Modeling Virus and Bacteria Populations in Europa’s Subsurface Ocean
Abstract
:1. Introduction
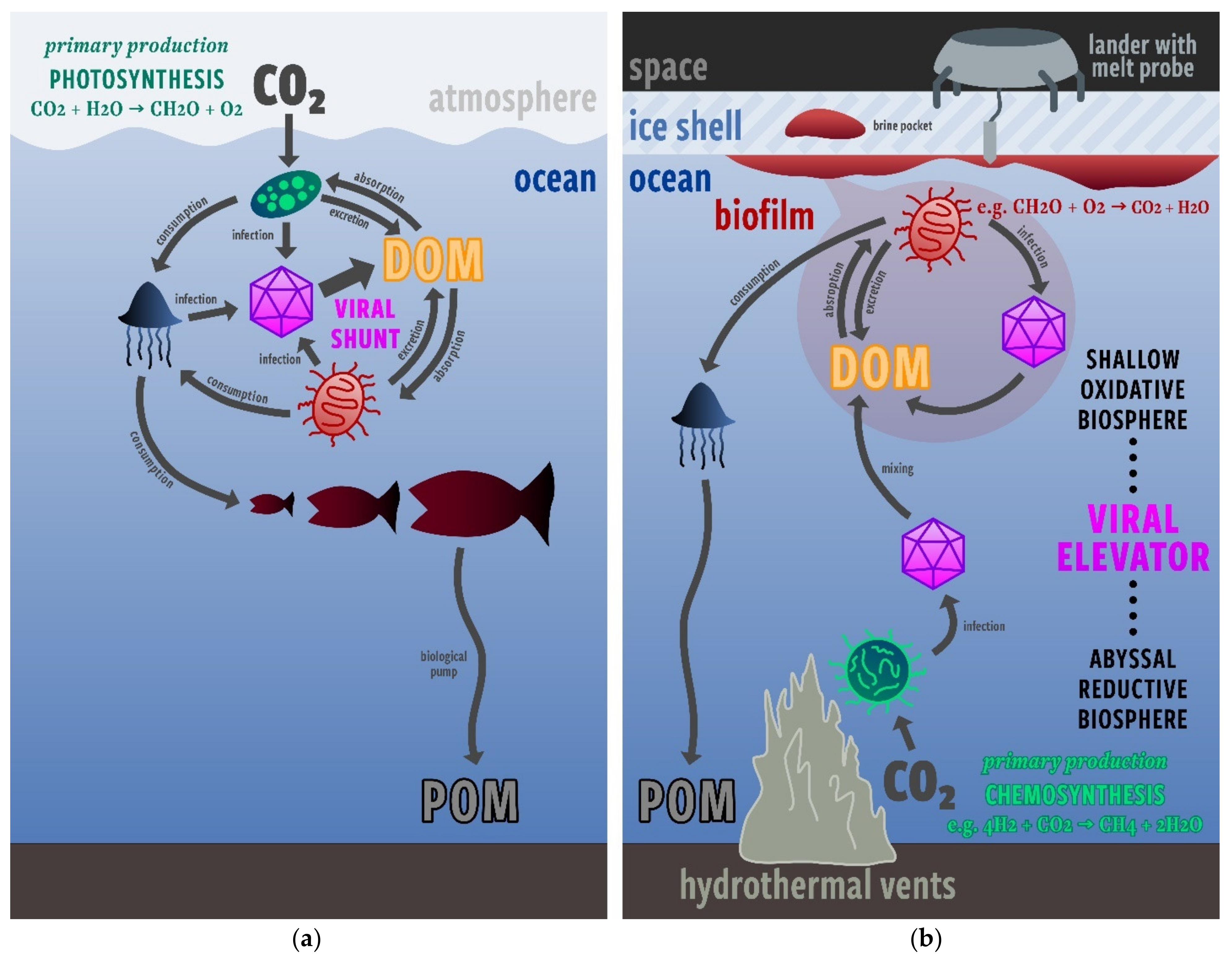
2. The Viral Elevator Hypothesis
3. Methods
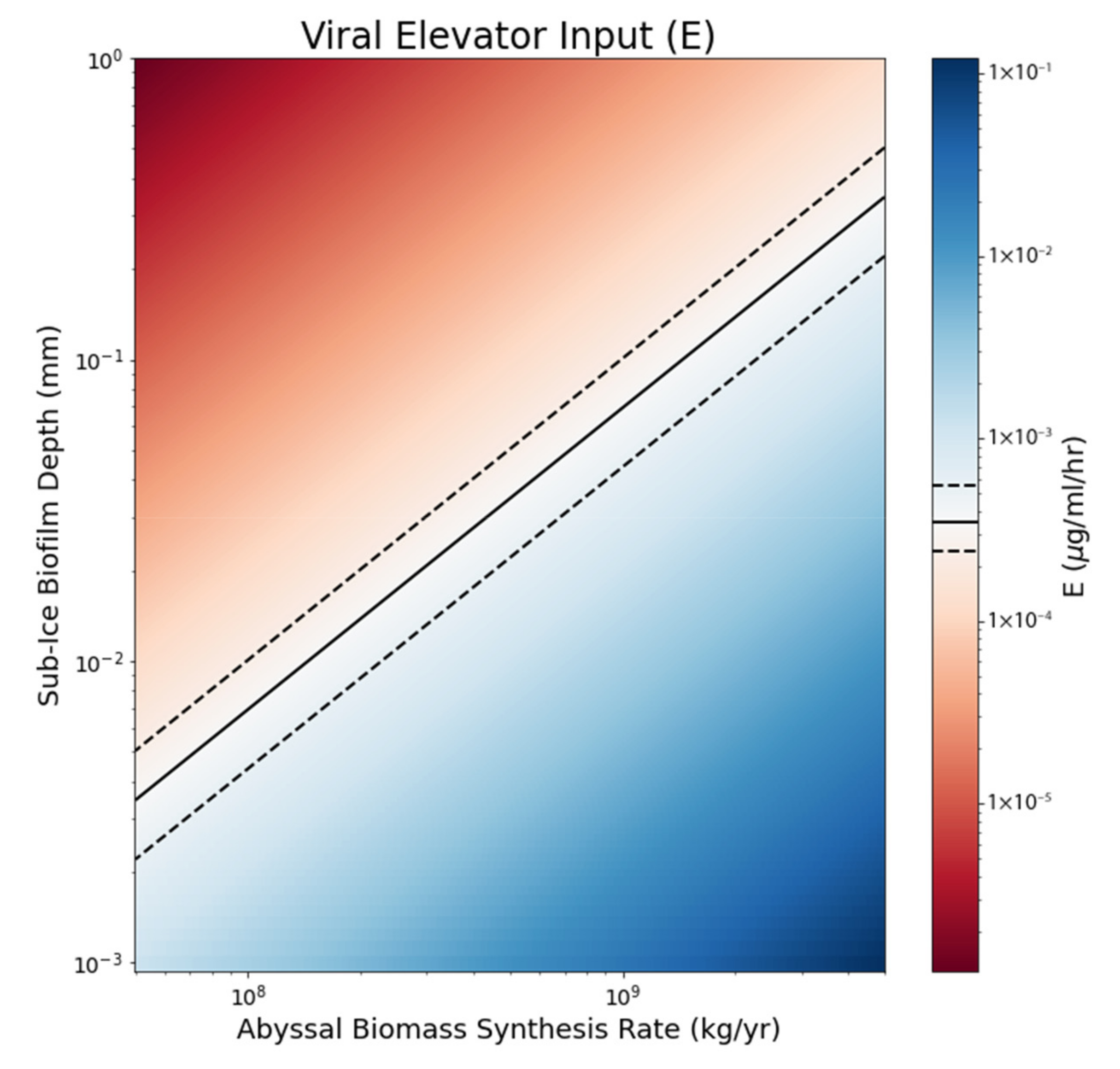
3.1. Plausibility of the Viral Elevator
3.2. Model and Parameters
| Parameter | Units | Description | Europa-Proxy Value * | Source |
|---|---|---|---|---|
| Initial amount of DOM (nutrient pool) | [30] | |||
| Initial bacterial population | [35] | |||
| Initial viral population | [30,35] | |||
| Temperature | [36] | |||
| ** | Uptake constant | [30] | ||
| Bacterial growth rate | [30] | |||
| Half-saturation constant | 0.022 | [30] | ||
| Constant of bacterial death | [37] | |||
| Viral decay rate | [40] | |||
| Fraction of uptake material recycled into nutrient pool as exudate | 0.02–0.2 | [38] | ||
| Fraction of viral lysis material recycling into nutrient pool | 0.99 | [39] | ||
| *** | Lytic vs. lysogenic fraction | [30] | ||
| Adsorption (infection) rate | [41] | |||
| Viral burst size | [41] | |||
| Conversion rate between grazed bacteria and DOM | This study |
4. Results
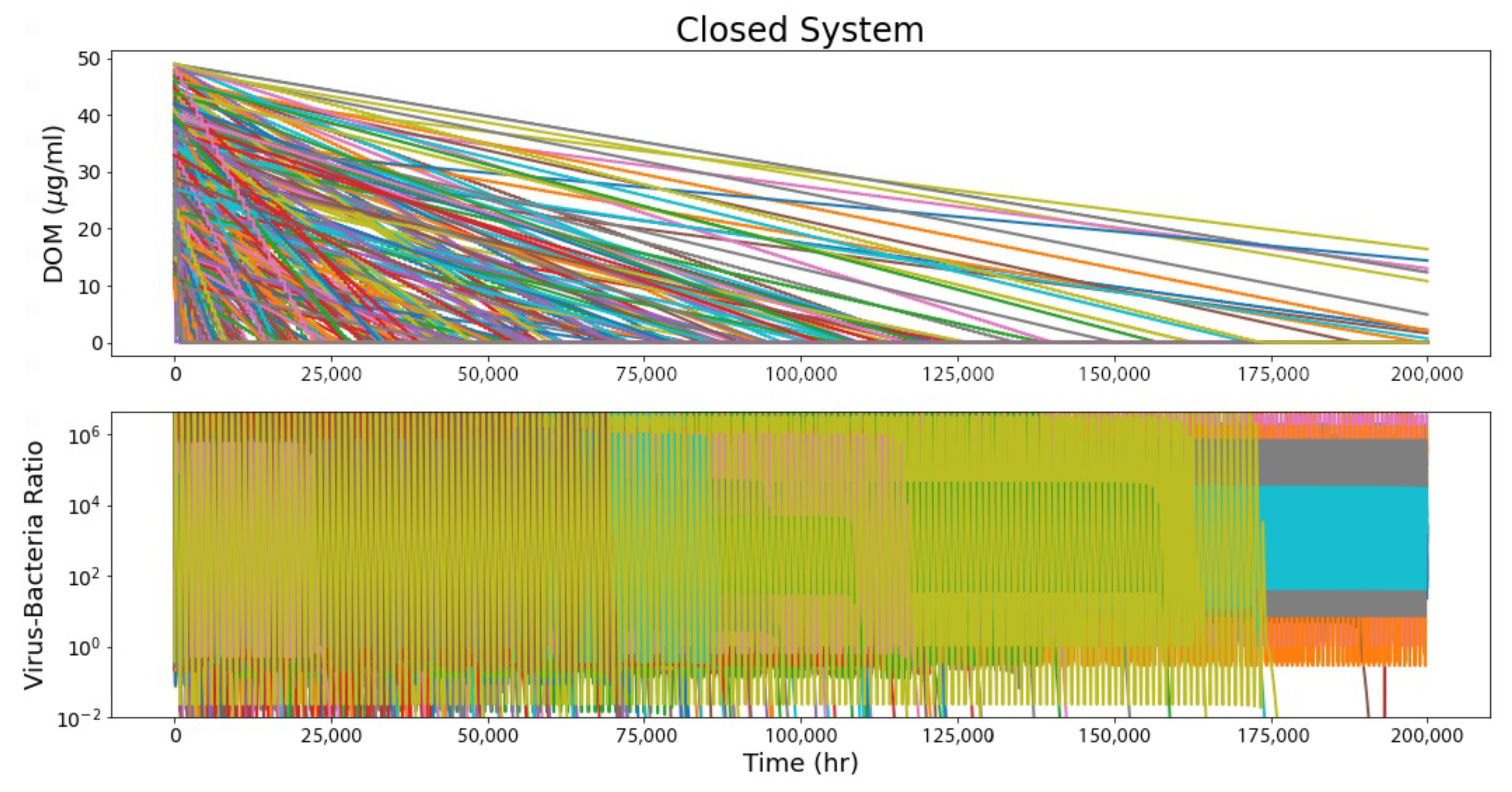
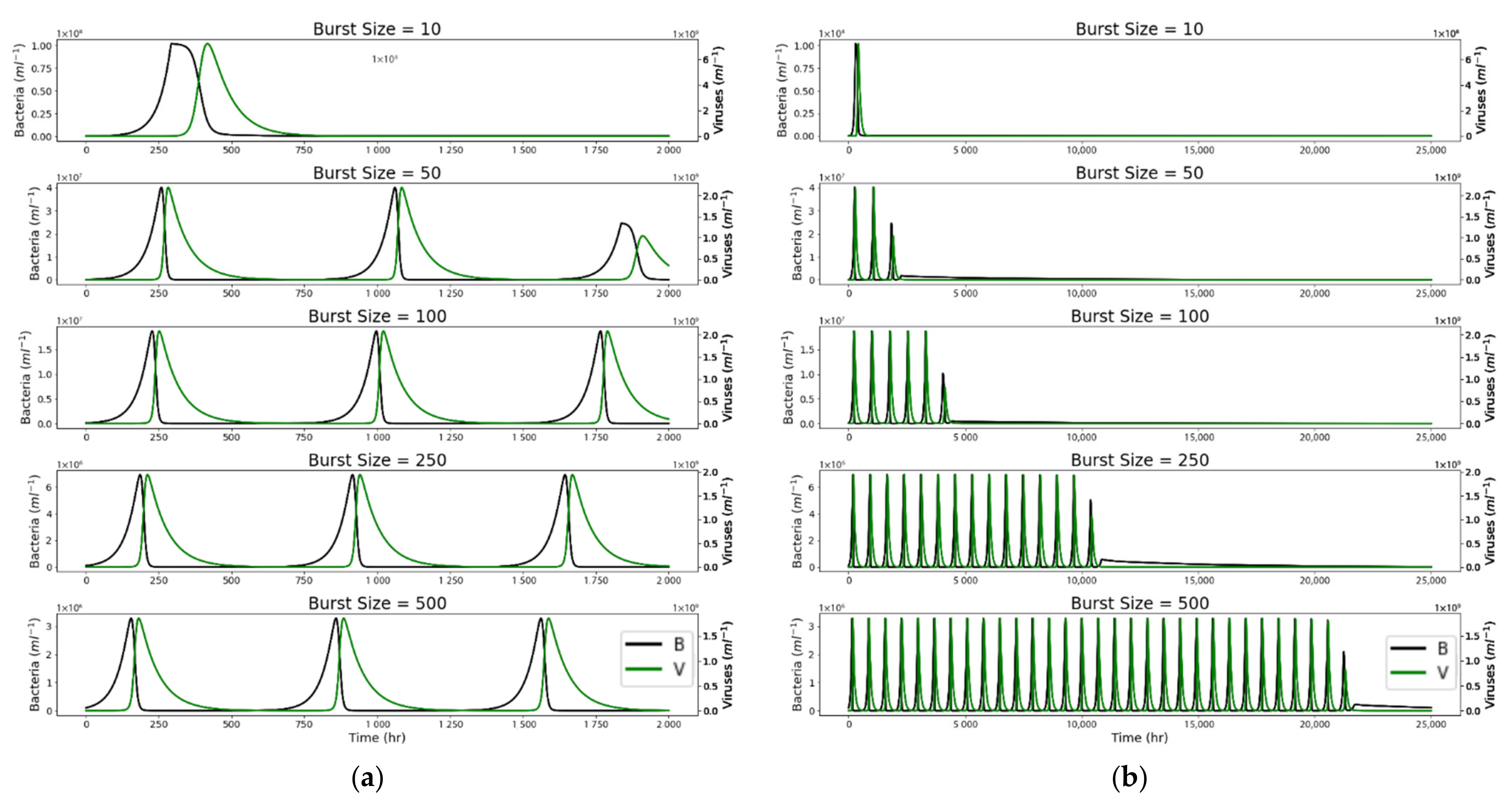
4.1. Closed System
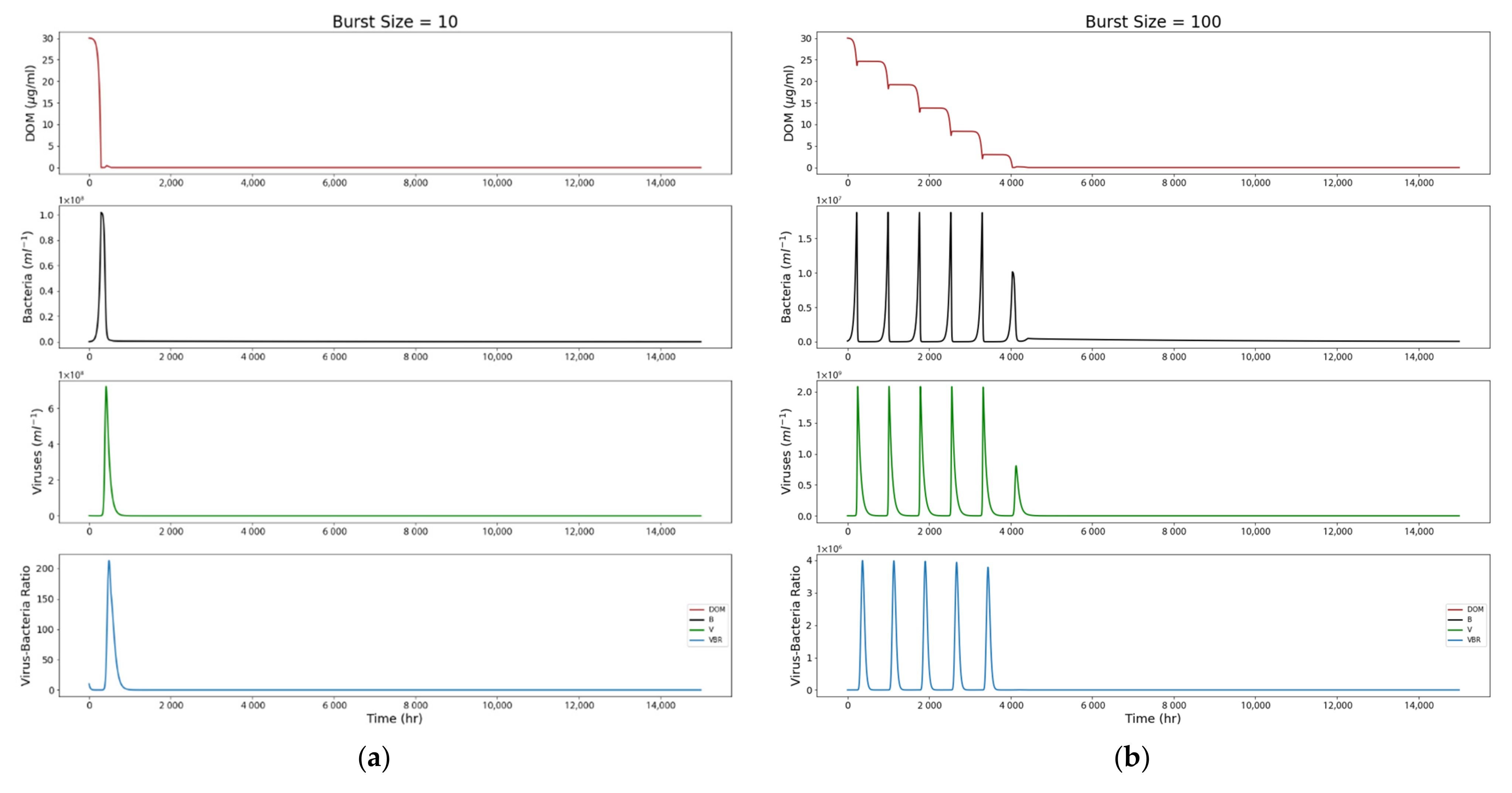
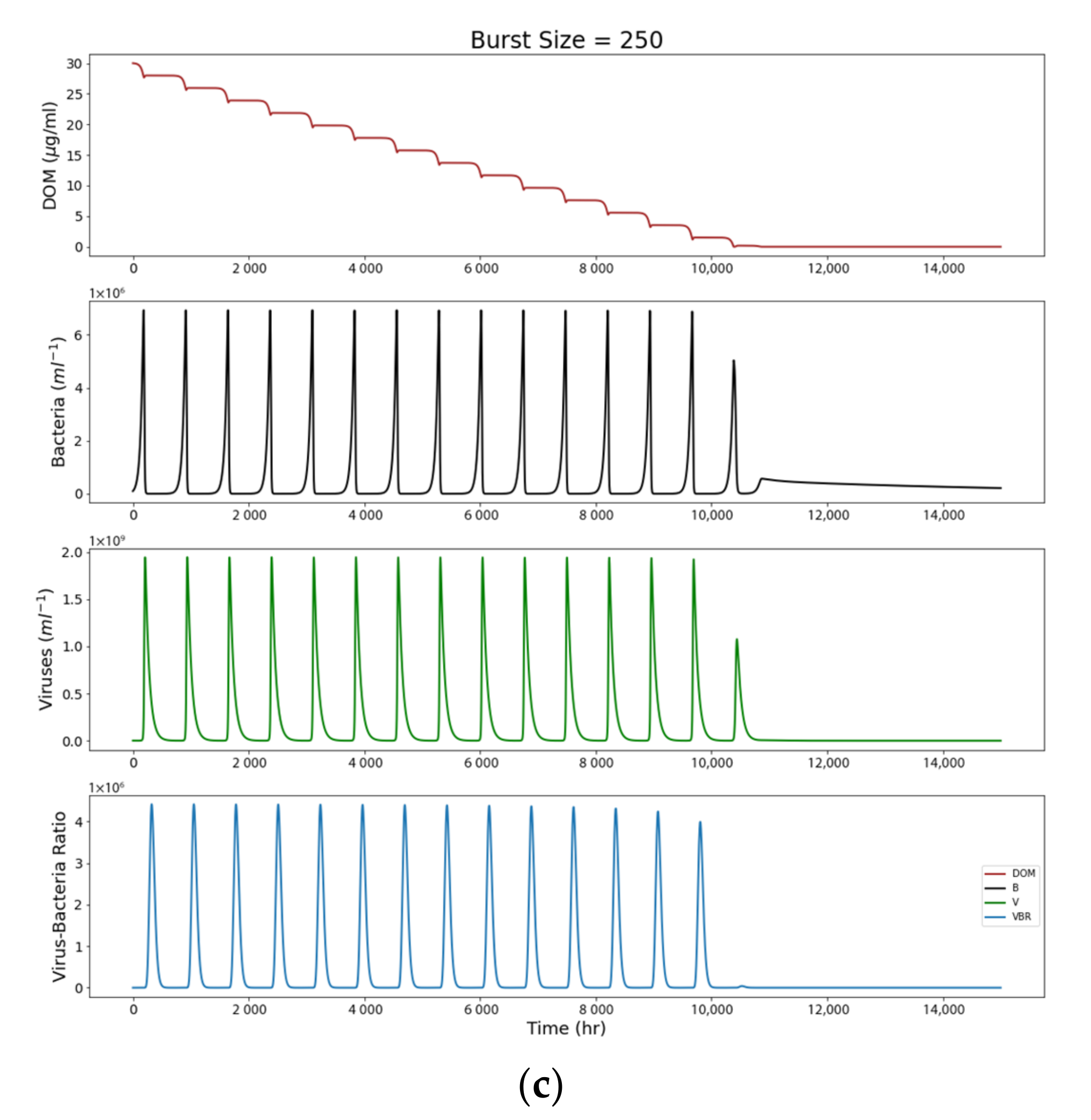
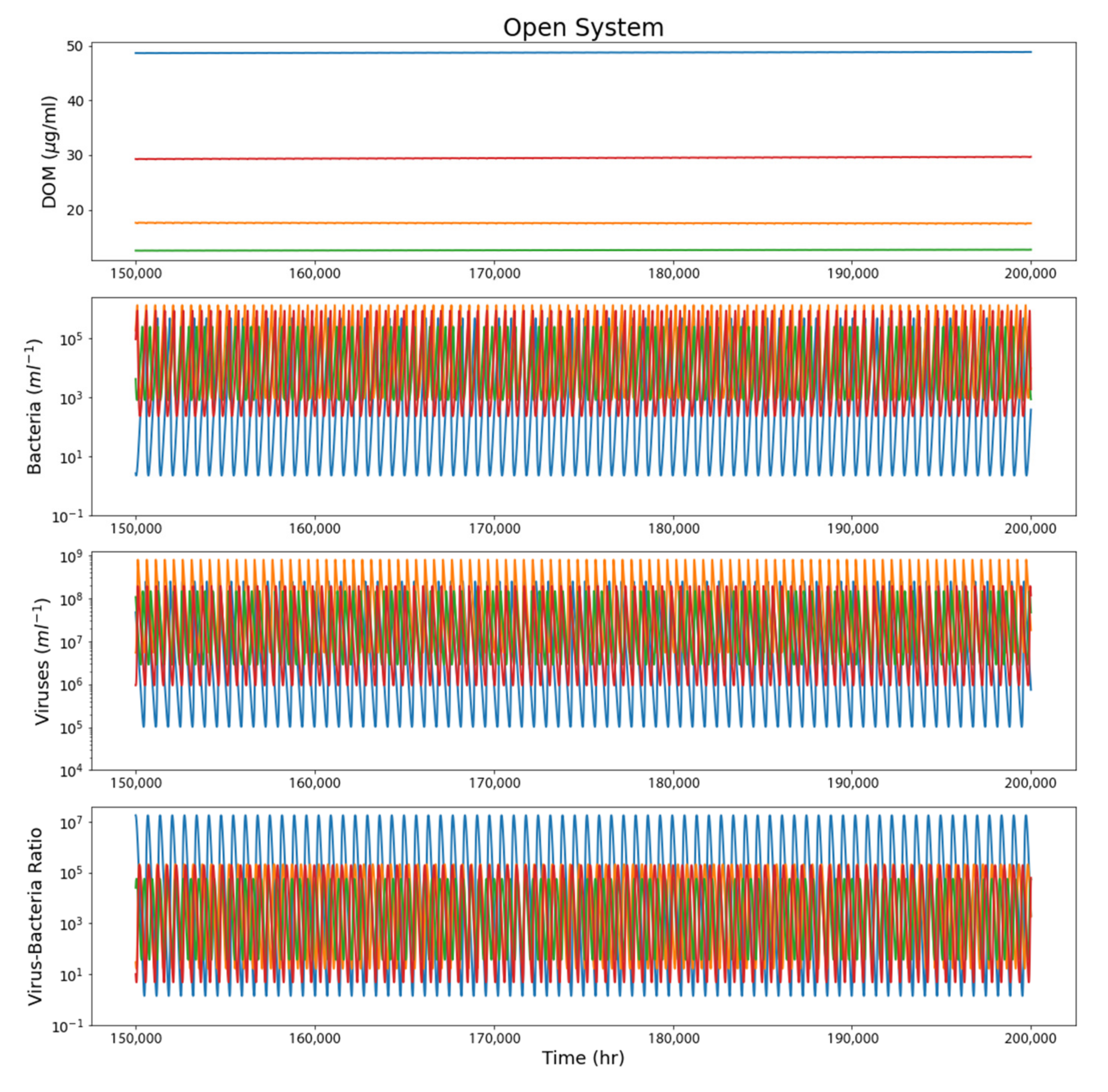
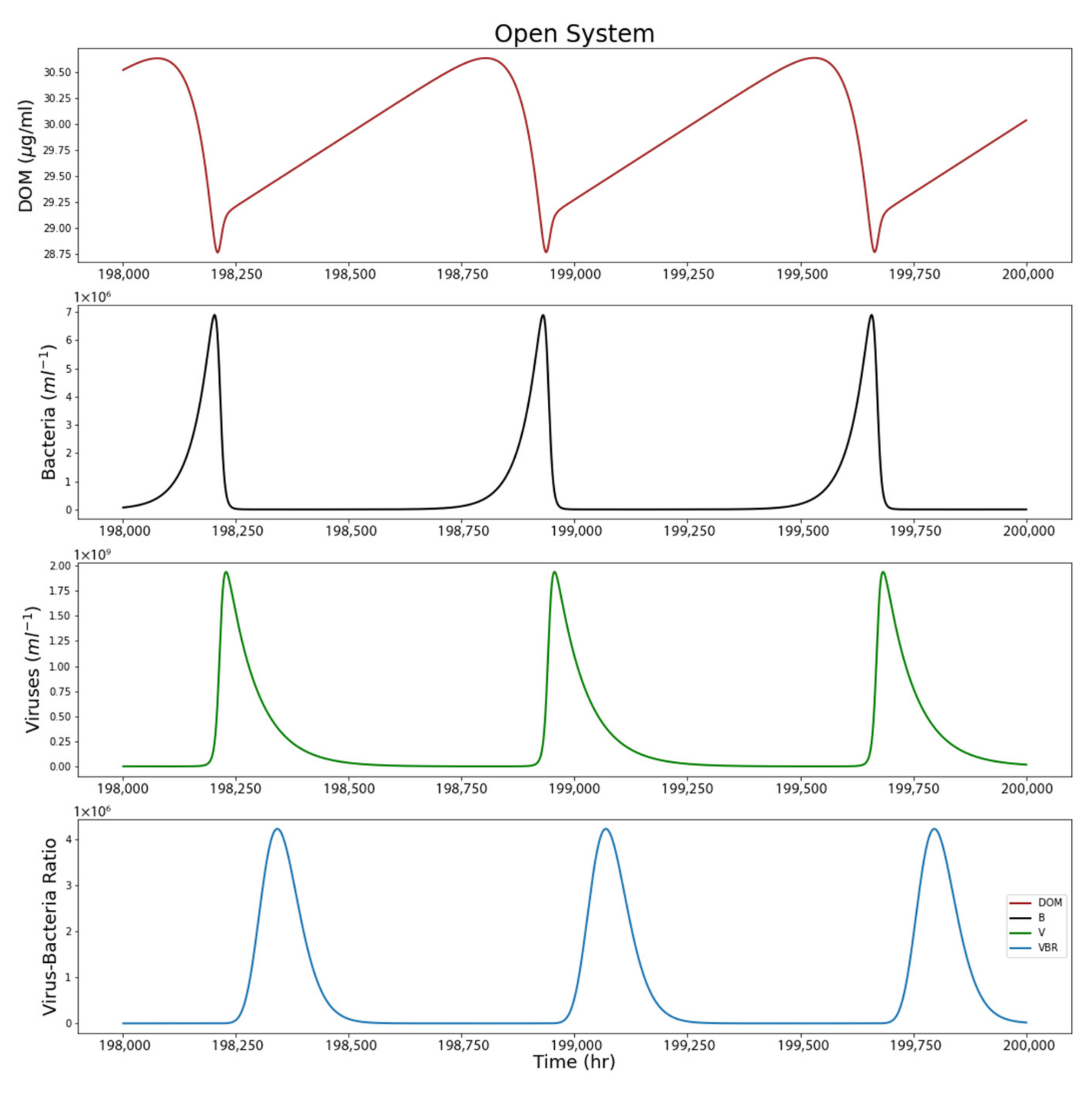
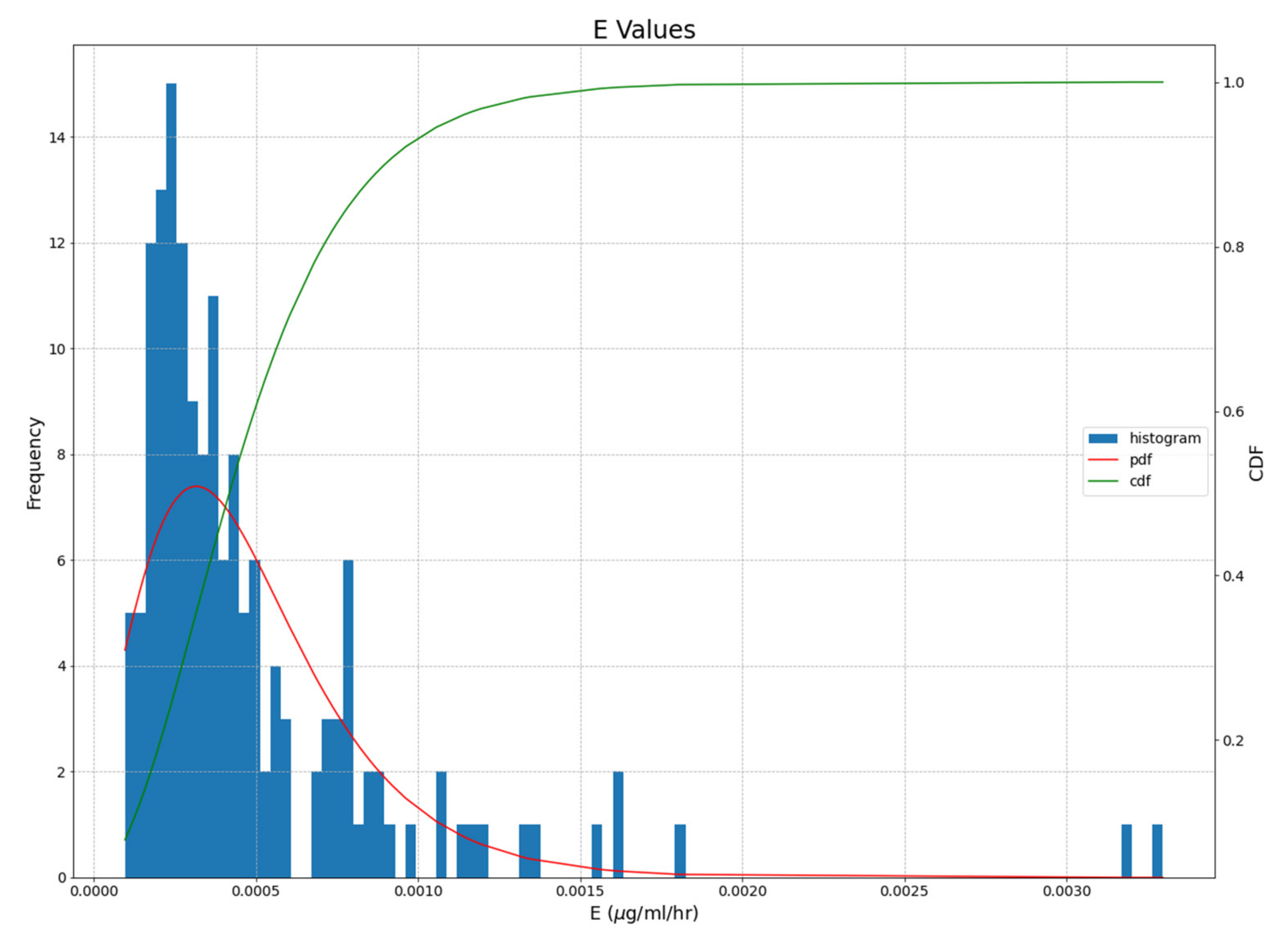
4.2. Open System
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Breitbart, M.; Bonnain, C.; Malki, K.; Sawaya, N.A. Phage puppet masters of the marine microbial realm. Nat. Microbiol. 2018, 3, 754–766. [Google Scholar] [CrossRef]
- Collins, R.E.; Deming, J.W. Abundant dissolved genetic material in Arctic Sea ice Part II: Viral dynamics during autumn freeze-up. Polar Biol. 2011, 34, 1831–1841. [Google Scholar] [CrossRef]
- Price, P.B.; Sowers, T. Temperature dependence of metabolic rates for microbial growth, maintenance, and survival. Proc. Natl. Acad. Sci. USA 2004, 101, 4631–4636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merino, N.; Aronson, H.S.; Bojanova, D.P.; Feyhl-Buska, J.; Wong, M.L.; Zhang, S.; Giovannelli, D. Living at the extremes: Extremophiles and the limits of life in a planetary context. Front. Microbiol. 2019, 10, 780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mykytczuk, N.C.S.; Foote, S.J.; Omelon, C.R.; Southam, G.; Greer, C.W.; Whyte, L.G. Bacterial growth at −15 °C; molecular insights from the permafrost bacterium Planococcus halocryophilus Or1. ISME J. 2013, 7, 1211–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frösler, J.; Panitz, C.; Wingender, J.; Flemming, H.C.; Rettberg, P. Survival of Deinococcus geothermalis in biofilms under desiccation and simulated space and martian conditions. Astrobiology 2017, 17, 431–447. [Google Scholar] [CrossRef] [PubMed]
- Berliner, A.J.; Mochizuki, T.; Stedman, K.M. Astrovirology: Viruses at Large in the Universe. Astrobiology 2018, 18, 207–223. [Google Scholar] [CrossRef] [PubMed]
- Trubl, G.; Stedman, K.; Bywaters, K.; Boston, P.J.; Kaelber, J.T.; Roux, S.; Rodríguez-Román, E. Astrovirology: Expanding the Search for Life. Bull. Am. Astron. Soc. 2021, 53, 516. [Google Scholar]
- Durzyńska, J.; Goździcka-Józefiak, A. Viruses and cells intertwined since the dawn of evolution. Virol. J. 2015, 12, 169. [Google Scholar] [CrossRef] [Green Version]
- Forterre, P. The origin of viruses and their possible roles in major evolutionary transitions. Virus Res. 2006, 117, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Forterre, P.; Prangishvili, D. The great billion-year war between ribosome-and capsid-encoding organisms (cells and viruses) as the major source of evolutionary novelties. Ann. N. Y. Acad. Sci. 2009, 1178, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Krupovic, M.; Dolja, V.V.; Koonin, E.V. Origin of viruses: Primordial replicators recruiting capsids from hosts. Nat. Rev. Genet. 2019, 17, 449–458. [Google Scholar] [CrossRef]
- Kivelson, M.G.; Khurana, K.K.; Joy, S.; Russell, C.T.; Southwood, D.J.; Walker, R.J.; Polanskey, C. Europa’s magnetic signature: Report from Galileo’s pass on 19 December 1996. Science 1997, 276, 1239–1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kivelson, M.G.; Khurana, K.K.; Russell, C.T.; Volwerk, M.; Walker, R.J.; Zimmer, C. Galileo Magnetometer Measurements: A Stronger Case for a Subsurface Ocean at Europa. Science 2000, 289, 1340–1343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chyba, C.F.; Phillips, C.B. Possible ecosystems and the search for life on Europa. Proc. Natl. Acad. Sci. USA 2001, 98, 801–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hand, K.P.; Carlson, R.W.; Chyba, C.F. Energy, Chemical Disequilibrium, and Geological Constraints on Europa. Astrobiology 2007, 7, 1006–1022. [Google Scholar] [CrossRef] [Green Version]
- Greenberg, R. Transport rates of radiolytic substances into Europa’s ocean: Implications for the potential origin and maintenance of life. Astrobiology 2010, 10, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.J.; Murray, A.E.; Hand, K.P. The Possible Emergence of Life and Differentiation of a Shallow Biosphere on Irradiated Icy Worlds: The Example of Europa. Astrobiology 2017, 17, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Kelley, D.S.; Karson, J.A.; Früh-Green, G.L.; Yoerger, D.R.; Shank, T.M.; Butterfield, D.A.; Hayes, J.M.; Schrenk, M.O.; Olson, E.J.; Proskurowski, G.; et al. A Serpentinite-Hosted Ecosystem: The Lost City Hydrothermal Field. Science 2005, 307, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Brazelton, W.J.; Schrenk, M.O.; Kelley, D.S.; Baross, J.A. Methane- and Sulfur-Metabolizing Microbial Communities Dominate the Lost City Hydrothermal Field Ecosystem. Appl. Environ. Microbiol. 2006, 72, 6257–6270. [Google Scholar] [CrossRef] [Green Version]
- López-García, P.; Vereshchaka, A.; Moreira, D. Eukaryotic diversity associated with carbonates and fluid—Seawater interface in Lost City hydrothermal field. Environ. Microbiol. 2006, 9, 546–554. [Google Scholar] [CrossRef]
- Vance, S.D.; Hand, K.P.; Pappalardo, R.T. Geophysical controls of chemical disequilibria in Europa. Geophys. Res. Lett. 2016, 43, 4871–4879. [Google Scholar] [CrossRef]
- Hand, K.P.; Carlson, R.W. H2O2 production by high-energy electrons on icy satellites as a function of surface temperature and electron flux. Icarus 2011, 215, 226–233. [Google Scholar] [CrossRef]
- Teolis, B.D.; Plainaki, C.; Cassidy, T.A.; Raut, U. Water Ice Radiolytic O2, H2, and H2 O2 Yields for Any Projectile Species, Energy, or Temperature: A Model for Icy Astrophysical Bodies. J. Geophys. Res. Planets 2017, 122, 1996–2012. [Google Scholar] [CrossRef]
- Galli, A.; Vorburger, A.; Wurz, P.; Pommerol, A.; Cerubini, R.; Jost, B.; Poch, O.; Tulej, M.; Thomas, N. 0.2 to 10 keV electrons interacting with water ice: Radiolysis, sputtering, and sublimation. Planet. Space Sci. 2018, 155, 91–98. [Google Scholar] [CrossRef]
- Li, J.; Gudipati, M.S.; Mishra, Y.N.; Liang, M.-C.; Yung, Y.L. Oxidant generation in the ice under electron irradiation: Simulation and application to Europa. Icarus 2021, 373, 114760. [Google Scholar] [CrossRef]
- Hesse, M.A.; Jordan, J.S.; Vance, S.D.; Oza, A.V. Downward Oxidant Transport Through Europa’s Ice Shell by Density-Driven Brine Percolation. Geophys. Res. Lett. 2022, 49, e2021GL095416. [Google Scholar] [CrossRef]
- Soderlund, K.M. Ocean Dynamics of Outer Solar System Satellites. Geophys. Res. Lett. 2019, 46, 8700–8710. [Google Scholar] [CrossRef] [Green Version]
- Roukaerts, A.; Deman, F.; Van der Linden, F.; Carnat, G.; Bratkic, A.; Moreau, S.; Fripiat, F. The biogeochemical role of a microbial biofilm in sea ice: Antarctic landfast sea ice as a case study. Elem. Sci. Anth. 2021, 9, 00134. [Google Scholar] [CrossRef]
- Showalter, G.M. Acquisition, Degradation, and Cycling of Organic Matter within Sea-Ice Brines by Bacteria and Their Viruses; University of Washington: Seattle, WA, USA, 2020. [Google Scholar]
- Soderlund, K.M.; Schmidt, B.E.; Wicht, J.; Blankenship, D.D. Ocean-driven heating of Europa’s icy shell at low latitudes. Nat. Geosci. 2013, 7, 16–19. [Google Scholar] [CrossRef]
- Zhu, P.; Manucharyan, G.E.; Thompson, A.F.; Goodman, J.C.; Vance, S.D. The influence of meridional ice transport on Europa’s ocean stratification and heat content. Geophys. Res. Lett. 2017, 44, 5969–5977. [Google Scholar] [CrossRef] [Green Version]
- Delgado, J. Molecular Diffusion Coefficients of Organic Compounds in Water at Different Temperatures. J. Phase Equilibria Diffus. 2007, 28, 427–432. [Google Scholar] [CrossRef]
- Kalousová, K.; Souček, O.; Tobie, G.; Choblet, G.; Čadek, O. Ice melting and downward transport of meltwater by two-phase flow in Europa’s ice shell. J. Geophys. Res. Planets 2014, 119, 532–549. [Google Scholar] [CrossRef]
- Nguyen, D.; Maranger, R. Respiration and bacterial carbon dynamics in Arctic Sea ice. Polar. Biol. 2011, 34, 1843–1855. [Google Scholar] [CrossRef]
- Melosh, H.J.; Ekholm, A.G.; Showman, A.P.; Lorenz, R.D. The temperature of Europa’s subsurface water ocean. Icarus 2004, 168, 498–502. [Google Scholar] [CrossRef]
- Servais, P.; Billen, G.; Rego, J.V. Rate of Bacterial Mortality in Aquatic Environments. Appl. Environ. Microbiol. 1985, 49, 1448–1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marx, J.G.; Carpenter, S.D.; Deming, J.W. Production of cryoprotectant extracellular polysaccharide substances (EPS) by the marine psychrophilic bacterium Colwellia psychrerythraea strain 34H under extreme conditions. Can. J. Microbiol. 2009, 55, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Jover, L.F.; Effler, T.C.; Buchan, A.; Wilhelm, S.W.; Weitz, J.S. The elemental composition of virus particles: Implications for marine biogeochemical cycles. Nat. Rev. Microbiol. 2014, 12, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Dell’Anno, A.; Corinaldesi, C.; Danovaro, R. Virus decomposition provides an important contribution to benthic deep-sea ecosystem functioning. Proc. Natl. Acad. Sci. USA 2015, 112, E2014–E2019. [Google Scholar] [CrossRef] [Green Version]
- Vaqué, D.; Lara, E.; Arrieta, J.; Holding, J.M.; Sà, E.L.; Hendriks, I.E.; Coello-Camba, A.; Álvarez, M.; Agusti, S.; Wassmann, P.F.; et al. Warming and CO2 Enhance Arctic Heterotrophic Microbial Activity. Front. Microbiol. 2019, 10, 494. [Google Scholar] [CrossRef] [Green Version]
- Suttle, C.A. Marine Viruses—Major Players in the Global Ecosystem. Nat. Rev. Microbiol. 2007, 5, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Lionard, M.; Péquin, B.; Lovejoy, C.; Vincent, W.F. Benthic Cyanobacterial Mats in the High Arctic: Multi-Layer Structure and Fluorescence Responses to Osmotic Stress. Front. Microbiol. 2012, 3, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohit, V.; Culley, A.; Lovejoy, C.; Bouchard, F.; Vincent, W.F. Hidden biofilms in a far northern lake and implications for the changing Arctic. NPJ Biofilms Microbiomes 2017, 3, 17. [Google Scholar] [CrossRef]
- Murga, R.; Stewart, P.S.; Daly, D. Quantitative analysis of biofilm thickness variability. Biotechnol. Bioeng. 1995, 45, 503–510. [Google Scholar] [CrossRef]
- Piculell, M.; Welander, P.; Jönsson, K.; Welander, T. Evaluating the effect of biofilm thickness on nitrification in moving bed biofilm reactors. Environ. Technol. 2015, 37, 732–743. [Google Scholar] [CrossRef]
- Suarez, C.; Piculell, M.; Modin, O.; Langenheder, S.; Persson, F.; Hermansson, M. Thickness determines microbial community structure and function in nitrifying biofilms via deterministic assembly. Sci. Rep. 2019, 9, 5110. [Google Scholar] [CrossRef] [Green Version]
- Bartlett, S.; Wong, M.L. Defining Lyfe in the Universe: From Three Privileged Functions to Four Pillars. Life 2020, 10, 42. [Google Scholar] [CrossRef] [Green Version]
- Waite, J.H.; Glein, C.R.; Perryman, R.S.; Teolis, B.D.; Magee, B.A.; Miller, G.; Grimes, J.; Perry, M.E.; Miller, K.E.; Bouquet, A.; et al. Cassini finds molecular hydrogen in the Enceladus plume: Evidence for hydrothermal processes. Science 2017, 356, 155–159. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.V. Phosphorus versus nitrogen limitation in the marine environment1. Limnol. Oceanogr. 1984, 29, 1149–1160. [Google Scholar] [CrossRef] [Green Version]
- Herbert, R.A. Nitrogen cycling in coastal marine ecosystems. FEMS Microbiol. Rev. 1999, 23, 563–590. [Google Scholar] [CrossRef]
- Pham, A.L.D. Understanding Ocean Iron Dynamics and Impacts on Marine Ecosystems. Ph.D. Thesis, Georgia Institute of Technology, Atlanta, GA, USA, 2019. [Google Scholar]
- Beckett, S.J.; Williams, H. Coevolutionary diversification creates nested-modular structure in phage–bacteria interaction networks. Interface Focus 2013, 3, 20130033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomez-Buckley, A.C.; Showalter, G.M.; Wong, M.L. Modeling Virus and Bacteria Populations in Europa’s Subsurface Ocean. Life 2022, 12, 620. https://doi.org/10.3390/life12050620
Gomez-Buckley AC, Showalter GM, Wong ML. Modeling Virus and Bacteria Populations in Europa’s Subsurface Ocean. Life. 2022; 12(5):620. https://doi.org/10.3390/life12050620
Chicago/Turabian StyleGomez-Buckley, Adriana C., Gordon M. Showalter, and Michael L. Wong. 2022. "Modeling Virus and Bacteria Populations in Europa’s Subsurface Ocean" Life 12, no. 5: 620. https://doi.org/10.3390/life12050620
APA StyleGomez-Buckley, A. C., Showalter, G. M., & Wong, M. L. (2022). Modeling Virus and Bacteria Populations in Europa’s Subsurface Ocean. Life, 12(5), 620. https://doi.org/10.3390/life12050620






