Exposure to Random Positioning Machine Alters the Mineralization Process and PTX3 Expression in the SAOS-2 Cell Line
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Simulation Experiment by RPM
2.3. Cell Viability Assessment
2.4. Evaluation of Calcification-like Structures by Toluidine Blue Staining
2.5. In Vitro Mineralization Measurement
2.6. Immunocytochemistry
2.7. Western Blotting Analysis
2.8. Statistical Analysis
3. Results
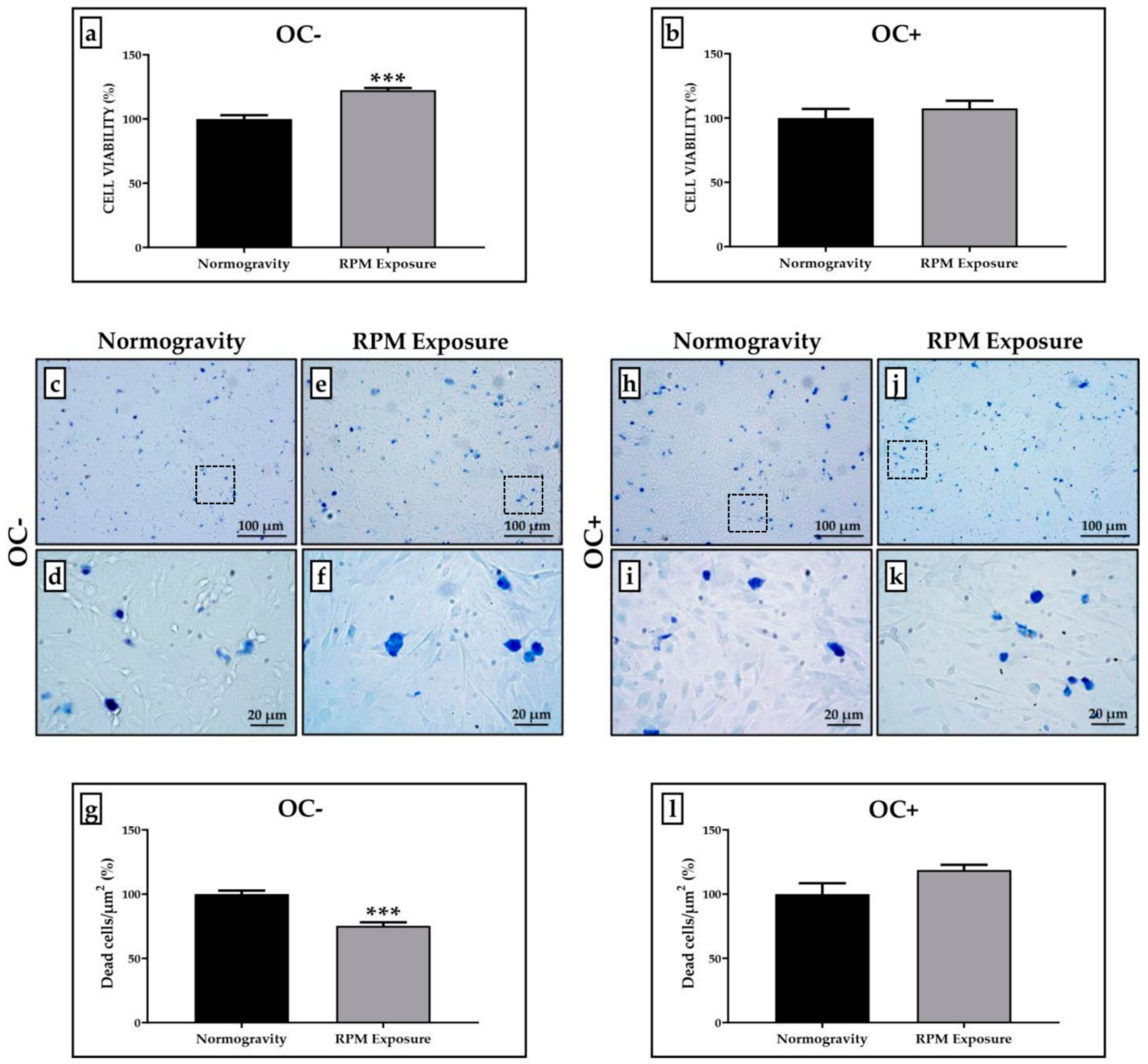
3.1. Cell Viability Evaluation after RPM Exposure
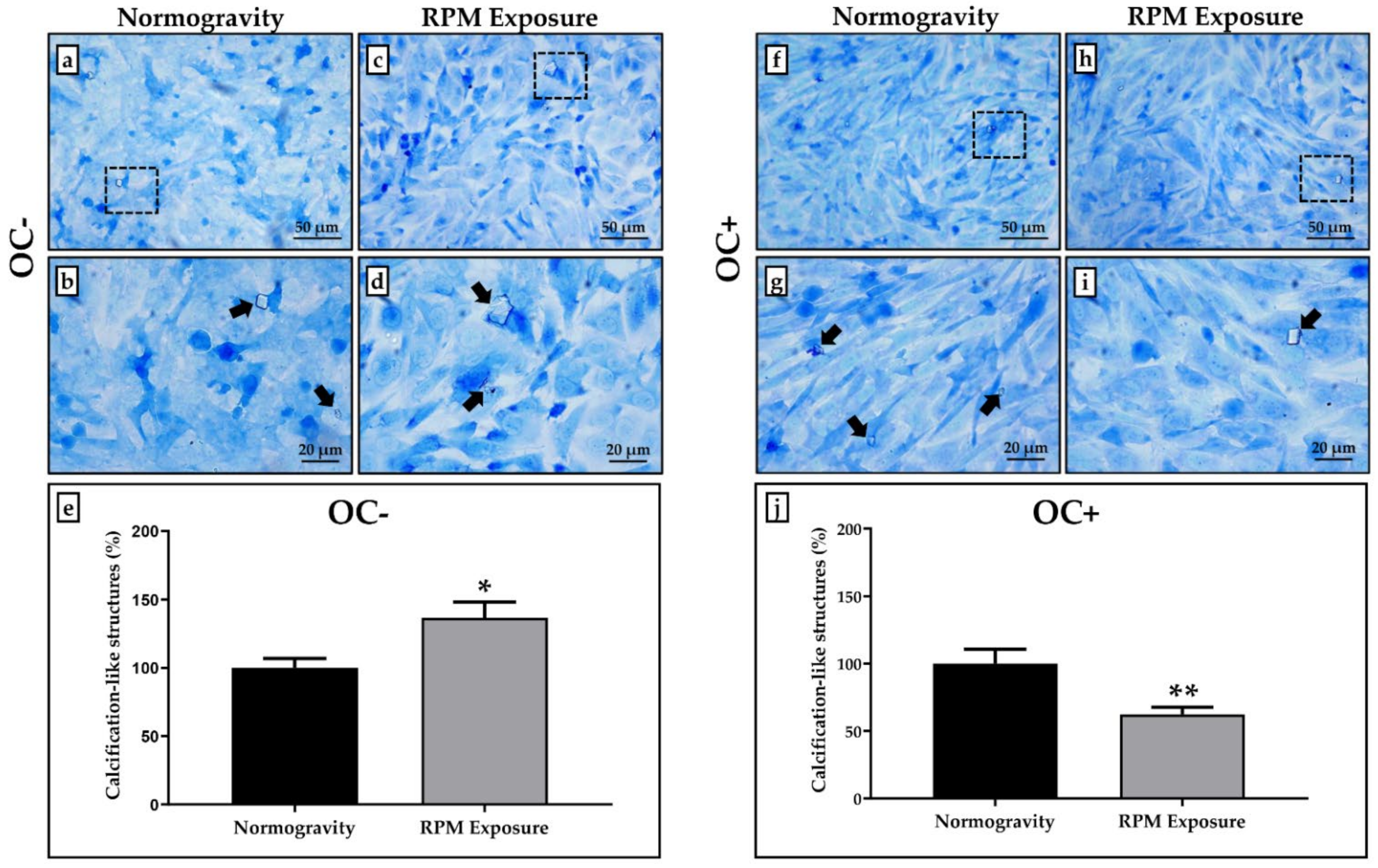
3.2. Calcification-like Structure Formation Assessed after Toluidine Blue Staining
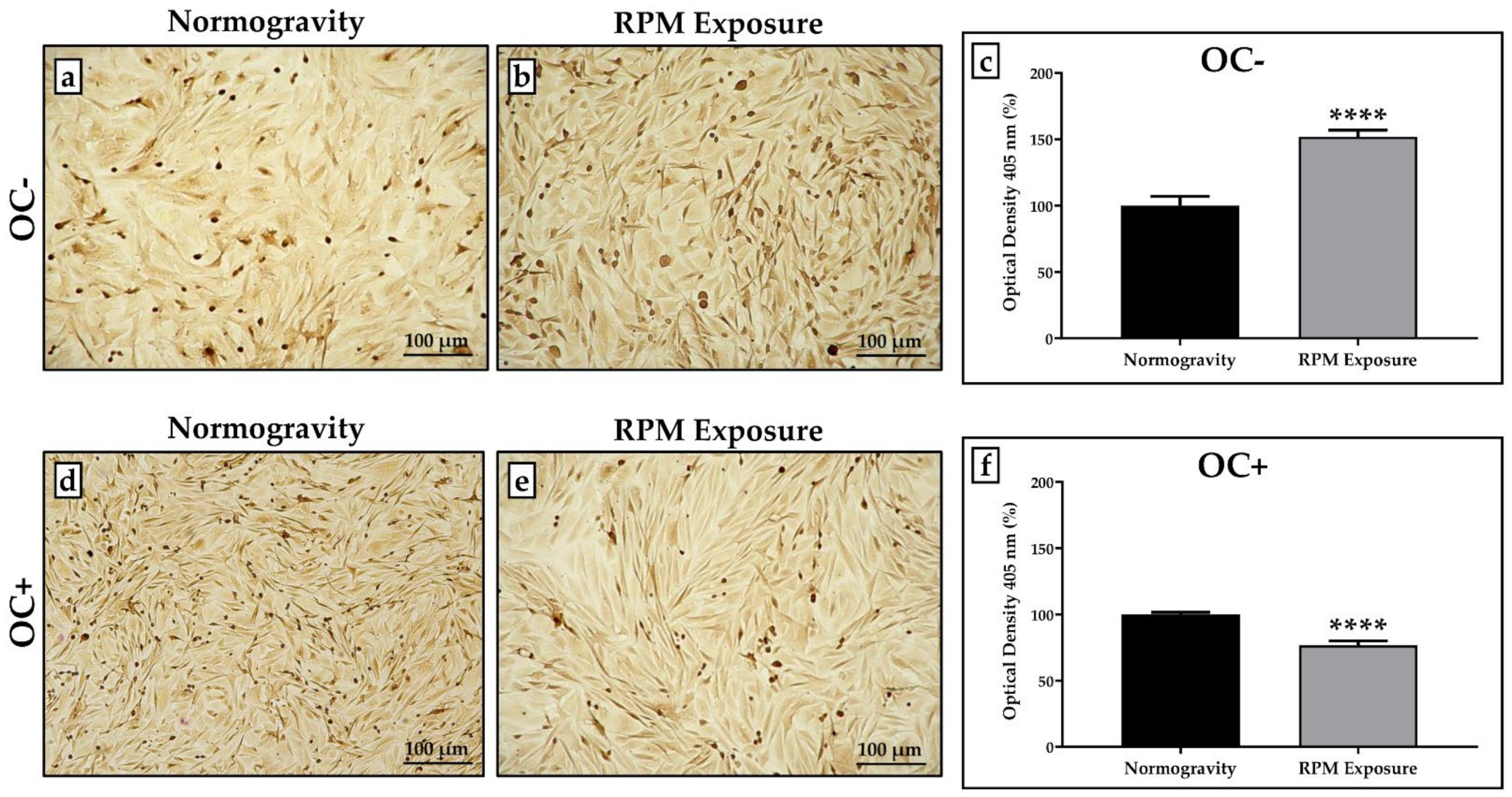
3.3. Effects of RPM Exposure on the Mineralization Process
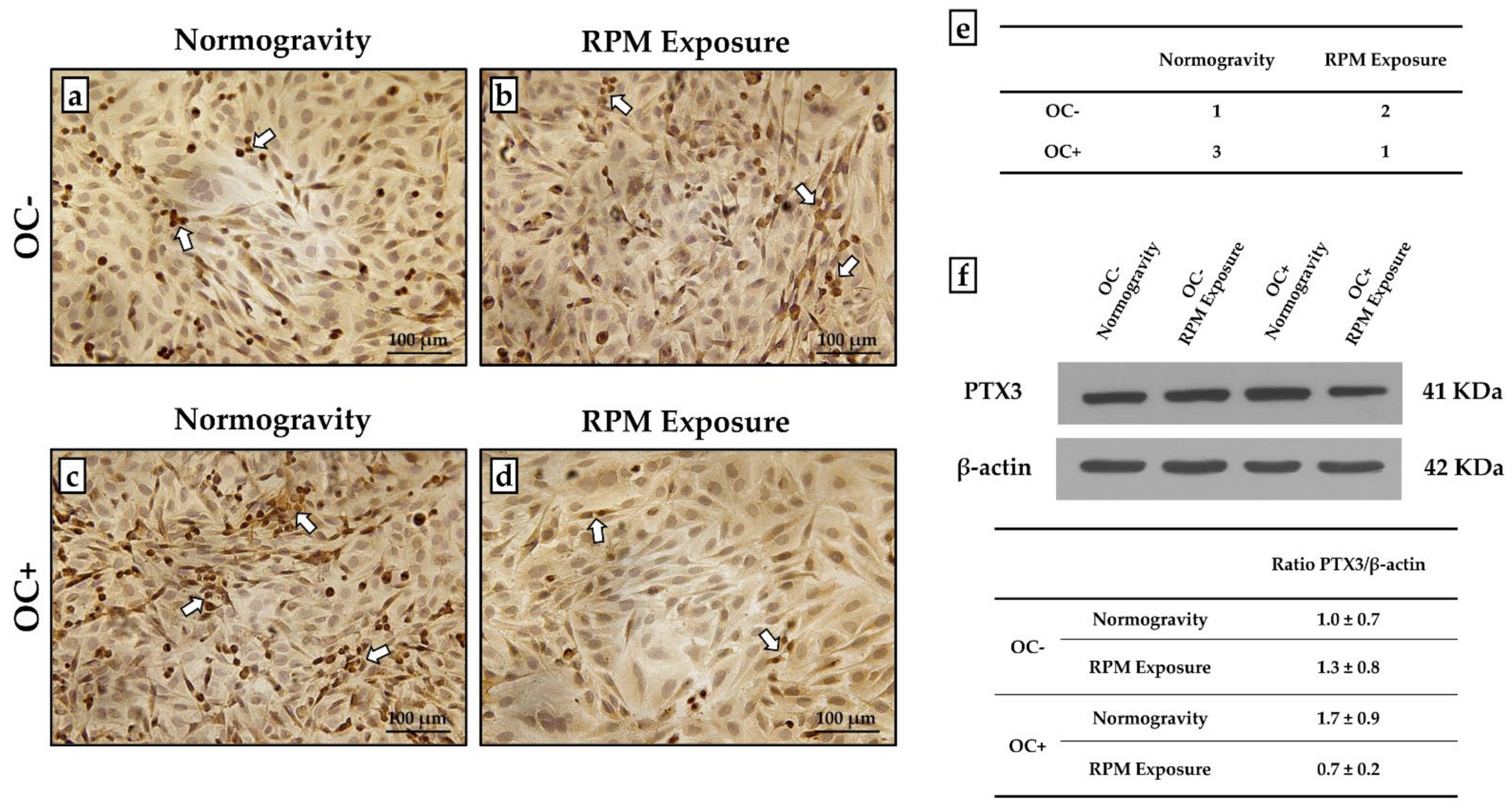
3.4. PTX3 Expression Analysis for Mineralization Process Characterization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tarantino, U.; Cariati, I.; Marini, M.; D’Arcangelo, G.; Tancredi, V.; Primavera, M.; Iundusi, R.; Gasbarra, E.; Scimeca, M. Effects of Simulated Microgravity on Muscle Stem Cells Activity. Cell. Physiol. Biochem. 2020, 54, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Lehar, A.; Meir, J.U.; Koch, C.; Morgan, A.; Warren, L.E.; Rydzik, R.; Youngstrom, D.W.; Chandok, H.; George, J.; et al. Targeting myostatin/activin A protects against skeletal muscle and bone loss during spaceflight. Proc. Natl. Acad. Sci. USA 2020, 117, 23942–23951. [Google Scholar] [CrossRef] [PubMed]
- Arfat, Y.; Xiao, W.-Z.; Iftikhar, S.; Zhao, F.; Li, D.-J.; Sun, Y.-L.; Zhang, G.; Shang, P.; Qian, A.-R. Physiological effects of microgravity on bone cells. Calcif. Tissue Int. 2014, 94, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Li, Y.; Zhou, H.; Cai, M.; Liu, J.; Gao, S.; Yang, J.; Tong, L.; Wang, J.; Zhou, S.; et al. Simulated microgravity reduces intracellular-free calcium concentration by inhibiting calcium channels in primary mouse osteoblasts. J. Cell. Biochem. 2019, 120, 4009–4020. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Heer, M.A.; Shackelford, L.C.; Sibonga, J.D.; Ploutz-Snyder, L.; Zwart, S.R. Benefits for bone from resistance exercise and nutrition in long-duration spaceflight: Evidence from biochemistry and densitometry. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2012, 27, 1896–1906. [Google Scholar] [CrossRef] [PubMed]
- Grimm, D.; Grosse, J.; Wehland, M.; Mann, V.; Reseland, J.E.; Sundaresan, A.; Corydon, T.J. The impact of microgravity on bone in humans. Bone 2016, 87, 44–56. [Google Scholar] [CrossRef]
- Smith, S.M.; Wastney, M.E.; O’Brien, K.O.; Morukov, B.V.; Larina, I.M.; Abrams, S.A.; Davis-Street, J.E.; Oganov, V.; Shackelford, L.C. Bone markers, calcium metabolism, and calcium kinetics during extended-duration space flight on the mir space station. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2005, 20, 208–218. [Google Scholar] [CrossRef]
- Calzia, D.; Ottaggio, L.; Cora, A.; Chiappori, G.; Cuccarolo, P.; Cappelli, E.; Izzotti, A.; Tavella, S.; Degan, P. Characterization of C2C12 cells in simulated microgravity: Possible use for myoblast regeneration. J. Cell. Physiol. 2020, 235, 3508–3518. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Y.; Zhao, F.; Yin, C.; Yang, C.; Wang, X.; Wu, Z.; Liang, S.; Li, D.; Lin, X.; et al. Recombinant Irisin Prevents the Reduction of Osteoblast Differentiation Induced by Stimulated Microgravity through Increasing β-Catenin Expression. Int. J. Mol. Sci. 2020, 21, 1259. [Google Scholar] [CrossRef]
- Ruden, D.M.; Bolnick, A.; Awonuga, A.; Abdulhasan, M.; Perez, G.; Puscheck, E.E.; Rappolee, D.A. Effects of Gravity, Microgravity or Microgravity Simulation on Early Mammalian Development. Stem Cells Dev. 2018, 27, 1230–1236. [Google Scholar] [CrossRef]
- Liu, L.; Cheng, Y.; Wang, J.; Ding, Z.; Halim, A.; Luo, Q.; Song, G. Simulated Microgravity Suppresses Osteogenic Differentiation of Mesenchymal Stem Cells by Inhibiting Oxidative Phosphorylation. Int. J. Mol. Sci. 2020, 21, 9747. [Google Scholar] [CrossRef] [PubMed]
- Morabito, C.; Guarnieri, S.; Cucina, A.; Bizzarri, M.; Mariggiò, M.A. Antioxidant Strategy to Prevent Simulated Microgravity-Induced Effects on Bone Osteoblasts. Int. J. Mol. Sci. 2020, 21, 3638. [Google Scholar] [CrossRef] [PubMed]
- Colaianni, G.; Mongelli, T.; Cuscito, C.; Pignataro, P.; Lippo, L.; Spiro, G.; Notarnicola, A.; Severi, I.; Passeri, G.; Mori, G.; et al. Irisin prevents and restores bone loss and muscle atrophy in hind-limb suspended mice. Sci. Rep. 2017, 7, 2811. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, U.; Greggi, C.; Cariati, I.; Visconti, V.V.; Gasparini, M.; Cateni, M.; Gasbarra, E.; Botta, A.; Salustri, A.; Scimeca, M. The Role of PTX3 in Mineralization Processes and Aging-Related Bone Diseases. Front. Immunol. 2021, 11, 622772. [Google Scholar] [CrossRef]
- Scimeca, M.; Salustri, A.; Bonanno, E.; Nardozi, D.; Rao, C.; Piccirilli, E.; Feola, M.; Tancredi, V.; Rinaldi, A.; Iolascon, G.; et al. Impairment of PTX3 expression in osteoblasts: A key element for osteoporosis. Cell Death Dis. 2017, 8, e3125. [Google Scholar] [CrossRef]
- Greggi, C.; Cariati, I.; Onorato, F.; Iundusi, R.; Scimeca, M.; Tarantino, U. PTX3 Effects on Osteogenic Differentiation in Osteoporosis: An In Vitro Study. Int. J. Mol. Sci. 2021, 22, 5944. [Google Scholar] [CrossRef]
- Grčević, D.; Sironi, M.; Valentino, S.; Deban, L.; Cvija, H.; Inforzato, A.; Kovačić, N.; Katavić, V.; Kelava, T.; Kalajzić, I.; et al. The Long Pentraxin 3 Plays a Role in Bone Turnover and Repair. Front. Immunol. 2018, 9, 417. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Zhou, X.-Z.; Li, N.; Guo, Y.-C.; Chen, T.-P. Pentraxin 3 promotes the osteoblastic differentiation of MC3T3-E1 cells through the PI3K/Akt signaling pathway. Biosci. Rep. 2020, 40, BSR20201165. [Google Scholar] [CrossRef]
- Prall, W.C.; Haasters, F.; Heggebö, J.; Polzer, H.; Schwarz, C.; Gassner, C.; Grote, S.; Anz, D.; Jäger, M.; Mutschler, W.; et al. Mesenchymal stem cells from osteoporotic patients feature impaired signal transduction but sustained osteoinduction in response to BMP-2 stimulation. Biochem. Biophys. Res. Commun. 2013, 440, 617–622. [Google Scholar] [CrossRef]
- Borst, A.G.; Van Loon, J.J.W.A. Technology and developments for the random positioning machine, RPM. Microgravity Sci. Technol. 2009, 21, 287–292. [Google Scholar] [CrossRef]
- Wuest, S.L.; Richard, S.; Kopp, S.; Grimm, D.; Egli, M. Simulated microgravity: Critical review on the use of random positioning machines for mammalian cell culture. Biomed Res. Int. 2015, 2015, 971474. [Google Scholar] [CrossRef] [PubMed]
- Kabakov, A.E.; Gabai, V.L. Cell Death and Survival Assays. Methods Mol. Biol. 2018, 1709, 107–127. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.-H.; Chang, J.-C. The static magnetic field accelerates the osteogenic differentiation and mineralization of dental pulp cells. Cytotechnology 2010, 62, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Gregory, C.A.; Gunn, W.G.; Peister, A.; Prockop, D.J. An Alizarin red-based assay of mineralization by adherent cells in culture: Comparison with cetylpyridinium chloride extraction. Anal. Biochem. 2004, 329, 77–84. [Google Scholar] [CrossRef]
- Tominari, T.; Ichimaru, R.; Taniguchi, K.; Yumoto, A.; Shirakawa, M.; Matsumoto, C.; Watanabe, K.; Hirata, M.; Itoh, Y.; Shiba, D.; et al. Hypergravity and microgravity exhibited reversal effects on the bone and muscle mass in mice. Sci. Rep. 2019, 9, 6614. [Google Scholar] [CrossRef]
- Roberts, D.R.; Asemani, D.; Nietert, P.J.; Eckert, M.A.; Inglesby, D.C.; Bloomberg, J.J.; George, M.S.; Brown, T.R. Prolonged Microgravity Affects Human Brain Structure and Function. Am. J. Neuroradiol. 2019, 40, 1878–1885. [Google Scholar] [CrossRef] [PubMed]
- Morita, H.; Abe, C.; Tanaka, K. Long-term exposure to microgravity impairs vestibulo-cardiovascular reflex. Sci. Rep. 2016, 6, 33405. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Han, C.; Lu, Z.; Ge, P.; Cui, Y.; Zhao, D.; Yang, X.; Wu, B.; Qiang, L.; Zhang, Y.; et al. Simulated microgravity suppresses MAPK pathway-mediated innate immune response to bacterial infection and induces gut microbiota dysbiosis. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 14631–14644. [Google Scholar] [CrossRef]
- Takahashi, H.; Nakamura, A.; Shimizu, T. Simulated microgravity accelerates aging of human skeletal muscle myoblasts at the single cell level. Biochem. Biophys. Res. Commun. 2021, 578, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Strollo, F.; Gentile, S.; Strollo, G.; Mambro, A.; Vernikos, J. Recent Progress in Space Physiology and Aging. Front. Physiol. 2018, 9, 1551. [Google Scholar] [CrossRef]
- Cariati, I.; Scimeca, M.; Bonanni, R.; Triolo, R.; Naldi, V.; Toro, G.; Marini, M.; Tancredi, V.; Iundusi, R.; Gasbarra, E.; et al. Role of Myostatin in Muscle Degeneration by Random Positioning Machine Exposure: An in vitro Study for the Treatment of Sarcopenia. Front. Physiol. 2022, 13, 782000. [Google Scholar] [CrossRef] [PubMed]
- Nagaraja, M.P.; Risin, D. The current state of bone loss research: Data from spaceflight and microgravity simulators. J. Cell. Biochem. 2013, 114, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Axpe, E.; Chan, D.; Abegaz, M.F.; Schreurs, A.-S.; Alwood, J.S.; Globus, R.K.; Appel, E.A. A human mission to Mars: Predicting the bone mineral density loss of astronauts. PLoS ONE 2020, 15, e0226434. [Google Scholar] [CrossRef]
- Hu, L.; Li, J.; Qian, A.; Wang, F.; Shang, P. Mineralization initiation of MC3T3-E1 preosteoblast is suppressed under simulated microgravity condition. Cell Biol. Int. 2015, 39, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Xie, Y.; He, J.; Zhou, J.; Gao, Y.; Wei, W.; Ding, N.; Ma, H.; Xian, C.J.; Chen, K.; et al. Microgravity induces inhibition of osteoblastic differentiation and mineralization through abrogating primary cilia. Sci. Rep. 2017, 7, 1866. [Google Scholar] [CrossRef] [PubMed]
- Czekanska, E.M.; Stoddart, M.J.; Richards, R.G.; Hayes, J.S. In search of an osteoblast cell model for in vitro research. Eur. Cell. Mater. 2012, 24, 1–17. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cariati, I.; Bonanni, R.; Scimeca, M.; Rinaldi, A.M.; Marini, M.; Tarantino, U.; Tancredi, V. Exposure to Random Positioning Machine Alters the Mineralization Process and PTX3 Expression in the SAOS-2 Cell Line. Life 2022, 12, 610. https://doi.org/10.3390/life12050610
Cariati I, Bonanni R, Scimeca M, Rinaldi AM, Marini M, Tarantino U, Tancredi V. Exposure to Random Positioning Machine Alters the Mineralization Process and PTX3 Expression in the SAOS-2 Cell Line. Life. 2022; 12(5):610. https://doi.org/10.3390/life12050610
Chicago/Turabian StyleCariati, Ida, Roberto Bonanni, Manuel Scimeca, Anna Maria Rinaldi, Mario Marini, Umberto Tarantino, and Virginia Tancredi. 2022. "Exposure to Random Positioning Machine Alters the Mineralization Process and PTX3 Expression in the SAOS-2 Cell Line" Life 12, no. 5: 610. https://doi.org/10.3390/life12050610
APA StyleCariati, I., Bonanni, R., Scimeca, M., Rinaldi, A. M., Marini, M., Tarantino, U., & Tancredi, V. (2022). Exposure to Random Positioning Machine Alters the Mineralization Process and PTX3 Expression in the SAOS-2 Cell Line. Life, 12(5), 610. https://doi.org/10.3390/life12050610







