A Spotlight on the Role of Radiomics and Machine-Learning Applications in the Management of Intracranial Meningiomas: A New Perspective in Neuro-Oncology: A Review
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Eligibility Criteria
2.3. Data Extraction
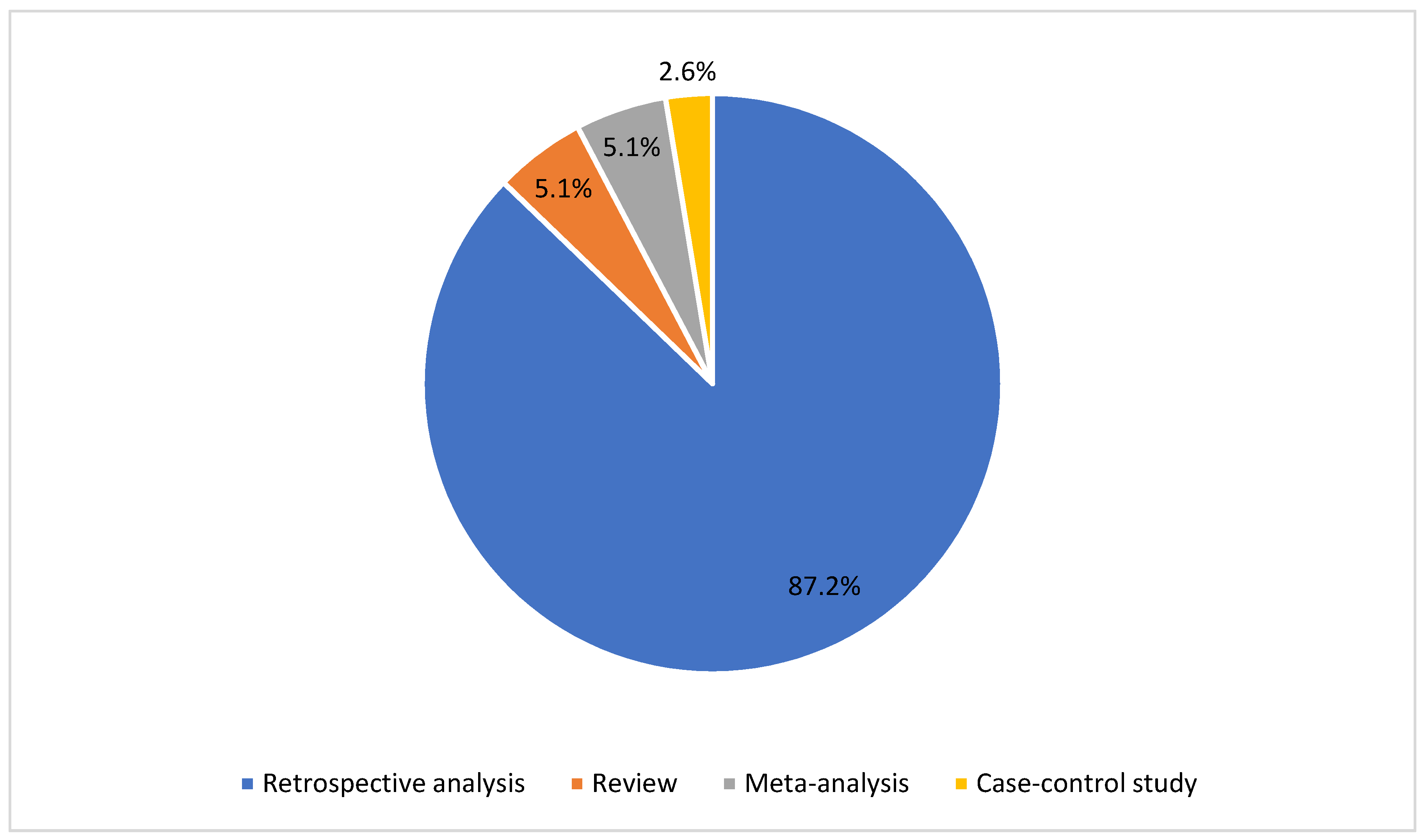
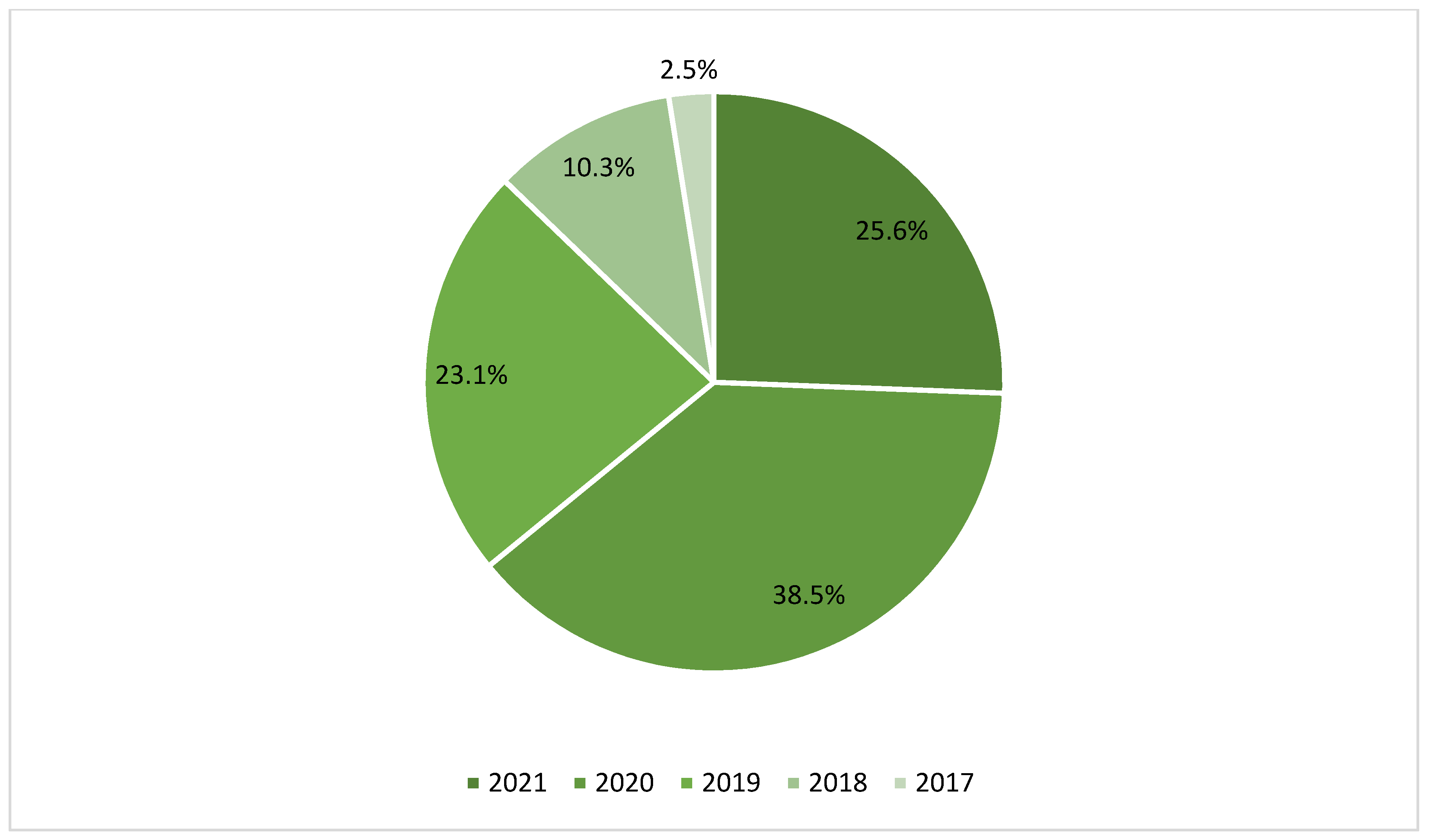
3. Results
4. Discussion
4.1. Preoperative Meningioma Grading
4.2. Preoperative Prediction of Ki-67 in Benign Meningioma
4.3. Prediction of Brain Invasion as an Indirect Tool for Recurrence and Poor Prognosis
4.4. Prediction of Meningioma Mass Consistency
4.5. Differential Diagnosis between Meningioma and Other CNS Tumors
4.6. Prognostic Implications
4.7. Limitations and Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Amisha Malik, P.; Pathania, M.; Rathaur, V.K. Overview of artificial intelligence in medicine. J. Fam. Med. Prim. Care 2019, 8, 2328–2331. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, N.; Matos, C.; Koh, D.M. How to develop a meaningful radiomic signature for clinical use in oncologic patients. Cancer Imaging 2020, 20, 33. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Scott, J.; Chaudhury, B.; Hall, L.; Goldgof, D.; Yeom, K.W.; Iv, M.; Ou, Y.; Kalpathy-Cramer, J.; Napel, S.; et al. Radiomics in Brain Tumor: Image Assessment, Quantitative Feature Descriptors, and Machine-Learning Approaches. Am. J. Neuroradiol. 2018, 39, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Van Timmeren, J.E.; Cester, D.; Tanadini-Lang, S.; Alkadhi, H.; Baessler, B. Radiomics in medical imaging-”how-to” guide and critical reflection. Insights Imaging 2020, 11, 91. [Google Scholar] [CrossRef]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef] [Green Version]
- Burrell, R.A.; McGranahan, N.; Bartek, J.; Swanton, C. The causes and consequences of genetic heterogeneity in cancer evolution. Nature 2013, 501, 338–345. [Google Scholar] [CrossRef]
- Fan, Y.; Feng, M.; Wang, R. Application of Radiomics in Central Nervous System Diseases: A Systematic literature review. Clin. Neurol. Neurosurg. 2019, 187, 105565. [Google Scholar] [CrossRef]
- Park, J.E.; Kim, H.S. Radiomics as a Quantitative Imaging Biomarker: Practical Considerations and the Current Standpoint in Neuro-oncologic Studies. Nucl. Med. Mol. Imaging 2018, 52, 99–108. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012–2016. Neuro-Oncology 2019, 21 (Suppl. 5), v1–v100. [Google Scholar] [CrossRef]
- Lin, D.D.; Lin, J.L.; Deng, X.Y.; Li, W.; Li, D.D.; Yin, B.; Lin, J.; Zhang, N.; Sheng, H.S. Trends in intracranial meningioma incidence in the United States, 2004–2015. Cancer Med. 2019, 8, 6458–6467. [Google Scholar] [CrossRef] [Green Version]
- Baldi, I.; Engelhardt, J.; Bonnet, C.; Bauchet, L.; Berteaud, E.; Grüber, A.; Loiseau, H. Epidemiology of meningiomas. Neurochirurgie 2018, 64, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Spille, D.C.; Sporns, P.B.; Heß, K.; Stummer, W.; Brokinkel, B. Prediction of High-Grade Histology and Recurrence in Meningiomas Using Routine Preoperative Magnetic Resonance Imaging: A Systematic Review. World Neurosurg. 2019, 128, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Zhang, X.; Di Russo, P.; Zhao, X.; Xu, T. The Current State of Radiomics for Meningiomas: Promises and Challenges. Front. Oncol. 2020, 10, 567736. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Manjila, S.; Sakla, N.; True, A.; Wardeh, A.H.; Beig, N.; Vaysberg, A.; Matthews, J.; Prasanna, P.; Spektor, V. Radiomics and radiogenomics in gliomas: A contemporary update. Br. J. Cancer 2021, 125, 641–657. [Google Scholar] [CrossRef] [PubMed]
- Katsila, T.; Matsoukas, M.-T.; Patrinos, G.P.; Kardamakis, D. Pharmacometabolomics Informs Quantitative Radiomics for Glioblastoma Diagnostic Innovation. OMICS J. Integr. Biol. 2017, 21, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Fan, Y.; Zhang, Z.; Tan, Z.; Yang, H.; Tu, W.; Wu, L.; Shen, X.; Guo, H.; Wu, Z.; et al. Three-Dimensional Radiomics Features From Multi-Parameter MRI Combined With Clinical Characteristics Predict Postoperative Cerebral Edema Exacerbation in Patients With Meningioma. Front. Oncol. 2021, 11, 625220. [Google Scholar] [CrossRef]
- Mayerhoefer, M.E.; Materka, A.; Langs, G.; Häggström, I.; Szczypiński, P.; Gibbs, P.; Cook, G. Introduction to Radiomics. J. Nucl. Med. 2020, 61, 488–495. [Google Scholar] [CrossRef]
- Hamerla, G.; Meyer, H.-J.; Schob, S.; Ginat, D.T.; Altman, A.; Lim, C.T.; Gihr, G.A.; Horvath-Rizea, D.; Hoffmann, K.-T.; Surov, A. Comparison of machine learning classifiers for differentiation of grade 1 from higher gradings in meningioma: A multicenter radiomics study. Magn. Reson. Imaging 2019, 63, 244–249. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, Y.; Li, M.; Liu, J.; Wang, F.; Weng, Q.; Wang, X.; Cao, D. Machine learning-based radiomics analysis in predicting the meningioma grade using multiparametric MRI. Eur. J. Radiol. 2020, 131, 109251. [Google Scholar] [CrossRef]
- Han, Y.; Wang, T.; Wu, P.; Zhang, H.; Chen, H.; Yang, C. Meningiomas: Preoperative predictive histopathological grading based on radiomics of MRI. Magn. Reson. Imaging 2021, 77, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Banzato, T.; Causin, F.; Della Puppa, A.; Cester, G.; Mazzai, L.; Zotti, A. Accuracy of deep learning to differentiate the histopathological grading of meningiomas on MR images: A preliminary study. J. Magn. Reson. Imaging 2019, 50, 1152–1159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, Y.W.; Oh, J.; You, S.C.; Han, K.; Ahn, S.S.; Choi, Y.S.; Chang, J.H.; Kim, S.H.; Lee, S.-K. Radiomics and machine learning may accurately predict the grade and histological subtype in meningiomas using conventional and diffusion tensor imaging. Eur. Radiol. 2019, 29, 4068–4076. [Google Scholar] [CrossRef] [PubMed]
- Ke, C.; Chen, H.; Lv, X.; Li, H.; Zhang, Y.; Chen, M.; Hu, D.; Ruan, G.; Zhang, Y.; Liu, L.; et al. Differentiation Between Benign and Nonbenign Meningiomas by Using Texture Analysis From Multiparametric MRI. J. Magn. Reson. Imaging 2020, 51, 1810–1820. [Google Scholar] [CrossRef]
- Kalasauskas, D.; Kronfeld, A.; Renovanz, M.; Kurz, E.; Leukel, P.; Krenzlin, H.; Brockmann, M.A.; Sommer, C.J.; Ringel, F.; Keric, N. Identification of High-Risk Atypical Meningiomas According to Semantic and Radiomic Features. Cancers 2020, 12, 2942. [Google Scholar] [CrossRef]
- Coroller, T.P.; Bi, W.L.; Huynh, E.; Abedalthagafi, M.; Aizer, A.A.; Greenwald, N.F.; Parmar, C.; Narayan, V.; Wu, W.W.; De Moura, S.M.; et al. Radiographic prediction of meningioma grade by semantic and radiomic features. PLoS ONE 2017, 12, e0187908. [Google Scholar] [CrossRef] [Green Version]
- Niu, L.; Zhou, X.; Duan, C.; Zhao, J.; Sui, Q.; Liu, X.; Zhang, X.J.Z. Differentiation Researches on the Meningioma Subtypes by Radiomics from Contrast-Enhanced Magnetic Resonance Imaging: A Preliminary Study. World Neurosurg. 2019, 126, e646–e652. [Google Scholar] [CrossRef]
- Ugga, L.; Perillo, T.; Cuocolo, R.; Stanzione, A.; Romeo, V.; Green, R.; Cantoni, V.; Brunetti, A. Meningioma MRI radiomics and machine learning: Systematic review, quality score assessment, and meta-analysis. Neuroradiology 2021, 63, 1293–1304. [Google Scholar] [CrossRef]
- Khanna, O.; Kazerooni, A.F.; Farrell, C.J.; Baldassari, M.P.; Alexander, T.D.; Karsy, M.; Greenberger, B.A.; Garcia, J.A.; Sako, C.; Evans, J.J.; et al. Machine Learning Using Multiparametric Magnetic Resonance Imaging Radiomic Feature Analysis to Predict Ki-67 in World Health Organization Grade I Meningiomas. Neurosurgery 2021, 89, 928–936. [Google Scholar] [CrossRef]
- Haddad, A.F.; Young, J.S.; Kanungo, I.; Sudhir, S.; Chen, J.-S.; Raleigh, D.R.; Magill, S.T.; McDermott, M.W.; Aghi, M.K. WHO Grade I Meningioma Recurrence: Identifying High Risk Patients Using Histopathological Features and the MIB-1 Index. Front. Oncol. 2020, 10, 1522. [Google Scholar] [CrossRef]
- Joo, L.; Park, J.E.; Park, S.Y.; Nam, S.J.; Kim, Y.-H.; Kim, J.H.; Kim, H.S. Extensive peritumoral edema and brain-to-tumor interface MRI features enable prediction of brain invasion in meningioma: Development and validation. Neuro-Oncology 2021, 23, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Mo, Y.; Huang, C.; Han, K.; He, M.; Wang, X.; Wen, J.; Yang, S.; Wu, H.; Dong, F.; et al. A Clinical Semantic and Radiomics Nomogram for Predicting Brain Invasion in WHO Grade II Meningioma Based on Tumor and Tumor-to-Brain Interface Features. Front. Oncol. 2021, 11, 752158. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.-C.; Zhang, Y.; Chen, J.-H.; Chang, K.-T.; Chen, T.-Y.; Lim, S.-W.; Wu, T.-C.; Su, M.-Y. Pre-operative MRI Radiomics for the Prediction of Progression and Recurrence in Meningiomas. Front. Neurol. 2021, 12, 636235. [Google Scholar] [CrossRef]
- Kandemirli, S.G.; Chopra, S.; Priya, S.; Ward, C.; Locke, T.; Soni, N.; Srivastava, S.; Jones, K.; Bathla, G. Presurgical detection of brain invasion status in meningiomas based on first-order histogram based texture analysis of contrast enhanced imaging. Clin. Neurol. Neurosurg. 2020, 198, 106205. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, K.; Liu, P.; Liu, Z.; Han, T.; Zhao, Z.; Cao, Y.; Zhang, G.; Zhang, J.; Tian, J.; et al. A radiomics model for preoperative prediction of brain invasion in meningioma non-invasively based on MRI: A multicentre study. eBioMedicine 2020, 58, 102933. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, J.; Han, T.; Zhao, Z.; Cao, Y.; Zhang, G.; Zhou, J. Radiomic features of magnetic resonance images as novel preoperative predictive factors of bone invasion in meningiomas. Eur. J. Radiol. 2020, 132, 109287. [Google Scholar] [CrossRef] [PubMed]
- Giammalva, G.R.; Ferini, G.; Musso, S.; Salvaggio, G.; Pino, M.A.; Gerardi, R.M.; Brunasso, L.; Costanzo, R.; Paolini, F.; Di Bonaventura, R.; et al. Intraoperative Ultrasound: Emerging Technology and Novel Applications in Brain Tumor Surgery. Front. Oncol. 2022, 12, 818446. [Google Scholar] [CrossRef] [PubMed]
- Giammalva, G.R.; Musso, S.; Salvaggio, G.; Pino, M.A.; Gerardi, R.M.; Umana, G.E.; Midiri, M.; Iacopino, D.G.; Maugeri, R. Coplanar Indirect-Navigated Intraoperative Ultrasound: Matching Un-navigated Probes With Neuronavigation During Neurosurgical Procedures. How We Do It. Oper. Neurosurg. 2021, 21, 485–490. [Google Scholar] [CrossRef]
- Cepeda, S.; Arrese, I.; García-García, S.; Velasco-Casares, M.; Escudero-Caro, T.; Zamora, T.; Sarabia, R. Meningioma Consistency Can Be Defined by Combining the Radiomic Features of Magnetic Resonance Imaging and Ultrasound Elastography. A Pilot Study Using Machine Learning Classifiers. World Neurosurg. 2020, 146, e1147–e1159. [Google Scholar] [CrossRef]
- Zhai, Y.; Song, D.; Yang, F.; Wang, Y.; Jia, X.; Wei, S.; Mao, W.; Xue, Y.; Wei, X. Preoperative Prediction of Meningioma Consistency via Machine Learning-Based Radiomics. Front. Oncol. 2021, 11, 657288. [Google Scholar] [CrossRef]
- Krivoshapkin, A.L.; Sergeev, G.S.; Kalneus, L.E.; Gaytan, A.S.; Murtazin, V.I.; Kurbatov, V.P.; Volkov, A.M.; Kostromskaya, D.V.; Pyatov, S.M.; Amelin, M.E.; et al. New Software for Preoperative Diagnostics of Meningeal Tumor Histologic Types. World Neurosurg. 2016, 90, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Maki, S.; Furuya, T.; Horikoshi, T.; Yokota, H.; Mori, Y.; Ota, J.; Kawasaki, Y.; Miyamoto, T.; Norimoto, M.; Okimatsu, S.; et al. A Deep Convolutional Neural Network with Performance Comparable to Radiologists for Differentiating Between Spinal Schwannoma and Meningioma. Spine 2020, 45, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Yu, M.; Miao, Y.; Shen, H.; Sui, Y.; Liu, Y.; Han, L.; Li, X.; Lin, M.; Guo, Y.; et al. Differential Diagnosis of Solitary Fibrous Tumor/Hemangiopericytoma and Angiomatous Meningioma Using Three-Dimensional Magnetic Resonance Imaging Texture Feature Model. BioMed Res. Int. 2020, 2020, 5042356. [Google Scholar] [CrossRef]
- Li, X.; Lu, Y.; Xiong, J.; Wang, D.; She, D.; Kuai, X.; Geng, D.; Yin, B. Presurgical differentiation between malignant haemangiopericytoma and angiomatous meningioma by a radiomics approach based on texture analysis. J. Neuroradiol. 2019, 46, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Li, L.; Han, Y.; Gu, D.; Chen, Q.; Wang, J.; Li, R.; Zhan, J.; Tian, J.; Zhou, D. Accurate Preoperative Distinction of Intracranial Hemangiopericytoma From Meningioma Using a Multihabitat and Multisequence-Based Radiomics Diagnostic Technique. Front. Oncol. 2020, 10, 534. [Google Scholar] [CrossRef] [PubMed]
- Morin, O.; Chen, W.; Nassiri, F.; Susko, M.; Magill, S.T.; Vasudevan, H.N.; Wu, A.; Vallières, M.; Gennatas, E.D.; Valdes, G.; et al. Integrated models incorporating radiologic and radiomic features predict meningioma grade, local failure, and overall survival. Neuro-Oncol. Adv. 2019, 1, vdz011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gennatas, E.D.; Wu, A.; Braunstein, S.E.; Morin, O.; Chen, W.C.; Magill, S.T.; Gopinath, C.; Villaneueva-Meyer, J.E.; Perry, A.; McDermott, M.W.; et al. Preoperative and postoperative prediction of long-term meningioma outcomes. PLoS ONE 2018, 13, e0204161. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Chen, J.-H.; Chen, T.-Y.; Lim, S.-W.; Wu, T.-C.; Kuo, Y.-T.; Ko, C.-C.; Su, M.-Y. Radiomics approach for prediction of recurrence in skull base meningiomas. Neuroradiology 2019, 61, 1355–1364. [Google Scholar] [CrossRef]
- Hale, A.T.; Stonko, D.P.; Wang, L.; Strother, M.K.; Chambless, L.B. Machine learning analyses can differentiate meningioma grade by features on magnetic resonance imaging. Neurosurg. Focus 2018, 45, E4. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Man, C.; Gong, L.; Dong, D.; Yu, X.; Wang, S.; Fang, M.; Wang, S.; Fang, X.; Chen, X.; et al. A deep learning radiomics model for preoperative grading in meningioma. Eur. J. Radiol. 2019, 116, 128–134. [Google Scholar] [CrossRef]
- Chen, C.; Guo, X.; Wang, J.; Guo, W.; Ma, X.; Xu, J. The Diagnostic Value of Radiomics-Based Machine Learning in Predicting the Grade of Meningiomas Using Conventional Magnetic Resonance Imaging: A Preliminary Study. Front. Oncol. 2019, 9, 1338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, H.; Lin, X.; He, J.; Pang, P.; Fan, B.; Lei, P.; Guo, D.; Ye, C. Value of MRI Radiomics Based on Enhanced T1WI Images in Prediction of Meningiomas Grade. Acad. Radiol. 2021, 28, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Laukamp, K.R.; Thiele, F.; Shakirin, G.; Zopfs, D.; Faymonville, A.; Timmer, M.; Maintz, D.; Perkuhn, M.; Borggrefe, J. Fully automated detection and segmentation of meningiomas using deep learning on routine multiparametric MRI. Eur. Radiol. 2019, 29, 124–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Won, S.Y.; Park, Y.W.; Ahn, S.S.; Moon, J.H.; Kim, E.H.; Kang, S.-G.; Chang, J.H.; Kim, S.H.; Lee, S.-K. Quality assessment of meningioma radiomics studies: Bridging the gap between exploratory research and clinical applications. Eur. J. Radiol. 2021, 138, 109673. [Google Scholar] [CrossRef] [PubMed]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G.M. Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD): The TRIPOD Statement. Br. J. Surg. 2015, 102, 148–158. [Google Scholar] [CrossRef] [Green Version]
- Zwanenburg, A.; Vallières, M.; Abdalah, M.A.; Aerts, H.J.W.L.; Andrearczyk, V.; Apte, A.; Ashrafinia, S.; Bakas, S.; Beukinga, R.J.; Boellaard, R.; et al. The Image Biomarker Standardization Initiative: Standardized Quantitative Radiomics for High-Throughput Image-based Phenotyping. Radiology 2020, 295, 328–338. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brunasso, L.; Ferini, G.; Bonosi, L.; Costanzo, R.; Musso, S.; Benigno, U.E.; Gerardi, R.M.; Giammalva, G.R.; Paolini, F.; Umana, G.E.; et al. A Spotlight on the Role of Radiomics and Machine-Learning Applications in the Management of Intracranial Meningiomas: A New Perspective in Neuro-Oncology: A Review. Life 2022, 12, 586. https://doi.org/10.3390/life12040586
Brunasso L, Ferini G, Bonosi L, Costanzo R, Musso S, Benigno UE, Gerardi RM, Giammalva GR, Paolini F, Umana GE, et al. A Spotlight on the Role of Radiomics and Machine-Learning Applications in the Management of Intracranial Meningiomas: A New Perspective in Neuro-Oncology: A Review. Life. 2022; 12(4):586. https://doi.org/10.3390/life12040586
Chicago/Turabian StyleBrunasso, Lara, Gianluca Ferini, Lapo Bonosi, Roberta Costanzo, Sofia Musso, Umberto E. Benigno, Rosa M. Gerardi, Giuseppe R. Giammalva, Federica Paolini, Giuseppe E. Umana, and et al. 2022. "A Spotlight on the Role of Radiomics and Machine-Learning Applications in the Management of Intracranial Meningiomas: A New Perspective in Neuro-Oncology: A Review" Life 12, no. 4: 586. https://doi.org/10.3390/life12040586
APA StyleBrunasso, L., Ferini, G., Bonosi, L., Costanzo, R., Musso, S., Benigno, U. E., Gerardi, R. M., Giammalva, G. R., Paolini, F., Umana, G. E., Graziano, F., Scalia, G., Sturiale, C. L., Di Bonaventura, R., Iacopino, D. G., & Maugeri, R. (2022). A Spotlight on the Role of Radiomics and Machine-Learning Applications in the Management of Intracranial Meningiomas: A New Perspective in Neuro-Oncology: A Review. Life, 12(4), 586. https://doi.org/10.3390/life12040586












