New Insights on Formyl Peptide Receptor Type 2 Involvement in Nociceptive Processes in the Spinal Cord
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drugs and Treatment Procedure
2.2. Animals and Experimental Procedures
2.3. Hot Plate Test
2.4. Tail Flick Test
2.5. Formalin Test
2.6. Carrageenan-Induced Thermal Hyperalgesia
2.7. Neuropathic Thermal Hyperalgesia
2.8. Cytokines and BDNF Assays
2.9. Data Analysis and Statistics
3. Results
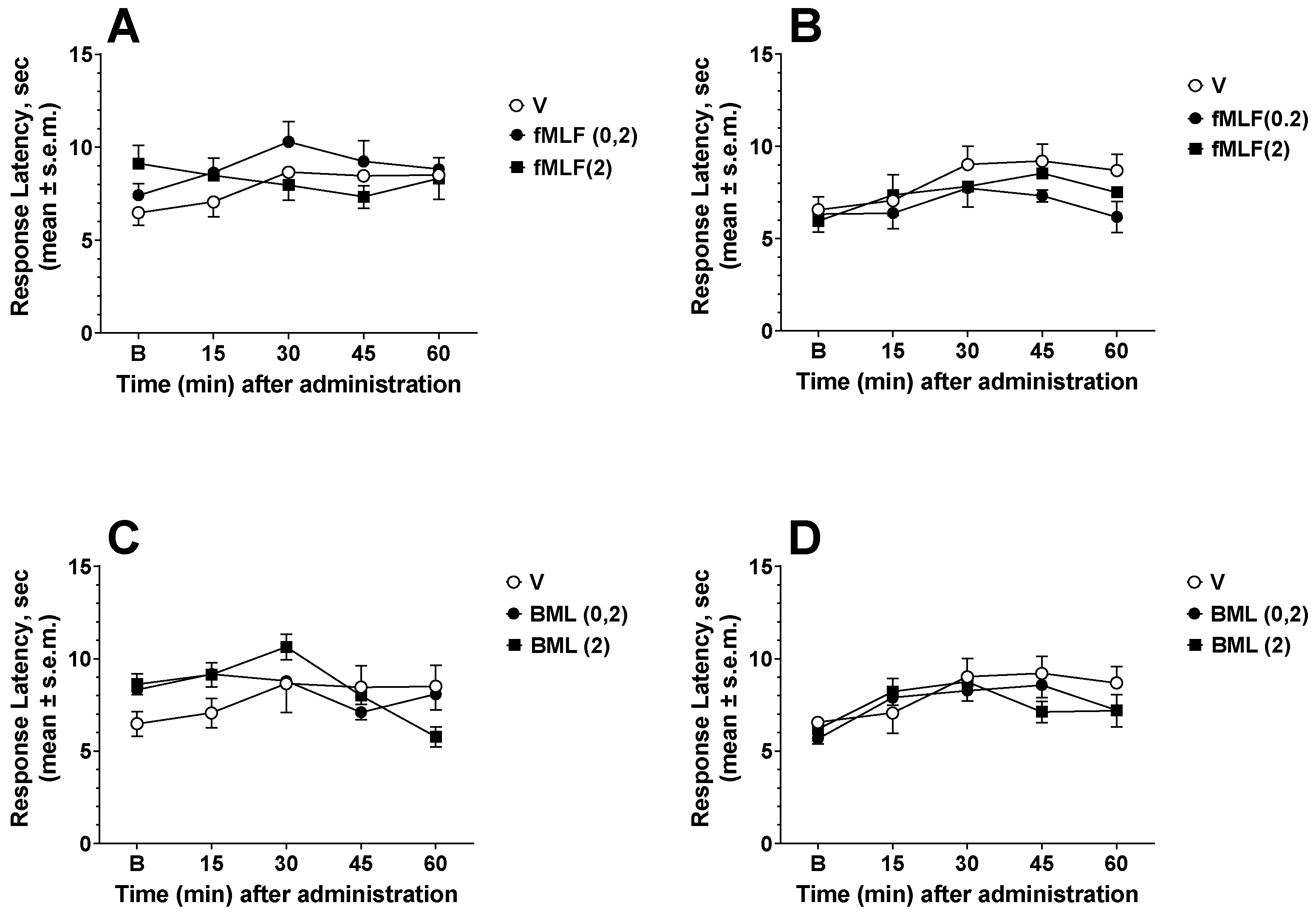
3.1. Effects of FPR Agonists and Antagonists in the Hot Plate and Tail Flick Tests
3.2. Effects of FPR Agonists and Antagonists in the Formalin Test
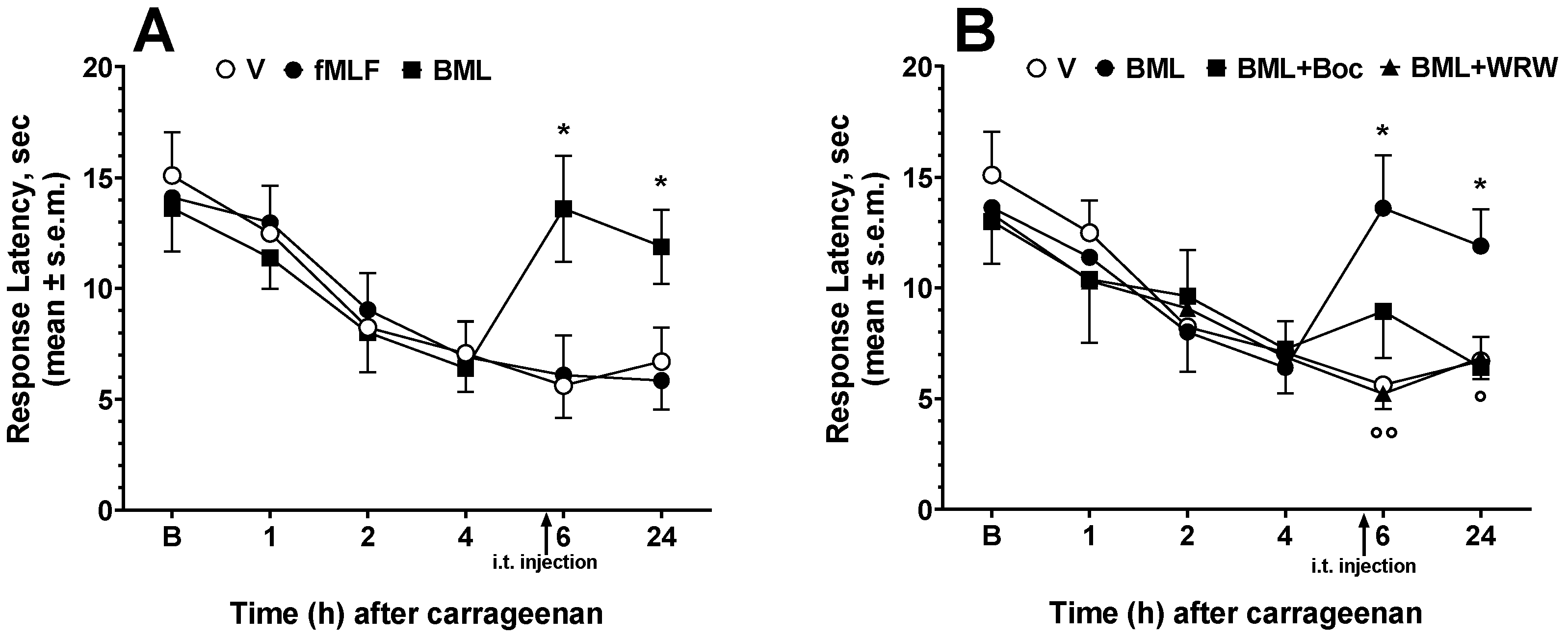
3.3. Effects of FPR Agonists and Antagonists on Carrageenan-Induced Thermal Hyperalgesia
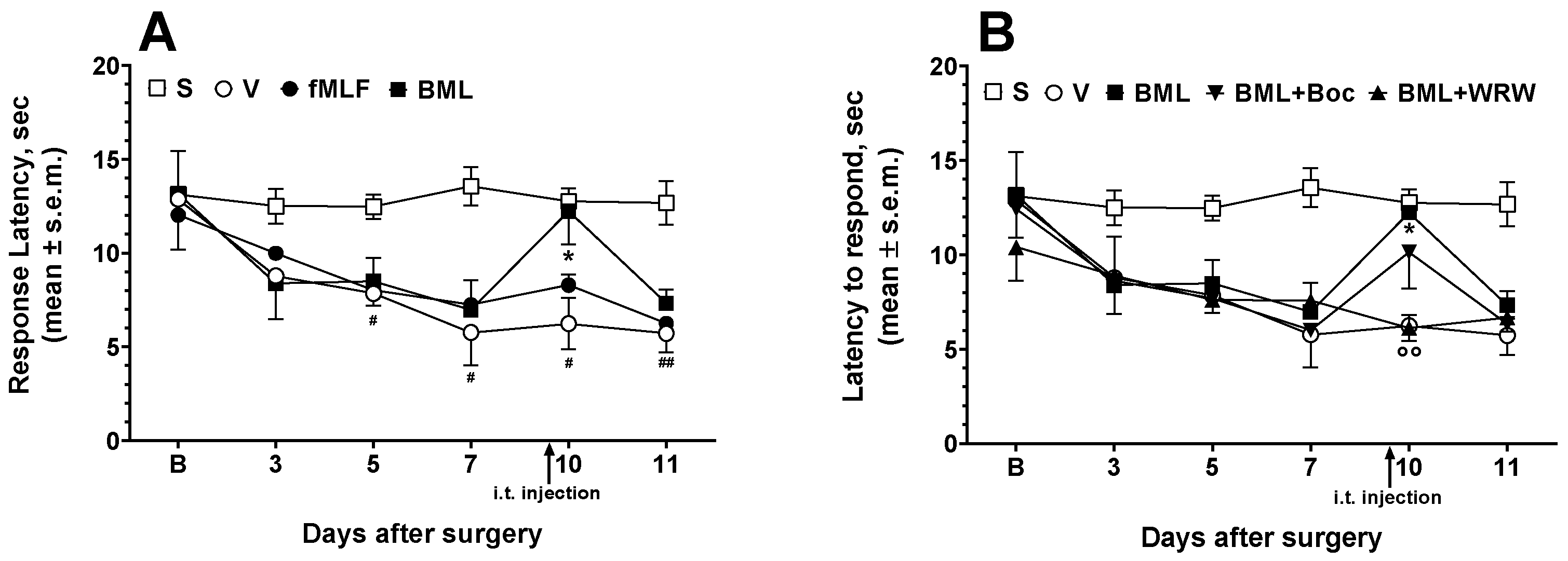
3.4. Effects of FPR Agonists and Antagonists on Neuropathic Hyperalgesia
3.5. Measurement of Cytokines and BDNF in the Spinal Cord
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, H.Q.; Ye, R.D. The formyl peptide receptors: Diversity of ligands and mechanism for recognition. Molecules 2017, 22, 455. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhao, J.; Chen, K.; Bian, X.; Wang, C.; Shi, Y.; Wang, J.M. G Protein-coupled Receptor FPR1 as a Pharmacologic Target in Inflammation and Human Glioblastoma. Int. Immunopharmacol. 2012, 14, 283–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruan, L.; Kong, Y.; Wang, J.M.; Le, Y. Chemoattractants and receptors in Alzheimer’s disease. Front. Biosci. (Schol. Ed.) 2010, 2, 504–514. [Google Scholar] [PubMed] [Green Version]
- Li, Y.; Ye, D. Molecular biology for formyl peptide receptors in human diseases. J. Mol. Med. 2013, 91, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.D.; Boulay, F.; Wang, J.M.; Dahlgren, C.; Gerard, C.; Parmentier, M.; Serhan, C.N.; Murphy, P.M. International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol. Rev. 2009, 61, 119–161. [Google Scholar] [CrossRef] [PubMed]
- He, H.Q.; Troksa, E.L.; Caltabiano, G.; Pardo, L.; Ye, R.D. Structural determinants for the interaction of formyl peptide receptor 2 with peptide ligands. J. Biol. Chem. 2014, 289, 2295–2306. [Google Scholar] [CrossRef] [Green Version]
- Sheikh, M.H.; Solito, E. Annexin A1: Uncovering the Many Talents of an Old Protein. Int. J. Mol. Sci. 2018, 19, 1045–1065. [Google Scholar] [CrossRef] [Green Version]
- Grewal, T.; Rentero, C.; Enrich, C.; Wahba, M.; Raabe, C.A.; Rescher, U. Annexin Animal Models-From Fundamental Principles to Translational Research. Int. J. Mol. Sci. 2021, 22, 3439. [Google Scholar] [CrossRef]
- Cattaneo, F.; Guerra, G.; Ammendola, R. Expression and Signaling of Formyl-Peptide Receptors in the Brain. Neurochem. Res. 2010, 35, 2018–2026. [Google Scholar] [CrossRef]
- Ferreira, S.H.; Cunha, F.Q.; Lorenzetti, B.B.; Michelin, M.A.; Perretti, M.; Flower, R.J.; Poole, S. Role of lipocortin-1 in the anti-hyperalgesic actions of dexamethasone. Br. J. Pharmacol. 1997, 121, 883–888. [Google Scholar] [CrossRef] [Green Version]
- Pieretti, S.; Di Giannuario, A.; De Felice, M.; Perretti, M.; Cirino, G. Stimulus-dependent specificity for annexin 1 inhibition of the inflammatory nociceptive response: The involvement of the receptor for formylated peptides. Pain 2004, 109, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, S.S.; Yazid, S.; Flower, R.J. Increased susceptibility of annexin-A1 null mice to nociceptive pain is indicative of a spinal antinociceptive action of annexin-A1. Br. J. Pharmacol. 2008, 154, 1135–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rittner, H.L.; Hackel, D.; Voigt, P.; Mousa, S.; Stolz, A.; Labuz, D.; Schäfer, M.; Schaefer, M.; Stein, C.; Brack, A. Mycobacteria attenuate nociceptive responses by formyl peptide receptor triggered opioid peptide release from neutrophils. PLoS Pathog. 2009, 5, e1000362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pei, L.; Zhang, J.; Zhao, F.; Su, T.; Wei, H.; Tian, J.; Li, M.; Shi, J. Annexin 1 exerts anti-nociceptive effects after peripheral inflammatory pain through formyl-peptide-receptor-like 1 in rat dorsal root ganglion. Br. J. Anaesth. 2011, 107, 948–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, C.; Ismail, N.; Koh, J.; Gunaseelan, S.; Low, Y.; Ng, Y.; Chua, J.; Ong, W. Localisation of formyl-peptide receptor 2 in the rat central nervous system and its role in axonal and dendritic outgrowth. Neurochem. Res. 2018, 43, 1587–1598. [Google Scholar] [CrossRef] [Green Version]
- Malcangio, M. Role of the immune system in neuropathic pain. Scand. J. Pain 2019, 20, 33–37. [Google Scholar] [CrossRef]
- Hylden, J.L.; Wilcox, G.L. Intrathecal morphine in mice: A new technique. Eur. J. Pharmacol. 1980, 67, 313–316. [Google Scholar] [CrossRef]
- Maione, F.; Colucci, M.; Raucci, F.; Mangano, G.; Marzoli, F.; Mascolo, N.; Crocetti, L.; Giovannoni, M.P.; Di Giannuario, A.; Pieretti, S. New insights on the arylpiperazinylalkyl pyridazinone ET1 as potent antinociceptive and anti-inflammatory agent. Eur. J. Pharmacol. 2020, 888, 173572. [Google Scholar] [CrossRef] [PubMed]
- Bennett, G.J.; Xie, Y.K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988, 33, 87–107. [Google Scholar] [CrossRef]
- Pieretti, S.; Ranjan, A.P.; Di Giannuario, A.; Mukerjee, A.; Marzoli, F.; Di Giovannandrea, R.; Vishwanatha, J.K. Curcumin-loaded Poly (d, l-lactide-co-glycolide) nanovesicles induce antinociceptive effects and reduce pronociceptive cytokine and BDNF release in spinal cord after acute administration in mice. Colloids Surf. B Biointerfaces 2017, 158, 379–386. [Google Scholar] [CrossRef]
- Le Bars, D.; Gozariu, M.; Cadden, S.W. Animal Models of Nociception. Pharmacol. Rev. 2001, 53, 597–652. [Google Scholar] [PubMed]
- Li, T.; Wanga, H.; Wanga, J.; Chena, Y.; Yanga, C.; Zhaoa, M.; Wang, G.; Yang, Z. Annexin 1 inhibits remifentanil-induced hyperalgesia and NMDA receptor phosphorylation via regulating spinal CXCL12/CXCR4 in rats. Neurosci. Res. 2019, 144, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, S.; Ke, X.; Yi, Y.; Yu, H.; Yu, D.; Li, Q.; Shang, Y.; Lu, Y.; Pei, L. The mechanism of Annexin A1 to modulate TRPV1 and nociception in dorsal root ganglion neurons. Cell Biosci. 2021, 11, 167–184. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Wang, H.; Fang, S.; Li, L.; Li, X.; Shi, J.; Zhu, M.; Tan, Z.; Lu, Z. Annexin-1 Mimetic Peptide Ac2-26 Suppresses Infammatory Mediators in LPS-Induced Astrocytes and Ameliorates Pain Hypersensitivity in a Rat Model of Infammatory Pain. Cell Mol. Neurobiol. 2020, 40, 569–585. [Google Scholar] [CrossRef] [PubMed]
- Vieira, C.; Salm, D.C.; Horewicz, V.V.; Ludtke, D.D.; Emer, A.A.; Koerich, J.F.; Mazzardo, G.; Elias, S.; Moré, A.O.O.; Mazzardo-Martins., L.; et al. Electroacupuncture decreases inflammatory pain through a pro-resolving mechanism involving the peripheral annexin A1-formyl peptide receptor 2/ALX-opioid receptor pathway. Pflugers Arch. 2021, 473, 683–695. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colucci, M.; Stefanucci, A.; Mollica, A.; Aloisi, A.M.; Maione, F.; Pieretti, S. New Insights on Formyl Peptide Receptor Type 2 Involvement in Nociceptive Processes in the Spinal Cord. Life 2022, 12, 500. https://doi.org/10.3390/life12040500
Colucci M, Stefanucci A, Mollica A, Aloisi AM, Maione F, Pieretti S. New Insights on Formyl Peptide Receptor Type 2 Involvement in Nociceptive Processes in the Spinal Cord. Life. 2022; 12(4):500. https://doi.org/10.3390/life12040500
Chicago/Turabian StyleColucci, Mariantonella, Azzurra Stefanucci, Adriano Mollica, Anna Maria Aloisi, Francesco Maione, and Stefano Pieretti. 2022. "New Insights on Formyl Peptide Receptor Type 2 Involvement in Nociceptive Processes in the Spinal Cord" Life 12, no. 4: 500. https://doi.org/10.3390/life12040500
APA StyleColucci, M., Stefanucci, A., Mollica, A., Aloisi, A. M., Maione, F., & Pieretti, S. (2022). New Insights on Formyl Peptide Receptor Type 2 Involvement in Nociceptive Processes in the Spinal Cord. Life, 12(4), 500. https://doi.org/10.3390/life12040500








