64.59 to −72.24) | 70.31 (65.76 to −74.85) |
| 15 min post-intervention | 16.19 (12.59 to −19.78) | 44.29 (39.03 to −49.54) |
| 24 h post-intervention | 13.83 (9.67 to −17.98) | 46.19 (42.26 to −50.11) |
| 48 h post-intervention | 14.44 (9.63 to −19.24) | 49.58 (44.52 to −54.63) |
| 72 h post-intervention | 11.64 (7.18 to −16.09) | 54.19 (46.98 to −61.39) |
| 7 days post-intervention | 9.06 (5.47 to −12.64) | 41.39 (34.67 to −48.10) |
| ROM (°) | Active PBMT-sMF (n = 36) | Placebo PBMT-sMF (n = 36) | |
|---|---|---|---|
| Neck | |||
| Flexion | Baseline 15 min post-intervention 7 days post-intervention | 54.03 (48.88–59.17) 55.81 (51.59–60.02) 59.33 (56.19–62.46) | 52.81 (49.16–56.45) 57.33 (54.05–60.60) 56.81 (53.33–60.28) |
| Extension | Baseline 15 min post-intervention 7 days post-intervention | 46.67 (42.42–50–91) 54.17 (50.40–57.93) 55.56 (52.40–58.71) | 51.03 (47.27–54.78) 56.14 (53.15–59.12) 57.64 (54.48–60.79) |
| Lateral—Right | Baseline 15 min post-intervention 7 days post-intervention | 40.69 (36.81–44.56) 45.19 (42.21–48.16) 44.33 (41.07–47.58) | 39.50 (36.21–42.78) 42.36 (39.38–45.33) 42.03 (38.87–45.18) |
| Lateral—Left | Baseline 15 min post-intervention 7 days post-Intervention | 41.89 (38.07–45.70) 42.36 (39.90–44.81) 43.17 (41.09–45.24) | 38.00 (35.34–40.65) 43.22 (40.46–45.97) 44.61 (41.54–47.67) |
| Rotation—Right | Baseline 15 min post-intervention 7 days post-intervention | 57.64 (53.90–61.37) 70.97 (67.66–74.27) 69.97 (66.18–73.75) | 63.42 (59.43–67.40) 68.56 (65.28–71.83) 67.86 (63.78–71.93) |
| Rotation—Left | Baseline 15 min post-intervention 7 days post-intervention | 63.28 (60.26–66.29) 74.67 (71.35–77.98) 69.67 (65.27–74.06) | 67.36 (64.06–70.65) 71.47 (67.69–75.24) 71.11 (67.02–75.19) |
| Shoulder | |||
| Flexion—Right | Baseline 15 min post-intervention 7 days post-intervention | 159.58 (153.49–165.66) 166.44 (161.37–171.50) 170.83 (167.38–174.27) | 164.25 (157.74–170.75) 169.31 (165.10–173.51) 171.53 (167.78–175.27) |
| Flexion—Left | Baseline 15 min post-intervention 7 days post-intervention | 160.17 (152.56–167.77) 170.14 (164.89–175.38) 173.47 (169.23–177.70) | 166.58 (161.75–171.40) 171.47 (167.71–175.22) 172.08 (168.81–175.34) |
| Extension—Right | Baseline 15 min post-intervention 7 days post-intervention | 50.08 (46.88–53.27) 56.06 (51.87–60.24) 54.03 (50.22–57.83) | 48.75 (44.80–52.69) 53.47 (49.63–57.30) 52.36 (48.09–56.62) |
| Extension—Left | Baseline 15 min post-intervention 7 days post-intervention | 52.08 (47.75–56.40) 55.14 (51.37–58.90) 55.31 (51.87–58.74) | 50.36 (45.67–55.04) 56.53 (52.65–60.40) 57.36 (53.67–61.04) |
| External Rotation—Right | Baseline 15 min post-intervention 7 days post-intervention | 76.11 (72.16–80.05) 81.89 (79.10–84.67) 82.78 (79.22–86.33) | 73.69 (70.01–77.36) 81.11 (77.42~84.79) 81.86 (77.14–86.67) |
| External Rotation—Left | Baseline 15 min post-intervention 7 days post-intervention | 71.81 (67.38–76.23) 80.69 (76.84–84.53) 82.78 (78.91–86.64) | 74.36 (70.12–78.59) 78.83 (74.56–83.09) 79.06 (74.67–83.44) |
| Internal Rotation—Right | Baseline 15 min post-intervention 7 days post-intervention | 76.81 (72.44–81.17) 78.47 (74.17–82.76) 84.03 (80.55–87.50) | 76.64 (72.65–80.62) 76.86 (72.43–81.28) 82.31 (78.88–85.73) |
| Internal Rotation—Left | Baseline 15 min post-intervention 7 days post-intervention | 78.25 (75.23–81.26) 77.56 (73.58–81.53) 84.44 (90.98–87.89) | 77.92 (74.01–81.82) 80.69 (76.74–84.63) 83.33 (79.66–86.99) |
| Abduction—Right | Baseline 15 min post-intervention 7 days post-intervention | 147.47 (138.51–156.42) 161.67 (155.28–168.05) 165.83 (159.99–171.66) | 156.86 (147.90–165.81) 161.28 (154.07–168.48) 162.44 (155.06–169.81) |
| Abduction—Left | Baseline 15 min post-intervention 7 days post-intervention | 149.39 (141.73–157.01) 163.89 (156.83–170.94) 167.22 (160.92–173.51) | 162.44 (155.40–169.47) 163.61 (156.20–171.91) 165.67 (157.70–173.63) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixeira, A.M.; Leal-Junior, E.C.P.; Casalechi, H.L.; Vanin, A.A.; de Paiva, P.R.V.; Melo, F.H.C.; Johnson, D.S.; Tomazoni, S.S. Photobiomodulation Therapy Combined with Static Magnetic Field Reduces Pain in Patients with Chronic Nonspecific Neck and/or Shoulder Pain: A Randomized, Triple-Blinded, Placebo-Controlled Trial. Life 2022, 12, 656. https://doi.org/10.3390/life12050656
Teixeira AM, Leal-Junior ECP, Casalechi HL, Vanin AA, de Paiva PRV, Melo FHC, Johnson DS, Tomazoni SS. Photobiomodulation Therapy Combined with Static Magnetic Field Reduces Pain in Patients with Chronic Nonspecific Neck and/or Shoulder Pain: A Randomized, Triple-Blinded, Placebo-Controlled Trial. Life. 2022; 12(5):656. https://doi.org/10.3390/life12050656
Chicago/Turabian StyleTeixeira, Adeilson Matias, Ernesto Cesar Pinto Leal-Junior, Heliodora Leão Casalechi, Adriane Aver Vanin, Paulo Roberto Vicente de Paiva, Fernando Hess Câmara Melo, Douglas Scott Johnson, and Shaiane Silva Tomazoni. 2022. "Photobiomodulation Therapy Combined with Static Magnetic Field Reduces Pain in Patients with Chronic Nonspecific Neck and/or Shoulder Pain: A Randomized, Triple-Blinded, Placebo-Controlled Trial" Life 12, no. 5: 656. https://doi.org/10.3390/life12050656
APA StyleTeixeira, A. M., Leal-Junior, E. C. P., Casalechi, H. L., Vanin, A. A., de Paiva, P. R. V., Melo, F. H. C., Johnson, D. S., & Tomazoni, S. S. (2022). Photobiomodulation Therapy Combined with Static Magnetic Field Reduces Pain in Patients with Chronic Nonspecific Neck and/or Shoulder Pain: A Randomized, Triple-Blinded, Placebo-Controlled Trial. Life, 12(5), 656. https://doi.org/10.3390/life12050656






