Thermal Boost to Breast Tumor Bed—New Technique Description, Treatment Application and Example Clinical Results
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. MW Thermal Boost Planning
- Regular interstitial application is performed according to the GEC-ESTRO recommendations [30], with local institutional adjustments regarding accessible equipment (pre-BCT mammography and USG, complete post-surgical pathological report, EBRT planning CT, clinical examination, C-arm X-ray in the operating room, intraoperative USG).
- CT scanning with ≤3 mm thick layers.
- Regular 3D initial brachytherapy plan preparation in the TPS: structure reconstruction (patient body, lungs, heart, skin, chest wall, ribs, surgical clips, skin scar) and CTV—visible tumor bed (postoperative scarification) plus adequate safety margin according to pathological report and recommendations, as well as applicator reconstruction (Figure 2A,B and Figure 3A,B).
- The most critical stage is selection of the proper applicator to be inserted with MW antennas and thermometers (Scheme 1 and Scheme 2):
- −
- Three-dimensional presentation of the breast and interstitial application need to be inspected and assessed to determine if thermal boost is possible in a particular case;
- −
- One needs to answer the question of whether the applicators are implanted in a regular, equidistant, and parallel manner and whether the insertion of 4–6 antennas is possible while leaving free applicators for temperature measurement between antennas;
- −
- −
- One must make sure that the expected antenna configuration fits the tumor bed delineated as CTV in the TPS; this is the stage when thermal boost volume is adjusted to the brachytherapy boost volume, and it is only possible when based on a clinical inspection by the planning physician;
- −
- Referring to the scheme (Scheme 1, middle and right; Figure 2D and Figure 3D), applicators for antennas are selected to best cover the CTV, and then applicators for thermal probes are chosen to ensure relevant temperature monitoring, especially in spots anticipated to be the hottest (e.g., Scheme 1, right, applicator number 5);
- −
- Next, it is mandatory to define the exact positions of each antenna and thermometer in a dedicated applicator. For that purpose, in the TPS, the physicist activates source positions in each applicator protruding from the CTV (Figure 2C and Figure 3C):
- −
- −
- Then, the distance from the applicator tip end to the tip end of the antenna has to be measured and noted for further reference; the same process is performed for all antennas;
- −
- Having chosen the thermometer-dedicated applicators (Figure 2E and Figure 3E, green capital letter T), the planners decide on the thermal probe (sensor) tip position in the applicator lumen. The probe tip should be positioned in the middle of neighboring antennas’ active lengths, where the highest temperature is expected to be measured. In addition, according to recommendations [28,29], planners should provide sensors on the periphery (e.g., Scheme 1, right, applicator numbers 8, 12, 14, 15). The BSD-500 provides up to 8 sensors. Due to the limited number, their positions should be effectively chosen with care.
- −
- Then, the distance from the applicator tip end to the tip end of the probe has to be measured and noted for further reference; the same process is performed for all thermal probes.
- The HT session plan is ready.
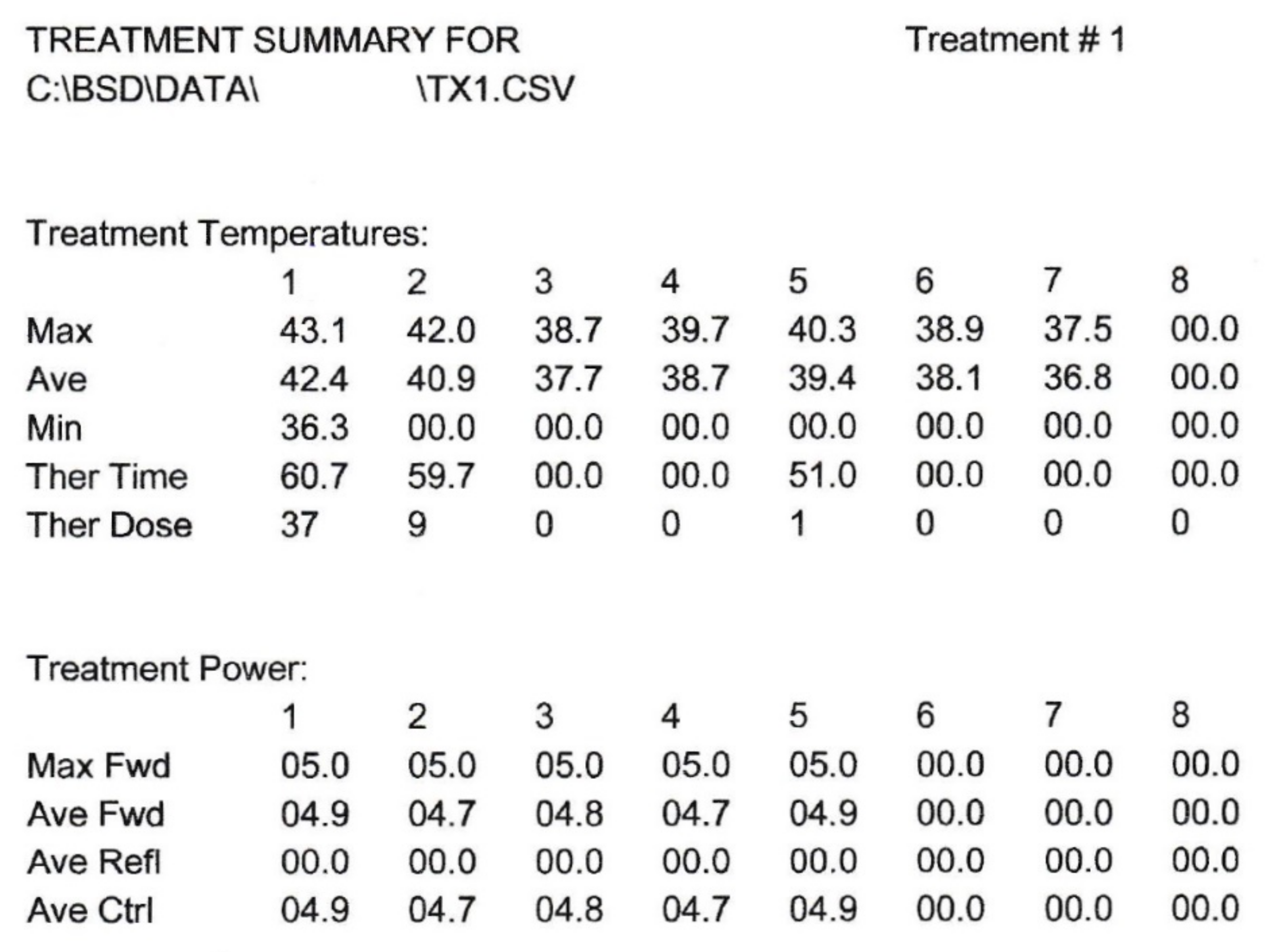
2.3. MW Thermal Boost Delivery




2.4. HDR Brachytherapy Boost Delivery
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fisher, B.; Anderson, S.; Bryant, J.; Margolese, R.G.; Deutsch, M.; Fisher, E.R.; Jeong, J.H.; Wolmark, N. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N. Engl. J. Med. 2002, 347, 1233–1341. [Google Scholar] [CrossRef] [PubMed]
- Darby, S.; McGale, P.; Correa, C.; Taylor, C.; Arriagada, R.; Clarke, M.; Cutter, D.; Davies, C.; Ewertz, M.; Godwin, J.; et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10,801 women in 17 randomized trials. Lancet 2011, 378, 1707–1716. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, U.; Cacinelli, N.; Mariani, L.; Greco, M.; Saccozzi, R.; Luini, A.; Aguilar, M.; Marubini, E. Twenty-year follow-up of a randomized trial comparing breast-conserving surgery with radical mastectomy for early breast cancer. N. Engl. J. Med. 2002, 347, 1227–1232. [Google Scholar] [CrossRef] [PubMed]
- Bartelink, H.; Horiot, J.C.; Poortmans, P.; Struikmans, H.; Bogaert, W.V.D.; Barillot, I.; Fourquet, A.; Borger, J.; Jager, J.; Hoogenraad, W.; et al. Recurrence rates after treatment of breast cancer with standard radiotherapy with or without additional radiation. N. Engl. J. Med. 2001, 345, 1378–1387. [Google Scholar] [CrossRef]
- Beaton, L.; Chan, E.K.; Tyldesley, S.; Gondara, L.; Speers, C.; Nichol, A. In the Era After the European Organisation for Research and Treatment of Cancer ‘Boost’ Study, is the Additional Radiotherapy to the Breast Tumour Bed Still Beneficial for Young Women? Clin. Oncol. 2020, 32, 373–381. [Google Scholar] [CrossRef]
- Hammer, J.; Mazeron, J.J.; van Limbergen, E. Breast boost—Why, how, when? Strahlenther. Onkol. 2001, 177, 33–36. [Google Scholar] [CrossRef]
- Skowronek, J.; Chicheł, A. Brachytherapy in breast cancer: An effective alternative. Prz. Menopauzalny 2014, 13, 48–55. [Google Scholar] [CrossRef]
- Polgár, C.; Major, T. Current status and perspectives of brachytherapy for breast cancer. Int. J. Clin. Oncol. 2009, 14, 7–24. [Google Scholar] [CrossRef]
- Polgár, C.; Jánváry, L.; Major, T.; Somogyi, A.; Takácsi-Nagy, Z.; Fröhlich, G.; Fodor, J. The role of high-dose-rate brachytherapy boost in breast-conserving therapy: Long-term results of the Hungarian National Institute of Oncology. Rep. Pract. Oncol. Radiother. 2010, 15, 1–7. [Google Scholar] [CrossRef]
- Medina, M.A.; Oza, G.; Sharma, A.; Arriaga, L.G.; Hernández, J.M.H.; Rotello, V.M.; Ramirez, J.T. Triple-Negative Breast Cancer: A Review of Conventional and Advanced Therapeutic Strategies. Int. J. Environ. Res. Public Health 2020, 17, 2078. [Google Scholar] [CrossRef]
- Polgár, C.; Fodor, J.; Orosz, Z.; Major, T.; Takácsi-Nagy, Z.; Mangel, L.C.; Sulyok, Z.; Somogyi, A.; Kásler, M.; Németh, G. Electron and high-dose-rate brachytherapy boost in the conservative treatment of stage I-II breast cancer first results of the randomized Budapest boost trial. Strahlenther. Onkol. 2002, 178, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Bartelink, H.; Horiot, J.C.; Poortmans, P.; Struikmans, H.; Bogaert, W.V.D.; Fourquet, A.; Jager, J.J.; Hoogenraad, W.J.; Oei, S.B.; Wárlám-Rodenhuis, C.C. Impact of a higher radiation dose on local control and survival in breast-conserving therapy of early breast cancer: 10-year results of the randomized boost vs. no boost EORTC 22881-10882 trial. J. Clin. Oncol. 2007, 25, 3259–3265. [Google Scholar] [CrossRef] [PubMed]
- Romestaing, P.; Lehingue, Y.; Carrie, C.; Coquard, R.; Montbarbon, X.; Ardiet, J.M.; Mamelle, N.; Gérard, J.P. Role of a 10-Gy boost in the conservative treatment of early breast cancer: Results of a randomized clinical trial in Lyon, France. J. Clin. Oncol. 1997, 15, 963–968. [Google Scholar] [CrossRef]
- Falk, M.H.; Issels, R.D. Hyperthermia in oncology. Int. J. Hyperther. 2001, 17, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Chicheł, A.; Skowronek, J.; Kubaszewska, M.; Kanikowski, M. Hyperthermia–Hyperthermia—Description of a method and a review of clinical applications. Rep. Pract. Oncol. Radiother. 2007, 12, 267–275. [Google Scholar] [CrossRef]
- Dewhirst, M.W.; Gibbs, F.A., Jr.; Roemer, R.B.; Samulski, T.V. Hyperthermia. In Clinical Radiation Oncology, 1st ed.; Gunderson, L.L., Tepper, J.E., Eds.; Churchill Livingstone: New York, NY, USA, 2000; Chapter 14; pp. 256–282. [Google Scholar]
- Glossary. Int. J. Hyperther. 2003, 19, 385–390. [CrossRef]
- Sneed, P.K.; Stauffer, P.R.; Li, G.C.; Stege, G.J.J. Hyperthermia. In Textbook of Radiation Oncology, 2nd ed.; Leibel, S.A., Phillips, T.L., Eds.; WB Saunders: Philadelphia, PA, USA, 2004; Chapter 70; pp. 1569–1596. [Google Scholar]
- Wust, P.; Hildebrandt, B.; Sreenivasa, G.; Rau, B.; Gellermann, J.; Riess, H.; Felix, R.; Schlag, P.M. Hyperthermia in combined treatment of cancer. Lancet Oncol. 2002, 3, 487–497. [Google Scholar] [CrossRef]
- Overgaard, J.; Horsman, M.R. Hyperthermia. In Basic Clinical Radiobiology, 2nd ed.; Steel, G.G., Ed.; Edward Arnold: London, UK, 1997; pp. 212–221. [Google Scholar]
- Jones, E.L.; Samulski, T.V.; Vujaskovic, Z.; Prosnitz, L.R.; Dewhrist, M.W. Hyperthermia. In Principles and Practise of Radiation Oncology, 4th ed.; Perez, C.A., Brady, L.W., Halperin, E.C., Schmidt-Ullrich, R.K., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2004; Chapter 24; pp. 699–735. [Google Scholar]
- Kapp, D.S.; Hahn, G.M.; Carlson, R.W. Principles of Hyperthermia. In Cancer Medicine e.5, 5th ed.; Bast, R.C., Jr., Kufe, D.W., Pollock, R.E., Weichselbaum, R.R., Holland, J.F., Frei, E., Eds.; B.C. Decker Inc.: Hamilton, ON, Canada, 2000. [Google Scholar]
- Dooley, W.C.; Vargas, H.I.; Fenn, A.J.; Tomaselli, M.B.; Harness, J.K. Focused Microwave Thermotherapy for preoperative treatment of invasive breast cancer: A review of clinical studies. Ann. Surg. Oncol. 2010, 17, 1076–1093. [Google Scholar] [CrossRef]
- Van der Zee, J. Heating the patient: A promising approach? Ann. Oncol. 2002, 13, 1173–1184. [Google Scholar] [CrossRef]
- Chicheł, A.; Skowronek, J.; Kanikowski, M. Thermal boost combined with interstitial brachytherapy in breast-conserving therapy—Assessment of early toxicity. Rep. Pract. Oncol. Radiother. 2011, 16, 87–94. [Google Scholar] [CrossRef]
- Chichel, A.; Skowronek, J. Thermal Boost Combined with HDR Brachytherapy in Breast-Conserving Therapy—A Study Update After 7-Year Follow-Up. Brachytherapy 2015, 14 (Suppl. 1), S40. [Google Scholar] [CrossRef]
- Kubaszewska, M.; Dymnicka, M.; Skowronek, J.; Chicheł, A.; Kanikowski, M. CT-image based conformal high-dose-rate brachytherapy boost in the conservative treatment of stage I-II breast cancer—Introducing the procedure. Rep. Pract. Oncol. Radiother. 2008, 13, 227–239. [Google Scholar] [CrossRef]
- Emami, B.; Stauffer, P.; Dewhirst, M.; Prionas, S.; Ryan, T.; Corry, P.; Herman, T.; Kapp, D.; Myerson, R.; Samulski, T.; et al. RTOG quality assurance guidelines for interstitial hyperthermia. Int. J. Radiat. Oncol. Biol. Phys. 1991, 20, 1117–1124. [Google Scholar] [CrossRef]
- Dobšíček Trefná, H.; Schmidt, M.; van Rhoon, G.C.; Kok, H.P.; Gordeyev, S.S.; Lamprecht, U.; Marder, D.; Nadobny, J.; Ghadjar, P.; Abdel-Rahman, S.; et al. Quality assurance guidelines for interstitial hyperthermia. Int. J. Hyperther. 2019, 36, 277–294. [Google Scholar] [CrossRef] [PubMed]
- Strnad, V.; Major, T.; Polgar, C.; Lotter, M.; Guinot, J.-L.; Gutierrez-Miguelez, C.; Galalae, R.; Van Limbergen, E.; Guix, B.; Niehoff, P.; et al. ESTRO-ACROP guideline: Interstitial multi-catheter breast brachytherapy as Accelerated Partial Breast Irradiation alone or as boost—GEC-ESTRO Breast Cancer Working Group practical recommendations. Radiother. Oncol. 2018, 128, 411–420. [Google Scholar] [CrossRef]
- Hartmann, K.A.; Audretsch, W.; Carl, U.M.; Gripp, S.; Kolotas, C.; Muskalla, K.; Rezai, M.; Schnabel, T.; Waap, I.; Zamboglou, N.; et al. Peroperative irradiation and interstitial radiotherapy-hyperthermia boost in breast tumors > or = 3 cm. The Düsseldorf experience. Strahlenther. Onkol. 1997, 173, 519–523. [Google Scholar] [CrossRef]
- Ryan, T.P.; Brace, C.L. Interstitial microwave treatment for cancer: Historical basis and current techniques in antenna design and performance. Int. J. Hyperther. 2017, 33, 3–14. [Google Scholar] [CrossRef]
- Kok, H.P.; Cressman, E.N.K.; Ceelen, W.; Brace, C.L.; Ivkov, R.; Grüll, H.; Ter Haar, G.; Wust, P.; Crezee, J. Heating technology for malignant tumors: A review. Int. J. Hyperther. 2020, 37, 711–741. [Google Scholar] [CrossRef]
- Van Vulpen, M.; Raaymakers, B.W.; Lagendijk, J.J.; Crezee, J.; de Leeuw, A.A.; van Moorselaar, J.R.; Ligtvoet, C.M.; Battermann, J.J. Three-dimensional controlled interstitial hyperthermia combined with radiotherapy for locally advanced prostate carcinoma—A feasibility study. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 116–126. [Google Scholar] [CrossRef]
- Kukiełka, A.M.; Hetnał, M.; Brandys, P.; Walasek, T.; Dąbrowski, T.; Pluta, E.; Nahajowski, D.; Kudzia, R. Interstitial hyperthermia of the prostate in combination with brachytherapy: An evaluation of feasibility and early tolerance. Strahlenther. Onkol. 2013, 189, 467–475. [Google Scholar] [CrossRef]
- Kukiełka, A.M.; Hetnał, M.; Bereza, K. Evaluation of tolerance and toxicity of high-dose-rate brachytherapy boost combined with interstitial hyperthermia for prostate cancer. Int. J. Hyperther. 2016, 32, 324–330. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kukiełka, A.M.; Strnad, V.; Stauffer, P.; Dąbrowski, T.; Hetnał, M.; Nahajowski, D.; Walasek, T.; Brandys, P.; Matys, R. Salvage brachytherapy in combination with interstitial hyperthermia for locally recurrent prostate carcinoma following external beam radiation therapy: A prospective phase II study. J. Contemp. Brachyther. 2015, 7, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Kudzia, R.; Nahajowski, D.; Kukiełka, A.M.; Dąbrowski, T.; Dybek, D.; Brandys, P.; Waligórski, M.P.R. Does microwave interstitial hyperthermia prior to high-dose-rate brachytherapy change prostate volume or therapy plan parameters? Int. J. Hyperther. 2015, 31, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Kok, H.P.; Wust, P.; Stauffer, P.R.; Bardati, F.; van Rhoon, G.C.; Crezee, J. Current state of the art of regional hyperthermia treatment planning: A review. Radiat. Oncol. 2015, 10, 196. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, K.; Maccarini, P.F.; Craciunescu, O.I.; Schlorff, J.L.; Stauffer, P.R. Thermal characteristics of ThermoBrachytherapy Surface Applicators (TBSA) for treating chest wall recurrence. Phys. Med. Biol. 2010, 55, 1949–1969. [Google Scholar] [CrossRef][Green Version]
- Gardner, R.A.; Vargas, H.I.; Block, J.B.; Vogel, C.L.; Fenn, A.J.; Kuehl, G.V.; Doval, M. Focused microwave phased array thermotherapy for primary breast cancer. Ann. Surg. Oncol. 2002, 9, 326–332. [Google Scholar] [CrossRef]
- Vargas, H.I.; Dooley, W.C.; Gardner, R.A.; Gonzalez, K.D.; Venegas, R.; Heywang-Kobrunner, S.H.; Fenn, A.J. Focused microwave phased array thermotherapy for ablation of early-stage breast cancer: Results of thermal dose escalation. Ann. Surg. Oncol. 2004, 11, 139–146. [Google Scholar] [CrossRef]
- Vargas, H.I.; Dooley, W.C.; Gardner, R.A.; Gonzalez, K.; Heywang-Köbrunner, S.; Fenn, A. Success of sentinel lymph node mapping after breast cancer ablation with focused microwave phased array thermotherapy. Am. J. Surg. 2003, 186, 330–332. [Google Scholar] [CrossRef]
- Nguyen, P.T.; Abbosh, A.; Crozier, S. Three-Dimensional Microwave Hyperthermia for Breast Cancer Treatment in a Realistic Environment Using Particle Swarm Optimization. IEEE Trans. Biomed. Eng. 2017, 64, 1335–1344. [Google Scholar] [CrossRef]

| Feature | Suitable | Unsuitable |
|---|---|---|
| Skin-to-skin distance | ≥7 cm | <7 cm |
| Applicator tip– antenna tip/end distance | ≥1 cm | <1 cm |
| Number of planes | ≥3 | <3 |
| Minimum antenna–skin distance | ≥1 cm | <1 cm |
| Antenna–rib(bone) distance | ≥1 cm | <1 cm |
| Seroma/hematoma | absent | present |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chicheł, A.; Burchardt, W.; Chyrek, A.J.; Bielęda, G.; Zwierzchowski, G.; Stefaniak, P.; Malicki, J. Thermal Boost to Breast Tumor Bed—New Technique Description, Treatment Application and Example Clinical Results. Life 2022, 12, 512. https://doi.org/10.3390/life12040512
Chicheł A, Burchardt W, Chyrek AJ, Bielęda G, Zwierzchowski G, Stefaniak P, Malicki J. Thermal Boost to Breast Tumor Bed—New Technique Description, Treatment Application and Example Clinical Results. Life. 2022; 12(4):512. https://doi.org/10.3390/life12040512
Chicago/Turabian StyleChicheł, Adam, Wojciech Burchardt, Artur J. Chyrek, Grzegorz Bielęda, Grzegorz Zwierzchowski, Patrycja Stefaniak, and Julian Malicki. 2022. "Thermal Boost to Breast Tumor Bed—New Technique Description, Treatment Application and Example Clinical Results" Life 12, no. 4: 512. https://doi.org/10.3390/life12040512
APA StyleChicheł, A., Burchardt, W., Chyrek, A. J., Bielęda, G., Zwierzchowski, G., Stefaniak, P., & Malicki, J. (2022). Thermal Boost to Breast Tumor Bed—New Technique Description, Treatment Application and Example Clinical Results. Life, 12(4), 512. https://doi.org/10.3390/life12040512







