Capillaroscopy and Immunological Profile in Systemic Sclerosis
Abstract
1. Introduction
2. Materials and Methods
- We performed IIF on HEp-2 cells (IIFT: HEp-2, Biosystems, Spain) as the gold-standard screening method for the detection of ANA and determination of the immunofluorescent staining pattern (anti-cell pattern (AC)) according to the International Consensus on ANA Patterns (ICAP, https://www.anapatterns.org/31.01.2022, accessed on 31 January 2022). ANA titer 1:160 or higher was considered positive.
- A mixed pattern consisted of a 5-element (subcellular regions) compound staining pattern AC-29, Topo I-like pattern:
- (1)
- Prominent, fine-speckled AC-4 type nuclear staining in interphase cells.
- (2)
- Consistent, strong, fine-speckled staining of the condensed chromatin in mitotic cells that appeared homogeneous at the initial dilution 1:80.
- (3)
- Strong staining of nucleolar organizing region (NOR) associated with condensed chromosomes in mitotic cells. The NOR staining at dilution 1:80 was obscured by the bright homogeneous chromosomal staining.
- (4)
- Weak cytoplasmic staining in interphase (and mitotic) cells, depicting a delicate network radiating from the perinuclear area towards the plasma membrane.
- (5)
- A nucleolar staining that appeared as a punctate nucleolar staining in interphase cells.
- We analyzed the patients’ serum for the presence of SSc-associated autoantibodies using a commercial line immunoblot assay (Scleroderma (Nucleoli) Profile Euroline (IgG); Euroimmun). The line blot detects autoantibodies to 13 scleroderma-associated autoantigens: Scl-70, CENP A, CENP B, RP11/RNAP-III, RP155/RNAP-III, fibrillarin, NOR-90, Th/To, PM-Scl100, PM-Scl75, Ku, PDGFR, and Ro-52. A single operator performed the assays and analyses per the manufacturer’s instructions. We considered the positive and negative results as defined by the assay, and we reported results in the borderline range as negative.
Statistical Processing and Study Design
3. Results
4. Discussion
4.1. Associations between Capillaroscopic Changes and SSc-Associated Autoantibodies
4.2. Associations between Capillaroscopic Changes and ANA Titer; Significance of AC Pattern
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pattanaik, D.; Brown, M.; Postlethwaite, B.C.; Postlethwaite, A.E. Pathogenesis of systemic sclerosis. Front Immunol. 2015, 6, 272. [Google Scholar] [CrossRef] [PubMed]
- Van Den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A., Jr.; Carreira, P.E.; et al. 2013 classification criteria for systemic sclerosis: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann. Rheum. Dis. 2013, 71, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
- Van Den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A., Jr.; Carreira, P.E.; et al. 2013 classification criteria for systemic sclerosis: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2013, 65, 2737–2747. [Google Scholar] [CrossRef] [PubMed]
- Maricq, H.R.; LeRoy, E.C.; D’angelo, W.A.; Medsger, T.A., Jr.; Rodnan, G.P.; Sharp, G.C.; Wolfe, J.F. Diagnostic potential of in vivo capillary microscopy in scleroderma and related disorders. Arthritis Rheum. 1980, 23, 183–189. [Google Scholar] [CrossRef]
- Maricq, H.R.; Harper, F.E.; Khan, M.M.; Tan, E.M.; LeRoy, E.C. Microvascular abnormalities as possible predictors of disease subsets in Raynaud phenomenon and early connective tissue disease. Clin. Exp. Rheumatol. 1983, 1, 195–205. [Google Scholar]
- Bergman, R.; Sharony, L.; Schapira, D.; Nahir, M.A.; Balbir-Gurman, A. The handheld dermatoscope as a nail-fold capillaroscopic instrument. Arch. Dermatol. 2003, 139, 1027–1030. [Google Scholar] [CrossRef]
- Lambova, S.; Hermann, W.; Müller-Ladner, U. Capillaroscopic pattern at the toes of systemic sclerosis patients: Does it ‘“tell”’ more than those of fingers? J. Clin. Rheumatol. 2011, 17, 311–314. [Google Scholar] [CrossRef]
- Lambova, S.; Müller-Ladner, U. Capillaroscopic findings in systemic sclerosis—Are they associated with disease duration and presence of digital ulcers? Discov. Med. 2011, 12, 413–418. [Google Scholar]
- Kenik, J.G.; Maricq, H.R.; Bole, G.G. Blind evaluation of the diagnostic specificity of nailfold capillary microscopy in the connective tissue diseases. Arthritis Rheum. 1981, 24, 885–891. [Google Scholar] [CrossRef]
- Cutolo, M.; Sulli, A.; Pizzorni, C.; Accardo, S. Nailfold videocapillaroscopy assessment of microvascular damage in systemic sclerosis. J. Rheumatol. 2000, 27, 155–160. [Google Scholar]
- Cutolo, M.; Pizzorni, C.; Tuccio, M.; Burroni, A.; Craviotto, C.; Basso, M.; Seriolo, B.; Sulli, A. Nailfold videocapillaroscopic patterns and serum autoantibodies in systemic sclerosis. Rheumatol. Oxf. 2004, 43, 719–726. [Google Scholar] [CrossRef]
- Ingegnoli, F.; Ardoino, I.; Boracchi, P.; Cutolo, M.; Airò, P.; Ananieva, L.P.; Ancuta, C.; Andrade, L.E.; Becvar, R.; Benenati, A.; et al. Nailfold capillaroscopy in systemic sclerosis: Data from the EULAR scleroderma trials and research (EUSTAR) database. Microvasc. Res. 2013, 89, 122–128. [Google Scholar] [CrossRef]
- Van Leeuwen, N.M.; Wortel, C.M.; Fehres, C.M.; Bakker, J.A.; Scherer, H.U.; Toes, R.E.; Huizinga, T.W.; de Vries-Bouwstra, J.K. Association between centromere- and topoisomerase-specific immune responses and the degree of microangiopathy in systemic sclerosis. J. Rheumatol. 2021, 48, 402–409. [Google Scholar] [CrossRef]
- Pizzorni, C.; Giampetruzzi, A.R.; Mondino, C.; Facchiano, A.; Abeni, D.; Paolino, S.; Ruaro, B.; Smith, V.; Sulli, A.; Cutolo, M. Nailfold capillaroscopic parameters and skin telangiectasiapatterns in patients withsystemic sclerosis. Microvasc. Res. 2017, 111, 20–24. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Silver, R.M.; Ainsworth, S.K.; Dobson, R.L.; Rust, P.; Maricq, H.R. Association between fluorescent antinuclear antibodies, capillary patterns, and clinical features in scleroderma spectrum disorders. Am. J. Med. 1984, 77, 812–822. [Google Scholar] [CrossRef]
- Caramaschi, P.; Canestrini, S.; Martinelli, N.; Volpe, A.; Pieropan, S.; Ferrari, M.; Bambara, L.M.; Carletto, A.; Biasi, D. Scleroderma patients nailfold videocapillaroscopic patterns are associated with disease subset and disease severity. Rheumatol. Oxf. 2007, 46, 1566–1569. [Google Scholar] [CrossRef]
- Van Leeuwen, N.M.; Ciaffi, J.; Schoones, J.W.; Huizinga, T.W.J.; de Vries-Bouwstra, J.K. Contribution of sex and autoantibodies to microangiopathy assessed by nailfold videocapillaroscopy in systemic sclerosis: A systematic review of the literature. Arthritis Care Res. 2021, 73, 722–731. [Google Scholar] [CrossRef]
- Kurteva, E.K. New immunological parameters in the diagnostic and pathogenic mechanisms in systemic sclerosis. Ph.D. Thesis, Medical University of Sofia, Sofia, Bulgaria, 2019. [Google Scholar]
- Markusse, I.M.; Meijs, J.; de Boer, B.; Bakker, J.A.; Schippers, H.P.C.; Schouffoer, A.A.; Ajmone Marsan, N.; Kroft, L.J.; Ninaber, M.K.; Huizinga, T.W.; et al. Predicting cardiopulmonaryinvolvement in patients with systemic sclerosis: Complementary value of nailfold videocapillaroscopy patternand disease-specific autoantibodies. Rheumatol. Oxf. 2017, 56, 1081–1088. [Google Scholar]
- Tieu, J.; Hakendorf, P.; Woodman, R.J.; Patterson, K.; Walker, J.; Roberts-Thomson, P. The role of nailfold 461 capillary dropout on mortality in systemic sclerosis. Intern. Med. J. 2018, 48, 517–523. [Google Scholar] [CrossRef]
- Schmidt, J.A.; Caspary, L.; von Bierbrauer, A.; Ehrly, A.M.; Jünger, M.; Jung, F.; Lawall, H. Standardisierung der Nagelfalz-Kapillarmikroskopie in der Routiendiagnostik. Vasa 1997, 26, 5–10. [Google Scholar]
- Lambova, S.N.; Muller-Ladner, U. Inhomogeneity of capillaroscopic findings in systemic sclerosis. Int. J. Rheum. Dis. 2019, 23, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Callejas-Moraga, E.; Guillén-Del-Castillo, A.; Marín-Sánchez, A.; Fonollosa-Pia, V.; Balada, E.; Martin, J.; Simeon-Aznar, C.P. AB0752 Clinical features of systemic sclerosis associated to anti rna polymerase iii antibodies in valld’hebron hospital. Ann. Rheum. Dis. 2018, 77, 1513. [Google Scholar]
- Callejas-Moraga, E.; Guillén-Del-Castillo, A.; Marín-Sánchez, A.; Roca-Herrera, M.; Balada, E.; Tolosa-Vilella, C.; Fonollosa-Pla, V.; Simeón-Aznar, C.P. Clinical features of systemic sclerosis patients with anti-RNA polymerase III antibody in a single centre in Spain. Clin. Exp. Rheumatol. 2019, 119 (Suppl. S37), 41–48. [Google Scholar]
- Yang, C.; Tang, S.; Zhu, D.; Ding, Y.; Qiao, J. Classical disease-specific autoantibodies in systemic sclerosis: Clinical features, gene susceptibility, and disease stratification. Front Med. Lausanne 2020, 7, 587773. [Google Scholar] [CrossRef] [PubMed]
- Fava, A.; Cimbro, R.; Wigley, F.M.; Liu, Q.-R.; Rosen, A.; Boin, F. Frequency of circulating topoisomerase-I-specific CD4 T cells predicts presence and progression of interstitial lung disease in scleroderma. Arthritis Res. Ther. 2016, 18, 99. [Google Scholar] [CrossRef] [PubMed]
- Radić, M.; Martinović Kaliterna, D.; Ljutić, D. The level of anti-topoisomerase I antibodies highly correlates with metacarpophalangeal and proximal interphalangeal joints flexion contractures in patients with systemic sclerosis. Clin. Exp. Rheumatol. 2006, 24, 407–412. [Google Scholar]
- Filaci, G.; Cutolo, M.; Basso, M.; Murdaca, G.; Derchi, L.; Gianrossi, R.; Ropolo, F.; Zentilin, P.; Sulli, A.; Puppo, F.; et al. Long-term treatment of patients affected by systemic sclerosis with cyclosporin A. J. Rheumatol. 2001, 40, 1431–1432. [Google Scholar] [CrossRef]
- Caramaschi, P.; Volpe, A.; Pieropan, S.; Tinazzi, I.; Mahamid, H.; Bambara, L.M.; Biasi, D. Cyclophosphamide treatment improves microvessel damage in systemic sclerosis. Clin. Rheumatol. 2009, 28, 391–395. [Google Scholar] [CrossRef]
- Bellando-Randone, S.; Lepri, G.; Bruni, C.; Blagojevic, J.; Radicati, A.; Cometi, L.; De Paulis, A.; Matucci-Cerinic, M.; Guiducci, S. Combination therapy with Bosentan and Sildenafil improves Raynaud’s phenomenon and fosters the recovery of microvascular involvement in systemic sclerosis. Clin. Rheumatol. 2016, 35, 127–132. [Google Scholar] [CrossRef][Green Version]
- Sobanski, V.; Dauchet, L.; Lefevre, G.; Lambert, M.; Morell-Dubois, S.; Sy, T.; Hachulla, E.; Hatron, P.Y.; Launay, D.; Dubucquoi, S. Prevalence of anti-RNA polymerase III antibodies in systemic sclerosis: New data from a French cohort and a systematic review and meta-analysis. Arthritis Rheum. 2014, 66, 407–417. [Google Scholar] [CrossRef]
- Koenig, M.; Dieudé, M.; Senécal, J. Predictive value of antinuclear autoantibodies: The lessons of the systemic sclerosis autoantibodies. Autoimmun. Rev. 2008, 7, 588–593. [Google Scholar] [CrossRef]
- Reveille, J.D.; Solomon, D.H. Evidence-based guidelines for the use of immunologic tests:aAnticentromere, Scl-70, and nucleolar antibodies. Arthritis Rheum. 2003, 49, 399–412. [Google Scholar] [CrossRef]
- Meyer, O.C.; Fertig, N.; Lucas, M.; Somogyi, N.; Medsger, T.A., Jr. Disease subsets, antinuclear antibody profile, and clinical features in 127 French and 247 US adult patients with systemic sclerosis. J. Rheumatol. 2007, 34, 104–109. [Google Scholar]
- Wielosz, E.; Dryglewska, M.; Majdan, M. Clinical consequences of the presence of anti-RNA Pol III antibodies in systemic sclerosis. Adv. Dermatol. Allergol. 2020, 37, 909–914. [Google Scholar] [CrossRef]
- Northway, J.D.; Tan, E.M. Differentiation of antinuclear antibodies giving Speckled staining patterns in immunofluorescence. Clin. Immunol. Immunopathol. 1972, 1, 140–154. [Google Scholar] [CrossRef]
- Reimer, G.; Rose, K.M.; Scheer, U.; Tan, E.M. Autoantibody to RNA polymerase I in scleroderma sera. J. Clin. Investig. 1987, 79, 65–72. [Google Scholar] [CrossRef]
- Kuwana, M.; Kaburaki, J.; Mimori, T.; Tojo, T.; Homma, M. Autoantibody reactive with three classes of RNA polymerases in sera from patients with systemic sclerosis. J. Clin. Investig. 1993, 91, 1399–1404. [Google Scholar] [CrossRef]
- Yamasaki, Y.; Honkanen-Scott, M.; Hernandez, L.; Ikeda, K.; Barker, T.; Bubb, M.R.; Narain, S.; Richards, H.B.; Chan, E.K.; Reeves, W.H.; et al. Nucleolar staining cannot be used as a screening test for the scleroderma marker anti-RNA polymerase I/III antibodies. Arthritis Rheum. 2006, 54, 3051–3056. [Google Scholar] [CrossRef]
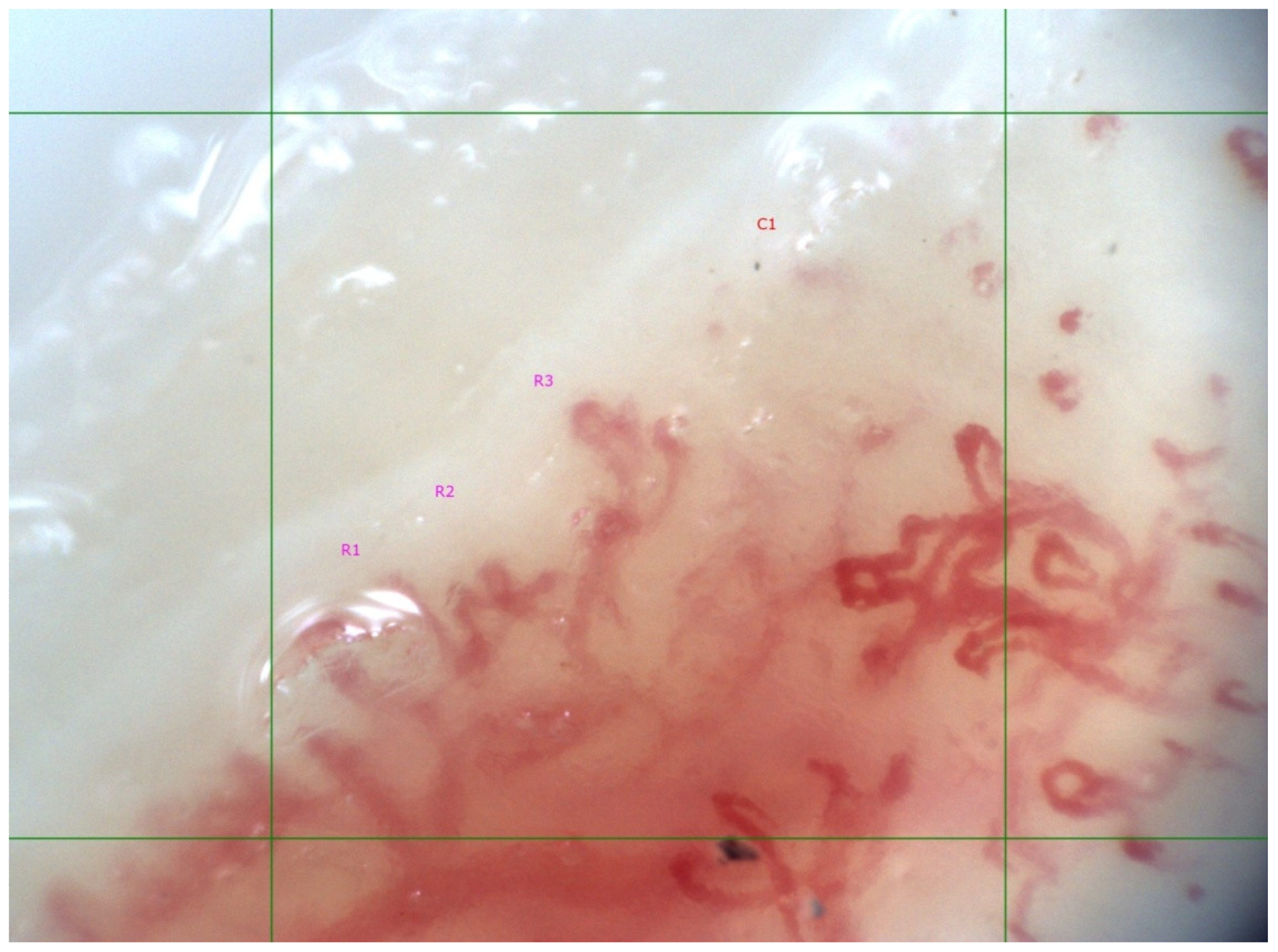
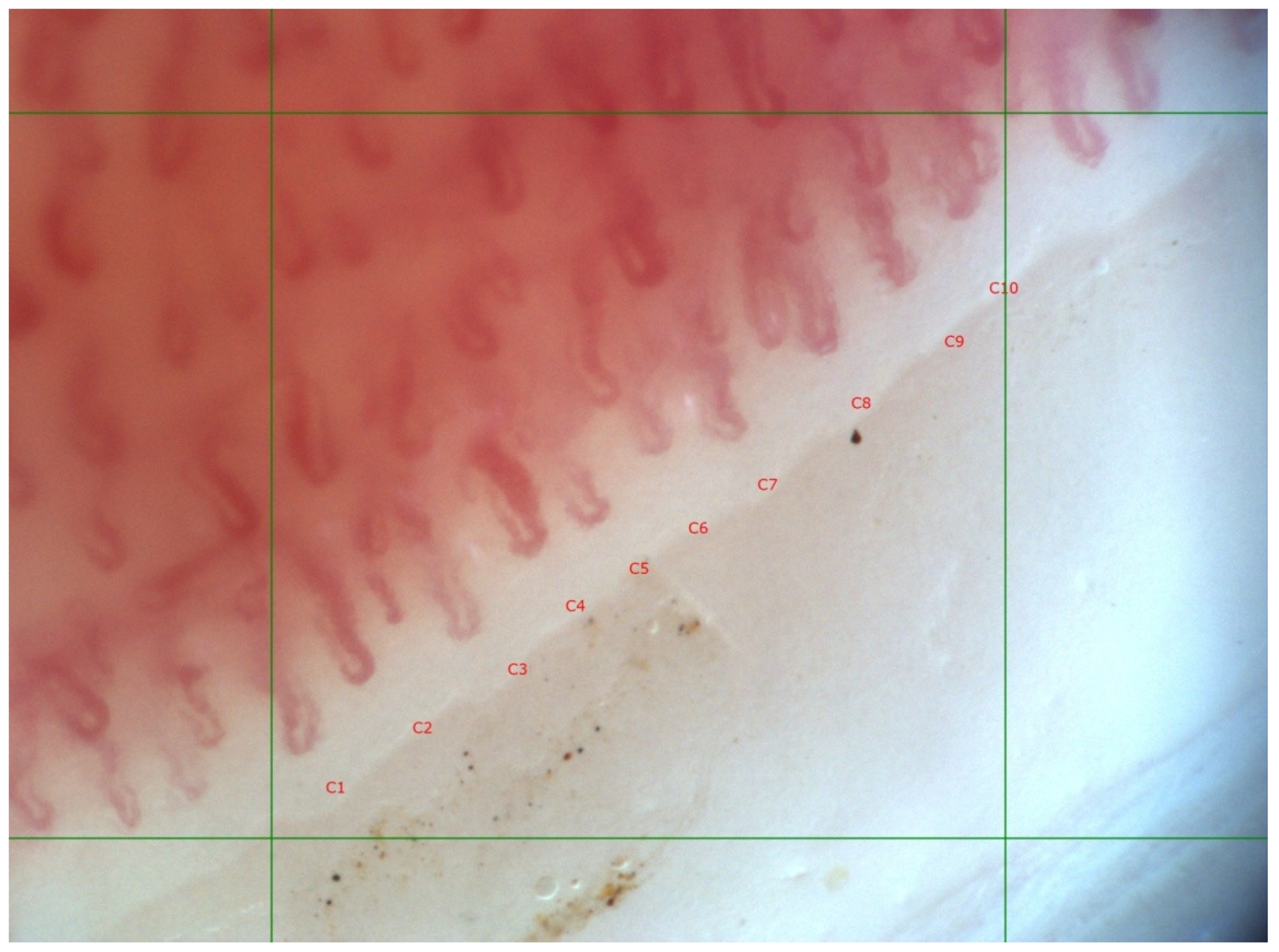
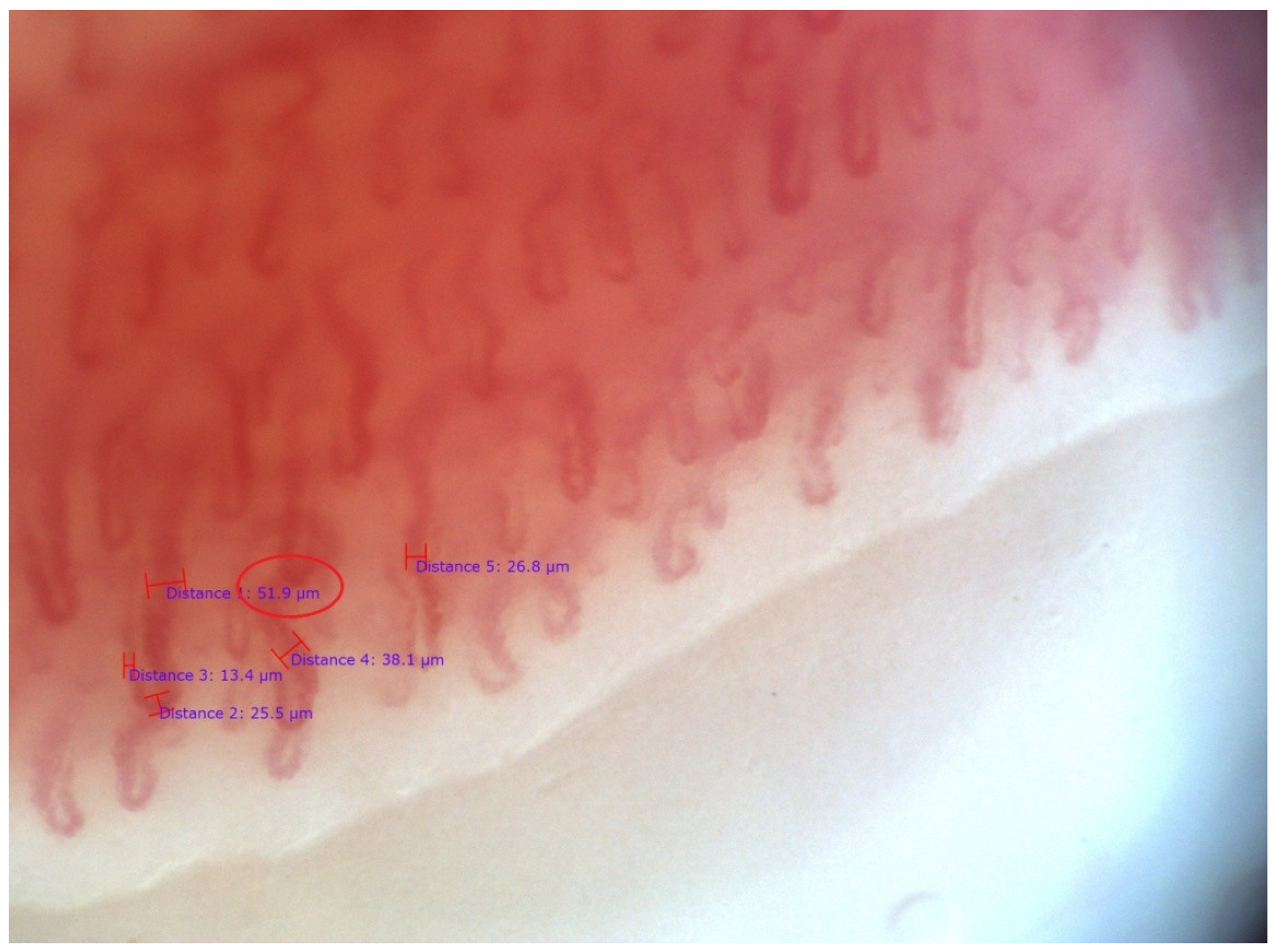
| SSc Patients, n = 19 | |
|---|---|
| Gender | 1 male, 18 females |
| Age | 51.56 ± 15.07 years |
| Type of cutaneous involvement | 16 limited, 3 diffuse cutaneous involvement |
| Presence of RP Digital ulcers | 100% 57% (11/19) |
| Other manifestations: | |
| Pulmonary Cardiac Joint Tendon | 57% (11/19) 10.5% (2/19) 42% (8/19) 21% (4/19) |
| Treatment in the last 6 months: | |
| Vasodilators Antiplatelet drugs Low-dose corticosteroids Cytotoxic drugs D-penicillamine | 79% (15/19) 26% (5/19 26% (5/19) 15.7% (3/19) 15.7% (3/19) |
| Case 1 | 20-year old male patient with limited cutaneous involvement, RP, joint and tendon involvement. Disease duration: 5 years. ANA titer 1:320, AC-5. The patient has received vasodilators in the last 6 months. Capillaroscopic examination revealed normal pattern. |
| Case 2 | 64-year old female patient with diffuse cutaneous involvement, RP, pulmonary involvement. Disease duration: 14 years. ANA titer 1:320, AC-5. The patient has received therapy with cyclophosphamide and corticosteroids in the last 6 months. The patient has received vasodilators, anti-platelet drug, and D-penicillamin in the last 6 months. Capillaroscopic examination revealed normal pattern. |
| Case 3 | 57-year old female patient with limited cutaneous involvement, RP, joint and tendon involvement. Disease duration: 13 years. ANA titer 1:160, AC-5. Capillaroscopic examination revealed “early” pattern. |
| Case 4 | 32-year old female patient with limited cutaneous involvement, RP, joint and tendon involvement. Disease duration: 15 years. ANA titer 1:1280, AC-5, AC-10. The patient has received vasodilators in the last 6 months. Capillaroscopic examination revealed normal pattern. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lambova, S.N.; Kurteva, E.K.; Dzhambazova, S.S.; Vasilev, G.H.; Kyurkchiev, D.S.; Geneva-Popova, M.G. Capillaroscopy and Immunological Profile in Systemic Sclerosis. Life 2022, 12, 498. https://doi.org/10.3390/life12040498
Lambova SN, Kurteva EK, Dzhambazova SS, Vasilev GH, Kyurkchiev DS, Geneva-Popova MG. Capillaroscopy and Immunological Profile in Systemic Sclerosis. Life. 2022; 12(4):498. https://doi.org/10.3390/life12040498
Chicago/Turabian StyleLambova, Sevdalina Nikolova, Ekaterina Krasimirova Kurteva, Sanie Syuleymanova Dzhambazova, Georgi Hristov Vasilev, Dobroslav Stanimirov Kyurkchiev, and Mariela Gencheva Geneva-Popova. 2022. "Capillaroscopy and Immunological Profile in Systemic Sclerosis" Life 12, no. 4: 498. https://doi.org/10.3390/life12040498
APA StyleLambova, S. N., Kurteva, E. K., Dzhambazova, S. S., Vasilev, G. H., Kyurkchiev, D. S., & Geneva-Popova, M. G. (2022). Capillaroscopy and Immunological Profile in Systemic Sclerosis. Life, 12(4), 498. https://doi.org/10.3390/life12040498







