Abbreviated MR Protocols in Prostate MRI
Abstract
:1. Introduction
2. Minimum Requirements for Multiparametric Prostate MRI
- A T2-weighted axial plane and at least one additional T2-weighted sequence in an orthogonal plane (i.e., sagittal or coronal) with a slice thickness of 3 mm (no gap), a Field of View (FoV) of 12–20 cm, and an in-plane resolution ≤0.7 × 0.4 mm;
- A diffusion-weighted sequence with at least one low b-value (preferably 50–100 s/mm²) and one intermediate b-value at 800–1000 s/mm². The “high b-value image” required for analysis (≥1400 s/mm²) can then be separately acquired or calculated from the two lower b-values. The slice thickness should be ≤4 mm (no gap), FoV of 16–22 cm, and an in-plane resolution ≤2.5 × 2.5 mm;
- A 3D T1-weighted gradient echo sequence (slice thickness 3 mm, no gap) with injection of a contrast agent, a temporal resolution ≤15 s, and a total observation time span of ≥2 min.
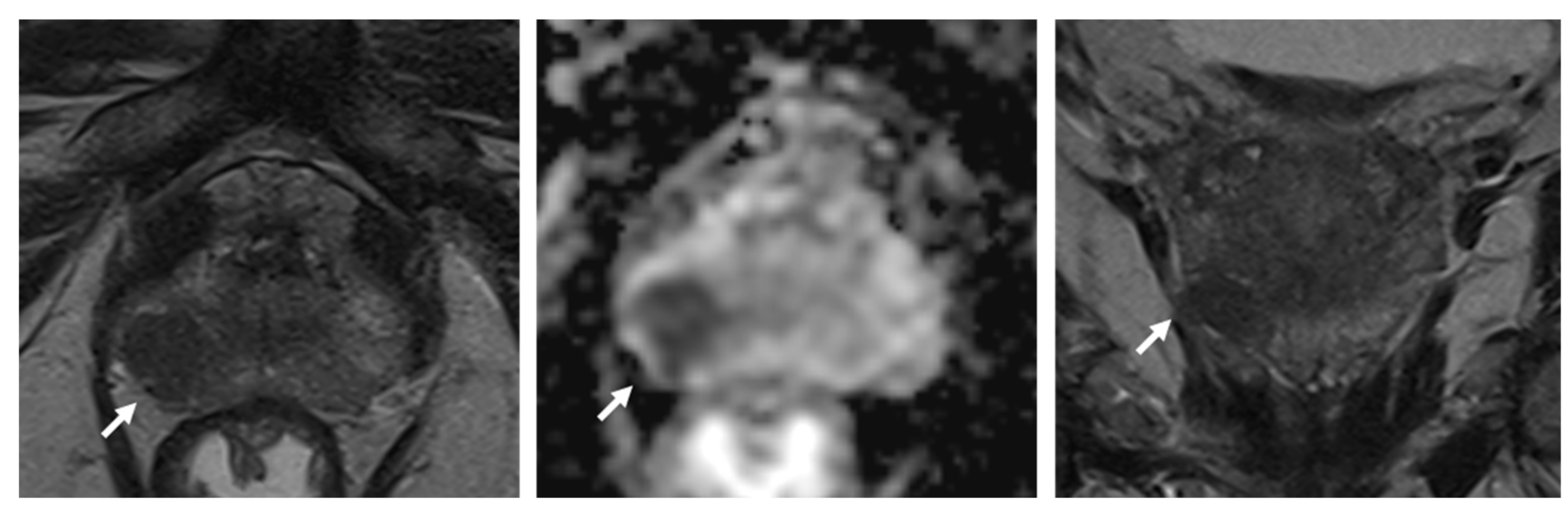
3. Areas of Possible Protocol Abbreviations
3.1. Use of Dynamic Contrast-Enhanced Sequences
3.2. Shortening T2-Weighted Acquisition Times
3.3. Shortening Diffusion-Weighted Imaging Acquisition Times
3.4. Optimizing Prostate MRI Workflow
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Kasivisvanathan, V.; Rannikko, A.S.; Borghi, M.; Panebianco, V.; Mynderse, L.A.; Vaarala, M.H.; Briganti, A.; Budäus, L.; Hellawell, G.; Hindley, R.G.; et al. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N. Engl. J. Med. 2018, 378, 1767–1777. [Google Scholar] [CrossRef]
- Ahmed, H.U.; El-Shater Bosaily, A.; Brown, L.C.; Gabe, R.; Kaplan, R.; Parmar, M.K.; Collaco-Moraes, Y.; Ward, K.; Hindley, R.G.; Freeman, A.; et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): A paired validating confirmatory study. Lancet 2017, 389, 815–822. [Google Scholar] [CrossRef] [Green Version]
- Turkbey, B.; Pinto, P.A.; Mani, H.; Bernardo, M.; Pang, Y.; McKinney, Y.L.; Khurana, K.; Ravizzini, G.C.; Albert, P.S.; Merino, M.J.; et al. Prostate cancer: Value of multiparametric MR imaging at 3 T for detection--histopathologic correlation. Radiology 2010, 255, 89–99. [Google Scholar] [CrossRef] [Green Version]
- Giganti, F.; Kirkham, A.; Allen, C.; Punwani, S.; Orczyk, C.; Emberton, M.; Moore, C.M. Update on Multiparametric Prostate MRI During Active Surveillance: Current and Future Trends and Role of the PRECISE Recommendations. Am. J. Roentgenol. 2021, 216, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Kortenbach, K.-C.; Boesen, L.; Løgager, V.; Thomsen, H.S. For men enrolled in active surveillance, pre-biopsy biparametric magnetic resonance imaging significantly reduces the risk of reclassification and disease progression after 1 year. Scand. J. Urol. 2021, 55, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Eldred-Evans, D.; Burak, P.; Connor, M.J.; Day, E.; Evans, M.; Fiorentino, F.; Gammon, M.; Hosking-Jervis, F.; Klimowska-Nassar, N.; McGuire, W.; et al. Population-Based Prostate Cancer Screening with Magnetic Resonance Imaging or Ultrasonography: The IP1-PROSTAGRAM Study. JAMA Oncol. 2021, 7, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Turkbey, B.; Rosenkrantz, A.B.; Haider, M.A.; Padhani, A.R.; Villeirs, G.; Macura, K.J.; Tempany, C.M.; Choyke, P.L.; Cornud, F.; Margolis, D.J.; et al. Prostate Imaging Reporting and Data System Version 2.1: 2019 Update of Prostate Imaging Reporting and Data System Version 2. Eur. Urol. 2019, 76, 340–351. [Google Scholar] [CrossRef]
- Mottet, N.; van den Bergh, R.C.; Briers, E.; van den Broeck, T.; Cumberbatch, M.G.; de Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer—2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef]
- Rosenkrantz, A.B.; Hemingway, J.; Hughes, D.R.; Duszak, R., Jr.; Allen, B., Jr.; Weinreb, J.C. Evolving Use of Prebiopsy Prostate Magnetic Resonance Imaging in the Medicare Population. J. Urol. 2018, 200, 89–94. [Google Scholar] [CrossRef]
- Liu, W.; Patil, D.; Howard, D.H.; Moore, R.H.; Wang, H.; Sanda, M.G.; Filson, C.P. Adoption of Prebiopsy Magnetic Resonance Imaging for Men Undergoing Prostate Biopsy in the United States. Urology 2018, 117, 57–63. [Google Scholar] [CrossRef]
- Vargas, H.A.; Hötker, A.M.; Goldman, D.A.; Moskowitz, C.S.; Gondo, T.; Matsumoto, K.; Ehdaie, B.; Woo, S.; Fine, S.W.; Reuter, V.E.; et al. Updated prostate imaging reporting and data system (PIRADS v2) recommendations for the detection of clinically significant prostate cancer using multiparametric MRI: Critical evaluation using whole-mount pathology as standard of reference. Eur. Radiol. 2016, 26, 1606–1612. [Google Scholar] [CrossRef] [Green Version]
- Kuhl, C.K.; Bruhn, R.; Krämer, N.; Nebelung, S.; Heidenreich, A.; Schrading, S. Abbreviated Biparametric Prostate MR Imaging in Men with Elevated Prostate-specific Antigen. Radiology 2017, 285, 493–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porter, K.K.; King, A.; Galgano, S.J.; Sherrer, R.L.; Gordetsky, J.B.; Rais-Bahrami, S. Financial implications of biparametric prostate MRI. Prostate Cancer Prostatic Dis. 2020, 23, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, A.H.; Zhao, Y.; Farooq, Z.; Prince, M.R. Immediate Allergic Reactions to Gadolinium-based Contrast Agents: A Systematic Review and Meta-Analysis. Radiology 2018, 286, 471–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulani, V.; Calamante, F.; Shellock, F.G.; Kanal, E.; Reeder, S.B. Gadolinium deposition in the brain: Summary of evidence and recommendations. Lancet Neurol. 2017, 16, 564–570. [Google Scholar] [CrossRef]
- Woo, S.; Suh, C.H.; Kim, S.Y.; Cho, J.Y.; Kim, S.H.; Moon, M.H. Head-to-Head Comparison between Biparametric and Multiparametric MRI for the Diagnosis of Prostate Cancer: A Systematic Review and Meta-Analysis. Am. J. Roentgenol. 2018, 211, W226–W241. [Google Scholar] [CrossRef]
- Kang, Z.; Min, X.; Weinreb, J.; Li, Q.; Feng, Z.; Wang, L. Abbreviated Biparametric Versus Standard Multiparametric MRI for Diagnosis of Prostate Cancer: A Systematic Review and Meta-Analysis. Am. J. Roentgenol. 2019, 212, 357–365. [Google Scholar] [CrossRef]
- Alabousi, M.; Salameh, J.-P.; Gusenbauer, K.; Samoilov, L.; Jafri, A.; Yu, H.; Alabousi, A. Biparametric vs multiparametric prostate magnetic resonance imaging for the detection of prostate cancer in treatment-naïve patients: A diagnostic test accuracy systematic review and meta-analysis. BJU Int. 2019, 124, 209–220. [Google Scholar] [CrossRef]
- Bosaily, A.E.-S.; Frangou, E.; Ahmed, H.U.; Emberton, M.; Punwani, S.; Kaplan, R.; Brown, L.C.; Freeman, A.; Jameson, C.; Hindley, R.; et al. Additional Value of Dynamic Contrast-enhanced Sequences in Multiparametric Prostate Magnetic Resonance Imaging: Data from the PROMIS Study. Eur. Urol. 2020, 78, 503–511. [Google Scholar] [CrossRef]
- Junker, D.; Steinkohl, F.; Fritz, V.; Bektic, J.; Tokas, T.; Aigner, F.; Herrmann, T.R.W.; Rieger, M.; Nagele, U. Comparison of multiparametric and biparametric MRI of the prostate: Are gadolinium-based contrast agents needed for routine examinations? World J. Urol. 2019, 37, 691–699. [Google Scholar] [CrossRef]
- Al Salmi, I.; Menezes, T.; El-Khodary, M.; Monteiro, S.; Haider, E.A.; Alabousi, A. Prospective evaluation of the value of dynamic contrast enhanced (DCE) imaging for prostate cancer detection, with pathology correlation. Can. J. Urol. 2020, 27, 10220–10227. [Google Scholar] [PubMed]
- Tamada, T.; Kido, A.; Yamamoto, A.; Takeuchi, M.; Miyaji, Y.; Moriya, T.; Sone, T. Comparison of Biparametric and Multiparametric MRI for Clinically Significant Prostate Cancer Detection with PI-RADS Version 2.1. J. Magn. Reson. Imaging 2021, 53, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Christophe, C.; Montagne, S.; Bourrelier, S.; Roupret, M.; Barret, E.; Rozet, F.; Comperat, E.; Coté, J.F.; Lucidarme, O.; Cussenot, O.; et al. Prostate cancer local staging using biparametric MRI: Assessment and comparison with multiparametric MRI. Eur. J. Radiol. 2020, 132, 109350. [Google Scholar] [CrossRef] [PubMed]
- Kamsut, S.; Reid, K.; Tan, N. Roundtable: Arguments in support of using multi-parametric prostate MRI protocol. Abdom. Radiol. 2020, 45, 3990–3996. [Google Scholar] [CrossRef]
- Padhani, A.R.; Schoots, I.; Villeirs, G. Contrast Medium or No Contrast Medium for Prostate Cancer Diagnosis. That Is the Question. J. Magn. Reson. Imaging 2021, 53, 13–22. [Google Scholar] [CrossRef]
- Schoots, I.G.; Barentsz, J.O.; Bittencourt, L.K.; Haider, M.A.; Macura, K.J.; Margolis, D.J.A.; Moore, C.M.; Oto, A.; Panebianco, V.; Siddiqui, M.M.; et al. PI-RADS Committee Position on MRI without Contrast Medium in Biopsy-Naive Men with Suspected Prostate Cancer: Narrative Review. Am. J. Roentgenol. 2021, 216, 3–19. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, G.; Shi, B.; Liu, Y.; Zou, T.; Yan, W.; Xiao, Y.; Xue, H.; Feng, F.; Lei, J.; et al. Comparison of biparametric and multiparametric MRI in the diagnosis of prostate cancer. Cancer Imaging 2019, 19, 90. [Google Scholar] [CrossRef]
- Gatti, M.; Faletti, R.; Calleris, G.; Giglio, J.; Berzovini, C.; Gentile, F.; Marra, G.; Misischi, F.; Molinaro, L.; Bergamasco, L.; et al. Prostate cancer detection with biparametric magnetic resonance imaging (bpMRI) by readers with different experience: Performance and comparison with multiparametric (mpMRI). Abdom. Radiol. 2019, 44, 1883–1893. [Google Scholar] [CrossRef]
- Muller, B.G.; van den Bos, W.; Brausi, M.; Fütterer, J.J.; Ghai, S.; Pinto, P.A.; Popeneciu, I.V.; de Reijke, T.M.; Robertson, C.; de la Rosette, J.J.M.C.H.; et al. Follow-up modalities in focal therapy for prostate cancer: Results from a Delphi consensus project. World J. Urol. 2015, 33, 1503–1509. [Google Scholar] [CrossRef]
- Kitajima, K.; Hartman, R.P.; Froemming, A.T.; Hagen, C.E.; Takahashi, N.; Kawashima, A. Detection of Local Recurrence of Prostate Cancer After Radical Prostatectomy Using Endorectal Coil MRI at 3 T: Addition of DWI and Dynamic Contrast Enhancement to T2-Weighted MRI. Am. J. Roentgenol. 2015, 205, 807–816. [Google Scholar] [CrossRef] [Green Version]
- Valle, L.F.; Greer, M.D.; Shih, J.H.; Barrett, T.; Law, Y.M.; Rosenkrantz, A.B.; Shebel, H.; Muthigi, A.; Su, D.; Merino, M.J.; et al. Multiparametric MRI for the detection of local recurrence of prostate cancer in the setting of biochemical recurrence after low dose rate brachytherapy. Diagn. Interv. Radiol. 2018, 24, 46–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abd-Alazeez, M.; Ramachandran, N.; Dikaios, N.; Ahmed, H.U.; Emberton, M.; Kirkham, A.; Arya, M.; Taylor, S.; Halligan, S.; Punwani, S. Multiparametric MRI for detection of radiorecurrent prostate cancer: Added value of apparent diffusion coefficient maps and dynamic contrast-enhanced images. Prostate Cancer Prostatic Dis. 2015, 18, 128–136. [Google Scholar] [CrossRef]
- Lotte, R.; Lafourcade, A.; Mozer, P.; Conort, P.; Barret, E.; Comperat, E.; Ezziane, M.; de Guibert, P.-H.J.; Tavolaro, S.; Belin, L.; et al. Multiparametric MRI for Suspected Recurrent Prostate Cancer after HIFU:Is DCE still needed? Eur. Radiol. 2018, 28, 3760–3769. [Google Scholar] [CrossRef] [PubMed]
- Luzurier, A.; Jouve De Guibert, P.-H.; Allera, A.; Feldman, S.F.; Conort, P.; Simon, J.M.; Mozer, P.; Compérat, E.; Boudghene, F.; Servois, V.; et al. Dynamic contrast-enhanced imaging in localizing local recurrence of prostate cancer after radiotherapy: Limited added value for readers of varying level of experience. J. Magn. Reson. Imaging 2018, 48, 1012–1023. [Google Scholar] [CrossRef]
- Bae, H.; Cho, N.H.; Park, S.Y. PI-RADS version 2: Optimal time range for determining positivity of dynamic contrast-enhanced MRI in peripheral zone prostate cancer. Clin. Radiol. 2019, 74, e27–e895. [Google Scholar] [CrossRef] [PubMed]
- Hötker, A.M.; Da Mutten, R.; Tiessen, A.; Konukoglu, E.; Donati, O.F. Improving workflow in prostate MRI: AI-based decision-making on biparametric or multiparametric MRI. Insights Imaging 2021, 12, 112. [Google Scholar] [CrossRef]
- van der Leest, M.; Israël, B.; Cornel, E.B.; Zámecnik, P.; Schoots, I.G.; van der Lelij, H.; Padhani, A.R.; Rovers, M.; van Oort, I.; Sedelaar, M.; et al. High Diagnostic Performance of Short Magnetic Resonance Imaging Protocols for Prostate Cancer Detection in Biopsy-naïve Men: The Next Step in Magnetic Resonance Imaging Accessibility. Eur. Urol. 2019, 76, 574–581. [Google Scholar] [CrossRef]
- Barth, B.K.; De Visschere, P.J.L.; Cornelius, A.; Nicolau, C.; Vargas, H.A.; Eberli, D.; Donati, O.F. Detection of Clinically Significant Prostate Cancer: Short Dual-Pulse Sequence versus Standard Multiparametric MR Imaging-A Multireader Study. Radiology 2017, 284, 725–736. [Google Scholar] [CrossRef]
- Weiss, J.; Martirosian, P.; Notohamiprodjo, M.; Kaufmann, S.; Othman, A.E.; Grosse, U.; Nikolaou, K.; Gatidis, S. Implementation of a 5-Minute Magnetic Resonance Imaging Screening Protocol for Prostate Cancer in Men with Elevated Prostate-Specific Antigen Before Biopsy. Investig. Radiol. 2018, 53, 186–190. [Google Scholar] [CrossRef]
- Polanec, S.H.; Lazar, M.; Wengert, G.J.; Bickel, H.; Spick, C.; Susani, M.; Shariat, S.; Clauser, P.; Baltzer, P.A.T. 3D T2-weighted imaging to shorten multiparametric prostate MRI protocols. Eur. Radiol. 2018, 28, 1634–1641. [Google Scholar] [CrossRef] [Green Version]
- Rosenkrantz, A.B.; Neil, J.; Kong, X.; Melamed, J.; Babb, J.S.; Taneja, S.S.; Taouli, B. Prostate cancer: Comparison of 3D T2-weighted with conventional 2D T2-weighted imaging for image quality and tumor detection. Am. J. Roentgenol. 2010, 194, 446–452. [Google Scholar] [CrossRef]
- Vidya Shankar, R.; Roccia, E.; Cruz, G.; Neji, R.; Botnar, R.; Prezzi, D.; Goh, V.; Prieto, C.; Dregely, I. Accelerated 3D T(2) w-imaging of the prostate with 1-mm isotropic resolution in less than 3 min. Magn. Reson. Med. 2019, 82, 721–731. [Google Scholar] [CrossRef] [Green Version]
- Caglic, I.; Povalej Brzan, P.; Warren, A.Y.; Bratt, O.; Shah, N.; Barrett, T. Defining the incremental value of 3D T2-weighted imaging in the assessment of prostate cancer extracapsular extension. Eur. Radiol. 2019, 29, 5488–5497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gassenmaier, S.; Afat, S.; Nickel, D.; Mostapha, M.; Herrmann, J.; Othman, A.E. Deep learning-accelerated T2-weighted imaging of the prostate: Reduction of acquisition time and improvement of image quality. Eur. J. Radiol. 2021, 137, 109600. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Ohno, Y.; Yamamoto, K.; Murayama, K.; Ikedo, M.; Yui, M.; Hanamatsu, S.; Tanaka, Y.; Obama, Y.; Ikeda, H.; et al. Deep Learning Reconstruction of Diffusion-weighted MRI Improves Image Quality for Prostatic Imaging. Radiology 2022, 204097. [Google Scholar] [CrossRef] [PubMed]
- Jendoubi, S.; Wagner, M.; Montagne, S.; Ezziane, M.; Mespoulet, J.; Comperat, E.; Estellat, C.; Baptiste, A.; Renard-Penna, R. MRI for prostate cancer: Can computed high b-value DWI replace native acquisitions? Eur. Radiol. 2019, 29, 5197–5204. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Hötker, A.M.; Muehlematter, U.J.; Burger, I.A.; Donati, O.F.; Barth, B.K. Value of bowel preparation techniques for prostate MRI: A preliminary study. Abdom. Radiol. 2021, 46, 4002–4013. [Google Scholar] [CrossRef]
- Brennan, D.L.; Lazarakis, S.; Lee, A.; Tan, T.H.; Chin, K.Y.; Oon, S.F. Do antispasmodics or rectal enemas improve image quality on multiparametric prostate MRI? An ‘Evidence-Based Practice’ review of the literature. Abdom. Radiol. 2021, 46, 2770–2778. [Google Scholar] [CrossRef]
- Barth, B.K.; Cornelius, A.; Nanz, D.; Eberli, D.; Donati, O.F. Diffusion-Weighted Imaging of the Prostate: Image Quality and Geometric Distortion of Readout-Segmented Versus Selective-Excitation Accelerated Acquisitions. Investig. Radiol. 2015, 50, 785–791. [Google Scholar] [CrossRef] [Green Version]
- Stocker, D.; Manoliu, A.; Becker, A.S.; Barth, B.K.; Nanz, D.; Klarhöfer, M.; Donati, O.F. Image Quality and Geometric Distortion of Modern Diffusion-Weighted Imaging Sequences in Magnetic Resonance Imaging of the Prostate. Investig. Radiol. 2018, 53, 200–206. [Google Scholar] [CrossRef] [Green Version]
- Gaur, S.; Lay, N.; Harmon, S.A.; Doddakashi, S.; Mehralivand, S.; Argun, B.; Barrett, T.; Bednarova, S.; Girometti, R.; Karaarslan, E.; et al. Can computer-aided diagnosis assist in the identification of prostate cancer on prostate MRI? a multi-center, multi-reader investigation. Oncotarget 2018, 9, 33804–33817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannini, V.; Mazzetti, S.; Armando, E.; Carabalona, S.; Russo, F.; Giacobbe, A.; Muto, G.; Regge, D. Multiparametric magnetic resonance imaging of the prostate with computer-aided detection: Experienced observer performance study. Eur. Radiol. 2017, 27, 4200–4208. [Google Scholar] [CrossRef]
- Zhu, L.; Gao, G.; Liu, Y.; Han, C.; Liu, J.; Zhang, X.; Wang, X. Feasibility of integrating computer-aided diagnosis with structured reports of prostate multiparametric MRI. Clin. Imaging 2020, 60, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Winkel, D.J.; Tong, A.; Lou, B.; Kamen, A.; Comaniciu, D.; Disselhorst, J.A.; Rodríguez-Ruiz, A.; Huisman, H.; Szolar, D.; Shabunin, I.; et al. A Novel Deep Learning Based Computer-Aided Diagnosis System Improves the Accuracy and Efficiency of Radiologists in Reading Biparametric Magnetic Resonance Images of the Prostate: Results of a Multireader, Multicase Study. Investig. Radiol. 2021, 56, 605–613. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hötker, A.M.; Vargas, H.A.; Donati, O.F. Abbreviated MR Protocols in Prostate MRI. Life 2022, 12, 552. https://doi.org/10.3390/life12040552
Hötker AM, Vargas HA, Donati OF. Abbreviated MR Protocols in Prostate MRI. Life. 2022; 12(4):552. https://doi.org/10.3390/life12040552
Chicago/Turabian StyleHötker, Andreas M., Hebert Alberto Vargas, and Olivio F. Donati. 2022. "Abbreviated MR Protocols in Prostate MRI" Life 12, no. 4: 552. https://doi.org/10.3390/life12040552
APA StyleHötker, A. M., Vargas, H. A., & Donati, O. F. (2022). Abbreviated MR Protocols in Prostate MRI. Life, 12(4), 552. https://doi.org/10.3390/life12040552





